Abstract
BACKGROUND & AIMS
Cigarette smoke has been identified as an independent risk factor for chronic pancreatitis (CP). Little is known about the mechanisms by which smoking promotes development of CP. We assessed the effects of aryl hydrocarbon receptor (AhR) ligands found in cigarette smoke on immune cell activation in humans and pancreatic fibrosis in animal models of CP.
METHODS
We obtained serum samples from patients with CP treated at Stanford University hospital and healthy individuals (controls) and isolated CD4+ T cells. Levels of interleukin-22 (IL22) were measured by enzyme-linked immunosorbent assay and smoking histories were collected. T cells from healthy nonsmokers and smokers were stimulated and incubated with AhR agonists (2,3,7,8-tetrachlorodibenzo-p-dioxin or benzo[a]pyrene) or antagonists and analyzed by flow cytometry. Mice were given intraperitoneal injections of caerulein or saline, with or without lipopolysaccharide, to induce CP. Some mice were given intraperitoneal injections of AhR agonists at the start of caerulein injection, with or without an antibody against IL22 (anti-IL22) starting 2 weeks after the first caerulein injection, or recombinant mouse IL22 or vehicle (control) intraperitoneally 4 weeks after the first caerulein injection. Mice were exposed to normal air or cigarette smoke for 6 h/d for 7 weeks and expression of AhR gene targets was measured. Pancreata were collected from all mice and analyzed by histology and quantitative reverse transcription polymerase chain reaction. Pancreatic stellate cells and T cells were isolated and studied using immunoblot, immunofluorescence, flow cytometry, and enzyme-linked immunosorbent analyses.
RESULTS
Mice given AhR agonists developed more severe pancreatic fibrosis (based on decreased pancreas size, histology, and increased expression of fibrosis-associated genes) than mice not given agonists after caerulein injection. In mice given saline instead of caerulein, AhR ligands did not induce fibrosis. Pancreatic T cells from mice given AhR agonists and caerulein were activated and expressed IL22, but not IL17 or interferon gamma. Human T cells exposed to AhR agonists up-regulated expression of IL22. In mice given anti-IL22, pancreatic fibrosis did not progress, whereas mice given recombinant IL22 had a smaller pancreas and increased fibrosis. Pancreatic stellate cells isolated from mouse and human pancreata expressed the IL22 receptor IL22RA1. Incubation of the pancreatic stellate cells with IL22 induced their expression of the extracellular matrix genes fibronectin 1 and collagen type I α1 chain, but not α2 smooth muscle actin or transforming growth factor–β. Serum samples from smokers had significantly higher levels of IL22 than those from nonsmokers.
CONCLUSIONS
AhR ligands found in cigarette smoke increase the severity of pancreatic fibrosis in mouse models of pancreatitis via up-regulation of IL22. This pathway might be targeted for treatment of CP and serve as a biomarker of disease.
Keywords: TCDD, BaP, Immune Regulation, PSC, CP
Chronic pancreatitis (CP) is a progressive inflammatory disease of the pancreas with gradual fibrotic replacement of the gland.1,2 Heavy and prolonged alcohol use is a common cause of CP, and most patients experience recurrent episodes of acute pancreatitis for several years before developing CP.3,4 Interestingly, only 3% of alcoholics develop CP, implying the presence of co-factors that amplify the effect of alcohol. Indeed, several studies have found smoking to be an independent risk factor for CP and its negative effects are additive with alcohol, accelerating CP progression,5–9 but the mechanisms remain elusive.
Pancreatic stellate cells (PSCs) are responsible for producing fibrous tissue in CP and could be activated by alcohol and cytokines released by injured acinar cells or local recruited leukocytes. Just recently, an in vitro study determined that PSCs could be activated by clinically relevant concentrations of cigarette smoke component, nicotine-derived nitrosamine ketone, and that PSCs express the nicotinic acetylcholine receptors, indicating a potential mechanism for smoking-induced CP progression.8
Besides nicotine, cigarette smoke also contains aryl hydrocarbon receptor (AhR) agonists, such as dioxin and benzo(a)pyrene (BaP).10,11 In addition, cigarette smoke was found to have an unexpectedly high dioxin-like potential that triggers AhR activation.12 In the past several years, AhR has been established as a critical ligand-dependent transcription factor involved in the regulation of the immune system, such as T-cell differentiation and dendritic cell function. Therefore, we sought to investigate the role of AhR ligands in cigarette smoke on immune activation and the pathogenesis of CP.
In this study, we investigated the effects of 2 well-described AhR ligands present in cigarette smoke on immune signaling and pancreatic fibrosis. The ligands led to leukocyte AhR activation and induced interleukin (IL) 22 generation, which promoted fibrosis in experimental CP. AhR deficiency had a protective role and IL22 neutralization suppressed pancreatic fibrosis. Importantly, PSCs had a functional IL22 receptor and up-regulated extracellular matrix (ECM) gene expression in response to exogenous IL22, both in vitro and in vivo. Interestingly, CP patients have higher circulating IL22 levels compared with healthy subjects. Among the CP patients, smokers had significantly higher circulating IL22 levels relative to non- or ex-smokers, further strengthening the link between smoking and IL22, as well as providing a potential immune target and biomarker for CP.
Material and Methods
Mice and Treatments
All animal protocols were approved by Institutional Animal Care and Use Committees of Stanford University. All mice including Balb/c, C57BL/6J, and AhRd were purchased from Jackson Laboratory (Bar Harbor, ME) and were housed under pathogen-free conditions. Experimental mice were age- and sex-matched. Bone marrow (BM) chimeric mice were generated by lethally irradiating mice with 9.5 Gy γ-radiation in 2 doses approximately 3 hours apart, followed by intravenous injection of 5 × 106 BM cells from wild-type (WT) or AhRd mice. Chimeric mice were left to engraft for at least 8 weeks before further experimental manipulation. CP was induced by repetitive caerulein injections13 with or without lipopolysaccharide (LPS).14 In brief, mice were given 6 hourly intraperitoneal injections of 50 μg/kg body weight caerulein (Sigma-Aldrich, St Louis, MO) 3 days per week, for a total of 4 or 6 weeks. Where indicated, control mice were injected with saline (instead of caerulein). In the caerulein + LPS model, LPS (3 mg/kg) was given once a week as an intraperitoneal injection 1 hour after the last dose of caerulein. Mice were then sacrificed and analyzed 3 days after the last caerulein injection.
Mice were given 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (1 or 10 μg/kg, weekly; AccuStandard Inc, New Haven, CT) intraperitoneally with or without BaP (5 or 25 mg/kg, daily; Sigma-Aldrich) orally at the start of caerulein injection for 4 weeks. For a neutralizing experiment, mice were treated with 2 weeks of isotype control or anti-IL22 (αIL22, 150 μg/mouse, 3 times/week; Genentech, South San Francisco, CA) together with TCDD (given weekly) starting at 2 weeks after first caerulein injection (ie, anti-IL22 treatment started at 2 weeks and after establishment of CP). Murine recombinant IL22 (rIL22) (100 ng/mouse, 5 times per week; Miltenyi Biotec, Bergisch Gladbach, Germany) or vehicle control was administrated intraperitoneally to mice at 4 weeks after first caerulein injection and mice were euthanized at 6 weeks. Pancreas from normal mice exposed to normal air or cigarette smoke for 6 h/ d for 7 weeks as described previously15 were used for Western blot or qualitative reverse transcription polymerase chain reaction.
Human Samples
Serum from healthy subjects and CP patients were obtained from Stanford Hospital with Institutional Review Board approval and patient consent.
Histology
Mice were euthanized by CO2 inhalation, and then pancreata were removed rapidly. Pancreas pieces were immediately fixed in 10% formalin, and then fixed tissues were sectioned and used for H&E and Trichrome or Sirius Red staining (performed by Histo-Tec Laboratory, Hayward, CA). Fibrotic area was quantified based on Sirus Red staining and Image J software analysis.
Quantitative Reverse Transcription Polymerase Chain Reaction
Total pancreas or isolated cells were lysed with Trizol reagent (Invitrogen, Carlsbad, CA) for total RNA preparation according to manufacturer’s instructions. Briefly, complementary DNA was generated using GoScript reverse transcription system (Promega, Madison, WI). Quantitative polymerase chain reaction was performed with an ABI Step One Platform (Applied Biosystems, Carlsbad, CA) using designed specific TaqMan probes and primers, as described previously.13 Transcripts levels were normalized to housekeeping gene Gapdh and displayed as fold induction over untreated controls unless otherwise stated.
Cell Preparation
Pancreatic leukocytes were isolated using collagenase digestion method as described previously for flow cytometry analysis.13,16 PSCs from CP mice or human surgical specimens were isolated by outgrowth method as described.13 Murine PSCs were cultured in Dulbecco’s modified Eagle medium/F12 (1:1) containing 10% fetal bovine serum and were ready for use after the second passage.
In Vitro T-Cell Differentiation
Human peripheral blood mononuclear cells were isolated from buffy coat (blood bank) or blood from volunteer healthy smokers or nonsmokers by Ficoll-Hypaque density gradient centrifugation, and then naïve CD4+ (from buffy coat) or total CD4+ (from smoker or nonsmoker healthy volunteer blood) T cells were purified with magnetic beads (Miltenyi Biotec) and cultured at 105 cells per well in 96-well round bottom plates. Enriched T cells were then stimulated for 5 days using plate-bound antibody to CD3 (1 μg/mL) plus soluble antibody to CD28 (2 μg/mL), together with indicated concentration of AhR agonists or antagonists.
Flow Cytometry
For surface staining, cells were stained with antibody to the following markers: CD45.2, CD4, CD19, NK1.1 CD11b, F4/80, CD44, and CD45RB (BioLegend, San Diego, CA). For intracellular cytokine staining, immediately after isolation, cells were cultured in RPMI complete medium and stimulated with phorbol myristate acetate (50 ng/mL) and ionomycin (1 μg/mL) in the presence of brefeldin A (10 μg/mL; eBioscience, San Diego, CA) for 4 hours. Cells were washed and subjected to surface staining. Cells were then fixed and permeabilized using eBioscience kit following manufacturer’s guidelines. Mouse phycoerythrin-IL22 (eBioscience), allophycocyanin-IL17α (BioLegend), AF700-interferon gamma (BD, San Jose, CA), and matched isotype controls were used for intracellular staining. For human T-cell intracellular staining, phycoerythrin-IL22, allophycocyanin-IL17α, and AF700-interferon gamma antibodies were all from BioLegend. Dead cells were excluded from analysis using violet viability stain (Invitrogen). Flow cytometry data collection was performed on Fortessa LSRII (BD) and analyzed using FlowJo software (Tree Star, Inc, Ashland, OR).
Western Blotting, Immunofluorescence, and Enzyme-Linked Immunosorbent Assay
Primary PSCs were treated with murine rIL22 and homogenized in RIPA buffer containing protein inhibitors and analyzed by Western blot, as described previously.16 Antibodies to IL22RA1, α–smooth muscle actin (αSMA; Abcam, Cambridge, MA), signal transducers and activators of transcription (STAT) 3, and phosphorylated STAT3 (Cell Signaling Technologies, Danvers, MA) were used. Antibodies to CYP1a1 (Novus Biologicals, Littleton, CO), β-tubulin (Cell Signaling Technologies), and actin (Sigma) were used in blots of pancreas homogenate from air- or cigarette smoke–exposed mice. Immunofluorescence staining for IL22RA1 and αSMA was performed according to manufacturer’s guideline and analyzed using confocal microscope. Serum samples from healthy subjects and CP patients were analyzed using enzyme-linked immunosorbent assay kit for human IL22 detection (eBioscience).
Statistical Analysis
One-way analysis of variance with Tukey’s post-hoc test was used to determine statistical significance between multiple groups unless otherwise indicated. Unpaired Student t test was used to determine statistical significance between 2 groups and P value <.05 was considered significant. Values are expressed as mean ± SEM or mean ± SD (Prism 5, GraphPad Software, San Diego, CA). Unless indicated, results are from at least 2–3 independent experiments with ≥4 mice per group.
Results
Aryl Hydrocarbon Receptor Activation Worsens Fibrosis in Chronic Pancreatitis
Cigarette smoking is an independent risk factor for accelerating CP,6,9 however, the mechanism remains elusive. Cigarette smoke contains AhR agonists, such as dioxin and BaP10,11; in addition, cigarette smoke was found to have an unexpectedly high dioxin-like potential that triggers AhR activation.12 Therefore, we sought to investigate the role of cigarette smoke AhR ligands on immune activation and on the pathogenesis of CP.
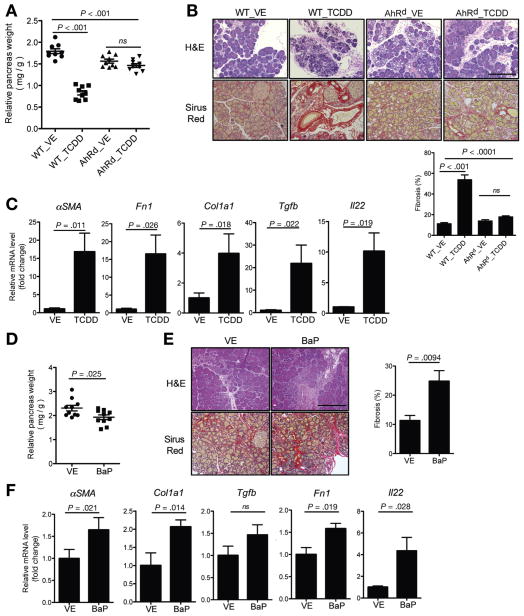
The well-characterized potent AhR agonist TCDD was administrated to mice undergoing caerulein-induced CP. Compared with the vehicle group, TCDD treatment worsened fibrosis in CP, as shown by decreased pancreas size, histology, and increased fibrosis-associated gene expression, such as αSMA (αSMA), Fn1 (fibronectin 1), Col1α1 (Collagen 1A1), and Tgfb (transforming growth factor–β [TGFβ]) (Figure 1A–C). However, AhR mutant or nonresponsive (AhRd) mice16 had little response to TCDD treatment (Figure 1A–C), indicating that pro-fibrotic effects of TCDD are mediated via AhR activation. Consistent with these findings, another AhR agonist (BaP) found in cigarette smoke also promoted fibrosis in CP (Figure 1D–F). BaP had less of a pronounced effect compared with TCDD. This is likely due to the fact that TCDD is one of the most potent AhR ligands, with an estimated EC50 (dose at which ligand leads to 50% of maximal target gene, such as cytochrome P450, CYP1A1, induction) of 10−12, whereas BaP’s EC50 is around 10−5 to 10−6.17 Because both AhR ligands coexist in cigarette smoke, we tested the effect of combined lower and higher doses of the AhR ligands in Balb/c mice because C57BL/6 mice develop severe fibrosis and may be difficult to detect smaller changes. Low-dose TCDD (1 μg/kg) did not increase the fibrotic effect of chronic caerulein administration, but in combination with low-dose BaP (5 mg/kg), fibrosis increased significantly, although a statistically significant change in gross pancreas weight was not observed (Supplementary Figure 1). As expected, a combination of the higher dose of TCDD and BaP led to a significant decrease in pancreas weight and increase in fibrosis. These results suggest that AhR ligands present in cigarette smoke have additive effects. In control mice chronically treated with saline instead of caerulein, AhR ligands did not induce fibrosis (Supplementary Figure 2), suggesting that AhR activation (cigarette smoke) alone is not sufficient, and additional pancreatic insults are required to induce CP.
Figure 1.
AhR activation worsens fibrosis in chronic pancreatitis. (A) Vehicle (VE) or AhR ligand (TCDD, 10 μg/kg, once per week) was administrated to C57BL/6J WT or AhRd mice right after starting CP induction with caerulein, and mice were euthanized and tissues harvested after 4 weeks of caerulein injection. Relative pancreas weights from indicated groups are shown (n = 9, pooled from 3 independent experiments, mean ± SEM, one-way analysis of variance [ANOVA], Tukey’s post-hoc test; ns, no significance). (B) Representative of pancreas H&E and Sirus Red staining. Scale bar = 200 μm. Quantitated fibrotic areas are shown in bar graph (mean ± SEM, one-way ANOVA, Tukey’s post-hoc test). (C) Quantitative reverse transcription polymerase chain reaction (RT-PCR) analysis of αSMA (α-SMA), Col1α1 (collagen1A1), Fn1 (fibronectin), Tgfb (TGFβ), and Il22 (IL22) gene expression in the pancreas from VE- or TCDD-treated WT mice (mean ± SEM, unpaired 2-tailed Student t test). (D) VE or BaP (25 mg/kg, daily) was orally administrated to Balb/c WT mice undergoing CP induction, and mice were euthanized and tissues harvested after 4 weeks of caerulein injection. Relative pancreas weights from indicated groups are shown (n = 10, pooled from 2 independent experiments, mean ± SEM, unpaired 2-tailed Student t test). (E) Representative of pancreas H&E and Sirus Red staining. Scale bar = 200 μm. Quantitated fibrotic areas are shown in bar graph (mean ± SEM). (F) Quantitative RT-PCR analysis of αSMA, Col1α1, Fn1, Tgfb, and Il22 expression in the pancreas from VE- or BaP-treated mice. Bar graphs show mean ± SEM (n = 9 per group, unpaired 2-tailed Student t test).
Fibrosis-promoting effects of AhR ligand TCDD are also seen in another CP model induced via chronic administration of caerulein and LPS (Supplementary Figure 3). Due to ubiquitous AhR expression pattern, we set up BM chimera studies to determine the contribution of hematopoietic cell AhR activation in the observed fibrosis progression. WT recipients were engrafted with WT or AhRd BM cells to generate WT→WT or WT→AhRd mice, respectively. After 8 weeks of engraftment, mice under-went caerulein-induced CP and were treated as mentioned previously, with either vehicle or TCDD. TCDD treatment promoted WT→WT mice pancreatic fibrosis similar to results seen in non-engrafted WT mice. WT mice engrafted with AhRd BM (WT→AhRd) had TCDD-mediated worsening of fibrosis similar to WT and not AhRd mice, indicating that TCDD pro-fibrotic effect is mainly mediated via hematopoietic or leukocyte AhR activation (Supplementary Figure 4).
Aryl Hydrocarbon Receptor Activation Induces Interleukin 22+ T Cells in the Pancreas and Neutralization of Interleukin 22 in Established Chronic Pancreatitis Suppresses Aryl Hydrocarbon Receptor–Mediated Pancreatic Fibrosis Promotion
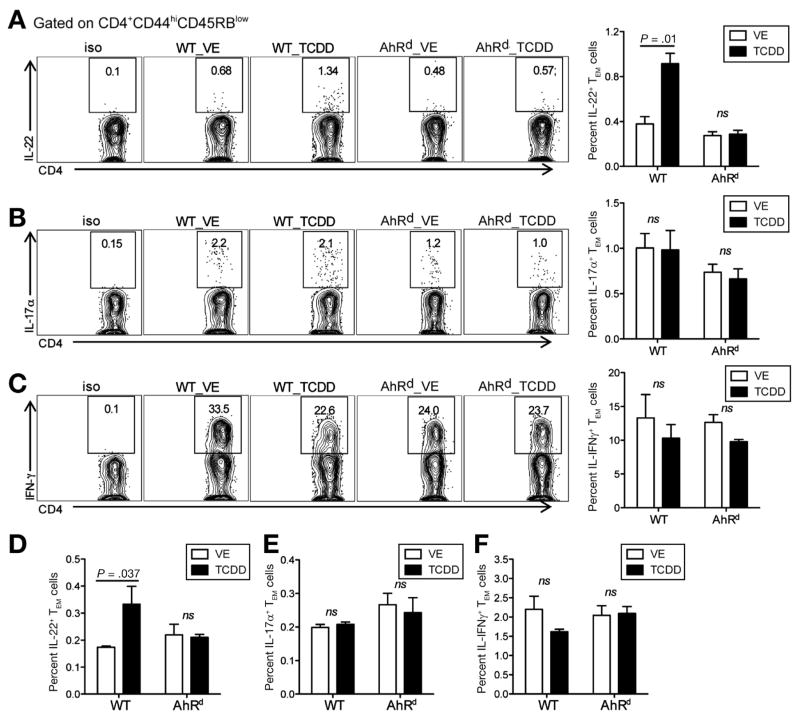
For decades, AhR has been implicated in mediating environmental toxicity mainly via nonhematopoietic cells.18 Recently, active research efforts have found a role for AhR in the immune system, particularly in T-cell differentiation and dendritic cell function.19–21 Several groups have established AhR as a critical ligand-dependent transcription factor for IL22 production in immune cells.16,22,23 Therefore, we investigated whether AhR activation by the agents found in cigarette smoke increases pancreatic IL22 in CP. As hypothesized, IL22 expression in the pancreas was dramatically increased in TCDD-treated CP mice relative to vehicle treatment by quantitative polymerase chain reaction analysis (Figure 1C). To confirm protein up-regulation, we isolated pancreatic leukocytes from vehicle- or TCDD-treated CP mice and analyzed IL22 expression by flow cytometry. It is well established that CP is associated with a marked increase in T cells.24 Therefore, we analyzed IL22 expression in pancreatic T cells and found that only pancreatic IL22, and not IL17 or interferon gamma, was up-regulated by CD4+ T effector/memory (TEM) cells in TCDD-treated mice during CP (Figure 2A–C). Similar results were found in splenocytes (Figure 2–F). Consistent with TCDD treatment, BaP also led to an increase in IL22+ CD4+ TEM cells in both pancreas and spleen (Supplementary Figure 5A–C). All in all, AhR activation promoted pancreatic fibrosis and was associated with a significant increase in IL22+ CD4+ TEM cells.
Figure 2.
TCDD induces IL22+ T cells in the pancreas and spleen. Pancreatic leukocytes were isolated and fluorescence-activated cell sorting analysis performed in VE- and TCDD-treated mice. (A–C) Frequency of IL22+, IL17α+, and interferon (IFN) gamma+ TEM (effector/memory, CD4+CD44hiCD45RBlow) cells in VE- or TCDD-treated WT or AhRd mice, at 4 weeks of CP induction. (D–F) Frequency of IL22+, IL17α+, and IFN gamma+ TEM in splenocytes in VE- or TCDD-treated WT or AhRd mice, at 4 weeks of CP induction. Bar graphs show mean ± SEM (n = 9 per group pooled from 3 independent experiments, unpaired 2-tailed Student t test; ns, no significance).
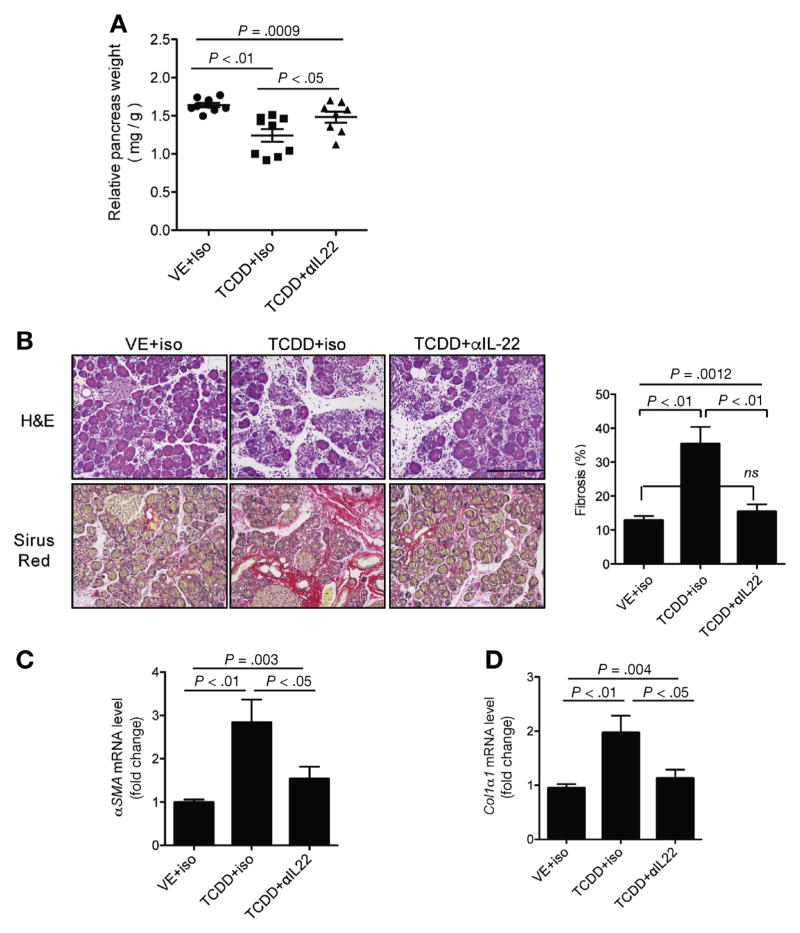
To investigate whether IL22 mediates TCDD’s fibrosis-promoting effects in CP, we used IL22-neutralizing antibody while we continued the TCDD treatment. Mice treated with anti-IL22 had attenuated pancreatic shrinkage (Figure 3A) and fibrosis (Figure 3B–D). These data suggest that TCDD and AhR activation-induced fibrogenesis was attributed, at least in part, to IL22 induction during CP progression.
Figure 3.
IL22 neutralization in established CP suppresses AhR fibrosis-promoting effects. (A) TCDD (10 μg/kg, once per week) and isotype control (iso) or anti-IL22 antibody (αIL22, 150 μg/mouse, 3 times per week) were administrated to Balb/c mice 2 weeks after starting CP induction. Relative pancreas weight from indicated groups is shown (n = 8 pooled from 2 independent experiments, mean ± SEM, one-way analysis of variance [ANOVA], Tukey’s post-hoc test). (B) Representative of pancreas H&E and Sirius Red staining. Scale bar = 200 μm. Quantitated fibrotic areas are shown in bar graph (mean ± SEM, one-way ANOVA, Tukey’s post-hoc test). (C, D) Quantitative reverse transcription polymerase chain reaction analysis of αSMA (α-SMA) and Col1α1 (collagen1A1) expression in the pancreas from indicated groups (n = 8 per group, mean ± SEM, unpaired 2-tailed Student t test).
Interleukin 22 Promotes Pancreatic Fibrosis Via Up-Regulation of Pancreatic Stellate Cell Extracellular Matrix Gene Expression
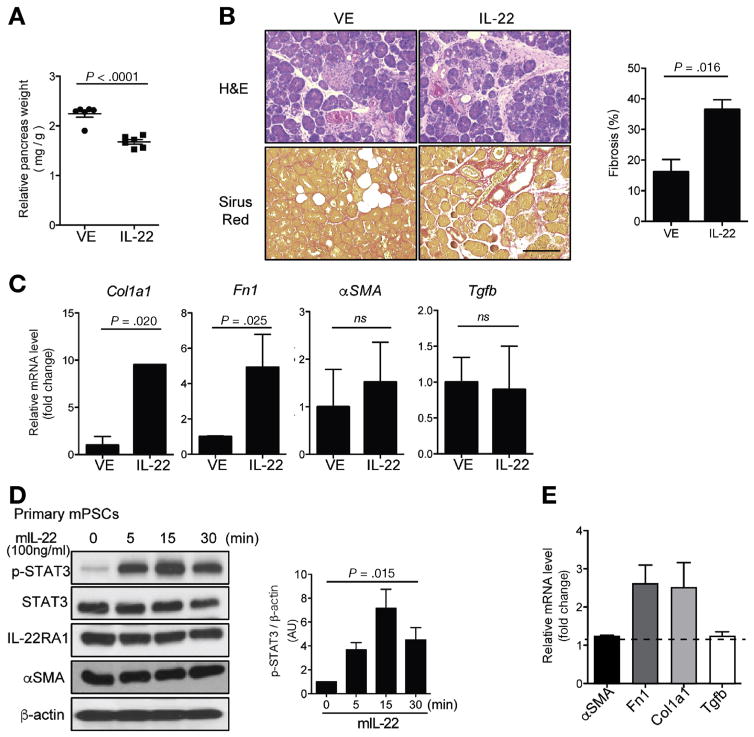
To evaluate the direct involvement of IL22 on fibrogenesis, mice undergoing CP induction were treated with either rIL22 or vehicle control. Mice treated with rIL22 during CP had smaller pancreas and increased fibrosis as compared with the vehicle-treated controls (Figure 4A and B). It is important to note that rIL22 up-regulated ECM genes, such as Fn1 and Col1α1, but not PSC activation-associated genes (αSMA and TGFβ) (Figure 4C). To assess the mechanism through which IL22 promotes fibrosis, we tested the effect of IL22 on PSCs isolated from CP mice. It is not known whether PSCs express IL22 receptor. Here we show that both mouse and human primary PSCs not only express IL22RA1 (Supplementary Figure 6), but also respond to IL22 by up-regulating phosphorylated STAT3 in a time-dependent manner (Figure 4D). Consistent with the in vivo findings (Figure 4C), IL22 induced PSCs ECM genes Fn1 and Col1α1 but not αSMA or Tgfb expression (Figure 4E).
Figure 4.
IL22 promotes pancreatic fibrosis via up-regulation of PSC ECM gene expression. Vehicle (VE) or rIL22 (100 ng/mouse, 5 times per week) was administrated to Balb/c mice starting at 4 weeks of CP induction and mice were euthanized after 6 weeks of caerulein injection. (A) Relative pancreas weight from VE- and IL22-treated mice is shown (n = 6 per group pooled from 2 independent experiments, mean ± SEM, unpaired 2-tailed Student t test). (B) Representative of pancreas H&E and Sirus Red staining. Scale bar = 200 μm. Quantitated fibrotic areas are shown in bar graph (mean ± SEM). (C) Quantitative reverse transcription polymerase chain reaction (RT-PCR) analysis of Col1α1 (collagen 1A1), Fn1 (fibronectin), αSMA (α-SMA), and Tgfb (TGFβ) expression in the pancreas from indicated mice. Bar graphs show mean ± SEM (n = 6, unpaired 2-tailed Student t test). (D) Primary murine PSCs (mPSCs) were treated with rIL22 (100 ng/mL) for indicated times and then lysed for immunoblotting with phosphorylated STAT3 (p-STAT), total STAT3, IL22RA1, and α-SMA. Data are representative of 3 independent experiments. Relative phosphorylated STAT3 levels (p-STAT3/β-actin) are shown as bar graph (mean ± SEM, one-way analysis of variance). (E) mPSCs were treated with VE or rIL22 (100 ng/mL) for 24 hours, expression of αSMA (α-SMA), Col1α1 (collagen 1A1), Fn1 (fibronectin), and Tgfb (TGFβ) were detected with quantitative RT-PCR. Results are shown as fold-change relative to VE group. Bar graph represents mean ± SEM (n = 3 independent experiments).
Circulating Interleukin 22 Is Elevated in Chronic Pancreatitis and Associated With Cigarette Smoking Among Chronic Pancreatitis Patients
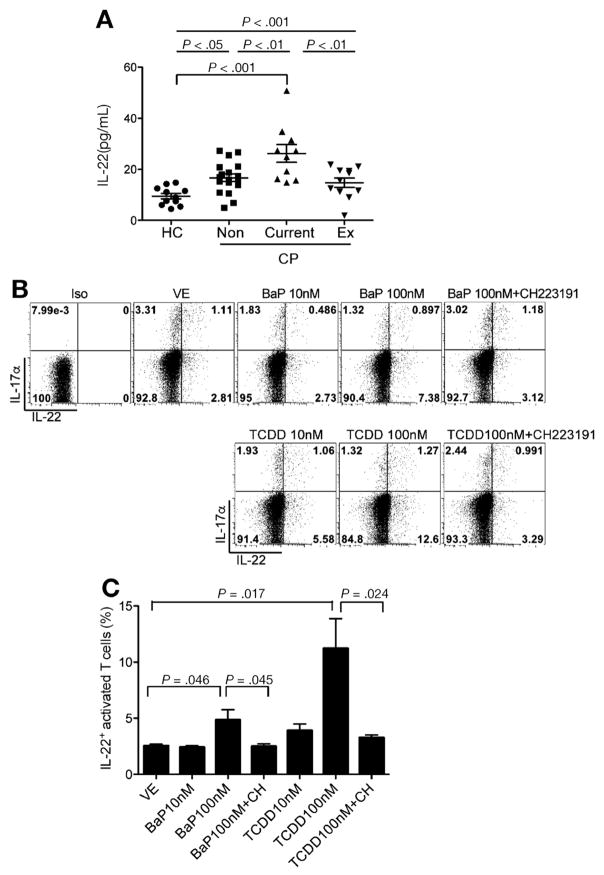
To further determine the significance of our findings, we evaluated and compared circulating IL22 level between healthy subjects and CP patients with or without a smoking history (Supplementary Table 1). Interestingly, CP patients, regardless of their smoking history, had higher serum IL22 compared with healthy subjects (Figure 5A). Among CP patients, however, current smokers had significantly higher circulating IL22 levels compared with those who either never smoked (nonsmokers) or had quit smoking for more than 1 year (ex-smokers) (Figure 5A). To further confirm that AhR ligands present in cigarette smoke could induce IL22 in human T cells, naïve CD4+ T cells were isolated from peripheral blood mononuclear cells of healthy volunteers and then treated with different AhR agonists in the presence or absence of AhR antagonist. TCDD and BaP induced generation of IL22+ CD4+ T cells in an AhR-dependent manner because the observed effect was blocked by the AhR antagonist CH223191 (Figure 5B and C). Although CD4+ T cells isolated from healthy smokers had slightly higher IL22 at baseline, they had similar induction responses to TCDD relative to respective vehicle control (Supplementary Figure 7).
Figure 5.
Circulating IL22 is elevated in CP and associated with cigarette smoking among CP patients. (A) Serum from health control subjects (HC, n = 11) and CP patients (CP) who were non-smokers (Non, n = 18), current-smokers (Current, n = 10), or ex-smokers (Ex, n = 11) were collected and IL22 analyzed using an enzyme-linked immunosorbent assay (mean ± SEM, one-way analysis of variance, Tukey’s post-hoc test). (B) Naïve CD4+ T cells were enriched using magnetic beads from peripheral blood mononuclear cells (PBMCs) of healthy blood donors (buffy coat), and then activated with antibodies to CD3 and CD28 in the presence of AhR agonist (TCDD or BaP) with or without the AhR antagonist (CH223191) for 5 days. Flow cytometry plots show frequency of IL17α and IL22 expression in activated CD4+ T cells. Data are representative of 4 independent experiments. (C) Bar graphs show frequency of IL22+ - activated T cells, presented as mean ± SD (n = 4, unpaired 2-tailed Student t test).
Taken together, our results demonstrate the fibrosis-promoting effect of AhR activation during CP, offering new insight into the mechanism of how cigarette smoking worsens and promotes CP progression. In addition, the identification of functional IL22RA1 expression on PSCs underlies a novel crosstalk between immune cells and PSCs during CP progression, which might be developed as a biomarker and therapeutic target.
Discussion
CP is a complex chronic inflammation disorder with links to genetic, metabolic, and environmental factors. Alcohol is the most established environmental risk factor for CP, while the independent effects and risks associated with smoking have not been elucidated until recently. In most populations, smoking is strongly associated with drinking alcohol,25 therefore, the independent effect of smoking can be difficult to assess. However, data from case-control26 and population-based studies5,27 show that there is an independent and dose-dependent association between smoking and CP. However, the mechanism by which smoking contributes to CP is still unknown. A clear understanding of the pathogenesis of CP may provide insights into the most effective targets to slow or reverse key aspects of CP. Thus, in this study, we sought to understand the mechanism by which cigarette smoking promotes fibrosis and progression of CP.
The presence of AhR ligands in cigarette smoking is well defined,11 and we used TCDD and BaP to investigate their effect in CP. With respect to tobacco smoke, BaP has been detected in concentrations ranging from 20 to 40 ng and 40 to 79 ng per cigarette in mainstream and sidestream cigarette smoking, respectively.28 One study reported a daily dioxin intake of 4.3 pg/kg body weight by smoking 20 cigarettes per day.10 However, as pointed out in a more recent study, older study calculations underestimated the toxic and dioxin effect because they depended on gas chromatography-mass spectrometric physicochemical assessment instead of bioassays that measure AhR activation, or xenobiotic-responsive element activating potential by measuring target CYP1A1 gene and enzyme activation.12 Several studies have linked AhR activation and toxicity to CYP1A1 induction.29,30 Dioxin in cigarette smoke has been reported in TCDD equivalent (0.4–2.4 pg TEQ/cigarette) based on physiochemical assessment, but a more recent study using xenobiotic-responsive element activating potential measurement, termed as BioTEQ/cigarette, found that cigarette smoking has a very high dioxin-like potential (18.5–51.2 ng BioTEQ/cigarette) that triggers AhR activation.12
These findings and a similar trend of pancreas CYP1A1 induction in TCDD-treated and cigarette smoke-exposed mice (Supplementary Figure 8A and B) support our model and relevance of the TCDD dosing used in this project. Once AhR ligands bind AhR, AhR translocates to the nucleus to bind the xenobiotic-responsive element after forming heterodimer with Arnt, and triggers induction of not only activating genes (eg, cyp1a1), but also regulating genes, such as AhR repressor (AhRR).31 Both TCDD treatment and cigarette smoke exposure also induced pancreas AhRR expression (Supplementary Figure 8C and D) with slightly higher effect in the latter group, likely due to the multiple AhR ligands present in cigarette smoke. There are also additional considerations that need to be made when comparing rodent with human studies on the basis of the notable difference in how AhR ligands, such as TCDD, are metabolized. Rodents metabolize TCDD much faster (half life of 12–31 days) compared with the much slower rate in humans, accounting for the very long TCDD half-life of approximately 5.8–14.1 years.32 Because chronic exposure over years in rodent studies is not feasible and until other models can be developed, doses used here and also used commonly in most experimental studies over relatively short period of times are helpful to study the pathophysiologic mechanisms associated with AhR activation as it pertains to cigarette smoking.
A number of recent studies have examined the functions of AhR in the immune system. The functions of AhR in T cells depend on a specific ligand. TCDD has also been reported to promote development of Foxp3+ regulatory T cells in an experimental model of multiple sclerosis (experimental autoimmune encephalomyelitis).19 In our CP study, in addition to inducing IL22+ T cells, TCDD also induced Foxp3+ regulatory T cells (data not shown). TGFβ, a well-defined pro-fibrotic cytokine, is also secreted by Foxp3+ regulatory T cells and may contribute, in part, to the TCDD-promoting effects of fibrosis in CP.33,34 This is consistent with the finding that IL22 treatment in CP, despite its fibrosis-promoting effects, did not alter pancreatic TGFβ. However, IL22 neutralization alone markedly suppressed fibrosis and abolished TCDD-mediated up-regulation of Col1α1 (collagen 1A1). It is also important to note that IL22-neutralizing antibody was administered at 2 weeks and after CP was established (where significant fibrosis and αSMA expression are present, as shown in our recent study13). Taken together, these findings support the potential therapeutic effect of blocking IL22 in CP. Intestinal epithelial cells express IL22 receptor and respond to IL22 by activating downstream STAT3 signaling in regulating intestinal inflammation.35 It was not known whether PSCs express IL22 receptor. In the present study, we found that functional IL22 receptor is expressed by PSCs, where IL22 activates STAT3 and induces ECM-related genes Fn1 and Col1α1. These findings add a new potential crosstalk mechanism between immune cells and PSCs in the promotion of fibrosis because immune cells are known to produce IL22 but lack IL22 receptor expression.
Besides CP, smoking has been described as a strong environmental risk factor for developing pancreatic cancer. Smoking accelerates the onset of pancreas cancer, especially in high-risk groups (eg, hereditary pancreatitis patients). 36,37 Activated PSCs are found not only around pancreatic ductal adenocarcinoma cells, but also around the pre-cancerous pancreatic intraepithelial neoplastic lesions, and are proposed to play a role in the different stages of pancreas cancer development.38 Our finding of PSC and immune cell interaction via IL22 signaling and STAT3 activation might also account for smoking-related acceleration of pancreatic carcinogenesis, an area for future investigation. Unlike in acute pancreatitis,16,39 IL22 has a fibrosis-promoting effect in CP, highlighting immune response differences between acute and chronic pancreatitis. This is also consistent with the opposing role of alternatively activated macrophages in acute vs chronic pancreatitis.13,40,41
The translational significance of our study is underscored by the findings of elevated circulating levels of IL22 in CP patients compared with healthy subjects and, in addition, the remarkable IL22 increases seen in association with CP patients who continue to smoke. CP patients who continued to smoke tended to be younger than those who either quit or never smoked and are, interestingly, more likely to have endocrine insufficiency. This finding is consistent with previous reports in which the diagnosis of pancreatitis was made about 5 years earlier in current smokers than in nonsmokers or ex-smokers.6 Future prospective studies with large cohorts of patients will be necessary to address the significance of these observations.
Early diagnosis of CP remains a major challenge in the field and chronic pancreatic inflammation and fibrosis develop in the apparent absence of symptoms or detectable clinical measures.42 Current imaging diagnostics, such as computed tomography scans and endoscopic ultrasounds, detect relatively late stages of the disease. Novel tests need to be considered for patients who are either at high risk for or suspected of having CP. Our finding here may provide a potential serum biomarker for CP and subclinical CP, and also offer a potential target to slow CP development and/or progression.
Supplementary Material
Acknowledgments
The authors thank Wenjun Ouyang from Genentech for providing anti-IL22 antibody. The authors thank Yi Wei for technical assistance.
Jing Xue and Qinglan Zhao contributed equally to this work. Walter Park and Aida Habtezion contributed equally to this work.
Funding
This work was supported by the National Pancreas Foundation Grant, National Institute of Health (NIH) grants DK092421 and DK105263 (Aida Habtezion); National Natural Science Foundation of China grant, China State Key Laboratory of Oncogenes and Related Gene (no. 91-15-15), Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning (no. TP2015007) (Jing Xue); Department of Veterans Affairs, and NIH CA163200 (Stephen J. Pandol), AA019996 and AA011999 (Stephen J. Pandol and Mouad Edderkaoui).
Abbreviations used in this paper
- AhR
aryl hydrocarbon receptor
- αSMA
α–smooth muscle actin
- BaP
benzo[a]pyrene
- BM
bone marrow
- CP
chronic pancreatitis
- ECM
extracellular matrix
- IL
interleukin
- LPS
lipopolysaccharide
- PSC
pancreatic stellate cell
- STAT
signal transducers and activators of transcription
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- TEM
T effector/memory
- TGFβ
transforming growth factor–β
- WT
wild-type
Footnotes
Conflicts of interest
The authors disclose no conflicts.
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at http://dx.doi.org/10.1053/j.gastro.2016.09.064.
References
- 1.Sarles H. Etiopathogenesis and definition of chronic pancreatitis. Dig Dis Sci. 1986;31(9 Suppl):91S–107S. doi: 10.1007/BF01295992. [DOI] [PubMed] [Google Scholar]
- 2.Witt H, Apte MV, Keim V, et al. Chronic pancreatitis: challenges and advances in pathogenesis, genetics, diagnosis, and therapy. Gastroenterology. 2007;132:1557–1573. doi: 10.1053/j.gastro.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Yadav D, Whitcomb DC. The role of alcohol and smoking in pancreatitis. Nat Rev Gastroenterol Hepatol. 2010;7:131–145. doi: 10.1038/nrgastro.2010.6. [DOI] [PubMed] [Google Scholar]
- 4.Gaisano HY, Gorelick FS. New insights into the mechanisms of pancreatitis. Gastroenterology. 2009;136:2040–2044. doi: 10.1053/j.gastro.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 5.Setiawan VW, Pandol SJ, Porcel J, et al. Prospective study of alcohol drinking, smoking, and pancreatitis: the multiethnic cohort. Pancreas. 2016;45:819–825. doi: 10.1097/MPA.0000000000000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maisonneuve P, Lowenfels AB, Mullhaupt B, et al. Cigarette smoking accelerates progression of alcoholic chronic pancreatitis. Gut. 2005;54:510–514. doi: 10.1136/gut.2004.039263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andriulli A, Botteri E, Almasio PL, et al. Smoking as a cofactor for causation of chronic pancreatitis: a meta-analysis. Pancreas. 2010;39:1205–1210. doi: 10.1097/MPA.0b013e3181df27c0. [DOI] [PubMed] [Google Scholar]
- 8.Lee AT, Xu Z, Pothula SP, et al. Alcohol and cigarette smoke components activate human pancreatic stellate cells: implications for the progression of chronic pancreatitis. Alcohol Clin Exp Res. 2015;39:2123–2133. doi: 10.1111/acer.12882. [DOI] [PubMed] [Google Scholar]
- 9.Tolstrup JS, Kristiansen L, Becker U, et al. Smoking and risk of acute and chronic pancreatitis among women and men: a population-based cohort study. Arch Intern Med. 2009;169:603–609. doi: 10.1001/archinternmed.2008.601. [DOI] [PubMed] [Google Scholar]
- 10.Muto H, Takizawa Y. Dioxins in cigarette smoke. Arch Environ Health. 1989;44:171–174. doi: 10.1080/00039896.1989.9935882. [DOI] [PubMed] [Google Scholar]
- 11.Stedman RL. The chemical composition of tobacco and tobacco smoke. Chem Rev. 1968;68:153–207. doi: 10.1021/cr60252a002. [DOI] [PubMed] [Google Scholar]
- 12.Kasai A, Hiramatsu N, Hayakawa K, et al. High levels of dioxin-like potential in cigarette smoke evidenced by in vitro and in vivo biosensing. Cancer Res. 2006;66:7143–7150. doi: 10.1158/0008-5472.CAN-05-4541. [DOI] [PubMed] [Google Scholar]
- 13.Xue J, Sharma V, Hsieh MH, et al. Alternatively activated macrophages promote pancreatic fibrosis in chronic pancreatitis. Nat Commun. 2015;6:7158. doi: 10.1038/ncomms8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lerch MM, Gorelick FS. Models of acute and chronic pancreatitis. Gastroenterology. 2013;144:1180–1193. doi: 10.1053/j.gastro.2012.12.043. [DOI] [PubMed] [Google Scholar]
- 15.Edderkaoui M, Xu S, Chheda C, et al. HDAC3 mediates smoking-induced pancreatic cancer. Oncotarget. 2016;7:7747–7760. doi: 10.18632/oncotarget.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xue J, Nguyen DT, Habtezion A. Aryl hydrocarbon receptor regulates pancreatic IL-22 production and protects mice from acute pancreatitis. Gastroenterology. 2012;143:1670–1680. doi: 10.1053/j.gastro.2012.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevens EA, Mezrich JD, Bradfield CA. The aryl hydrocarbon receptor: a perspective on potential roles in the immune system. Immunology. 2009;127:299–311. doi: 10.1111/j.1365-2567.2009.03054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu YZ, Hogenesch JB, Bradfield CA. The PAS super-family: sensors of environmental and developmental signals. Annu Rev Pharmacol Toxicol. 2000;40:519–561. doi: 10.1146/annurev.pharmtox.40.1.519. [DOI] [PubMed] [Google Scholar]
- 19.Quintana FJ, Basso AS, Iglesias AH, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 20.Veldhoen M, Hirota K, Westendorf AM, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 21.Yamazaki S, Inaba K, Tarbell KV, et al. Dendritic cells expand antigen-specific Foxp3+ CD25+ CD4+ regulatory T cells including suppressors of alloreactivity. Immunol Rev. 2006;212:314–329. doi: 10.1111/j.0105-2896.2006.00422.x. [DOI] [PubMed] [Google Scholar]
- 22.Monteleone I, Rizzo A, Sarra M, et al. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology. 2011;141:237–248. 248 e1. doi: 10.1053/j.gastro.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Zelante T, Iannitti RG, Cunha C, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39:372–385. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Goecke H, Forssmann U, Uguccioni M, et al. Macro-phages infiltrating the tissue in chronic pancreatitis express the chemokine receptor CCR5. Surgery. 2000;128:806–814. doi: 10.1067/msy.2000.108613. [DOI] [PubMed] [Google Scholar]
- 25.Yadav AK, Bracher A, Doran SF, et al. Mechanisms and modification of chlorine-induced lung injury in animals. Proc Am Thorac Soc. 2010;7:278–283. doi: 10.1513/pats.201001-009SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yadav D, Hawes RH, Brand RE, et al. Alcohol consumption, cigarette smoking, and the risk of recurrent acute and chronic pancreatitis. Arch Intern Med. 2009;169:1035–1045. doi: 10.1001/archinternmed.2009.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin Y, Tamakoshi A, Hayakawa T, et al. Cigarette smoking as a risk factor for chronic pancreatitis: a case-control study in Japan. Research Committee on Intractable Pancreatic Diseases Pancreas. 2000;21:109–114. doi: 10.1097/00006676-200008000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Kuller LH, Garfinkel L, Correa P, et al. Contribution of passive smoking to respiratory cancer. Environ Health Perspect. 1986;70:57–69. doi: 10.1289/ehp.867057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kellermann G, Shaw CR, Luyten-Kellerman M. Aryl hydrocarbon hydroxylase inducibility and bronchogenic carcinoma. N Engl J Med. 1973;289:934–937. doi: 10.1056/NEJM197311012891802. [DOI] [PubMed] [Google Scholar]
- 30.Kouri RE, McKinney CE, Slomiany DJ, et al. Positive correlation between high aryl hydrocarbon hydroxylase activity and primary lung cancer as analyzed in cryopreserved lymphocytes. Cancer Res. 1982;42:5030–5037. [PubMed] [Google Scholar]
- 31.Mimura J, Fujii-Kuriyama Y. Functional role of AhR in the expression of toxic effects by TCDD. Biochim Biophys Acta. 2003;1619:263–268. doi: 10.1016/s0304-4165(02)00485-3. [DOI] [PubMed] [Google Scholar]
- 32.Grassman JA, Masten SA, Walker NJ, et al. Animal models of human response to dioxins. Environ Health Perspect. 1998;106(Suppl 2):761–775. doi: 10.1289/ehp.98106761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagashio Y, Ueno H, Imamura M, et al. Inhibition of transforming growth factor beta decreases pancreatic fibrosis and protects the pancreas against chronic injury in mice. Lab Invest. 2004;84:1610–1618. doi: 10.1038/labinvest.3700191. [DOI] [PubMed] [Google Scholar]
- 34.Yoo BM, Yeo M, Oh TY, et al. Amelioration of pancreatic fibrosis in mice with defective TGF-beta signaling. Pancreas. 2005;30:e71–e79. doi: 10.1097/01.mpa.0000157388.54016.0a. [DOI] [PubMed] [Google Scholar]
- 35.Pickert G, Neufert C, Leppkes M, et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206:1465–1472. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lowenfels AB, Maisonneuve P, Whitcomb DC, et al. Cigarette smoking as a risk factor for pancreatic cancer in patients with hereditary pancreatitis. JAMA. 2001;286:169–170. doi: 10.1001/jama.286.2.169. [DOI] [PubMed] [Google Scholar]
- 37.Rulyak SJ, Lowenfels AB, Maisonneuve P, et al. Risk factors for the development of pancreatic cancer in familial pancreatic cancer kindreds. Gastroenterology. 2003;124:1292–1299. doi: 10.1016/s0016-5085(03)00272-5. [DOI] [PubMed] [Google Scholar]
- 38.Apte MV, Wilson JS, Lugea A, et al. A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology. 2013;144:1210–1219. doi: 10.1053/j.gastro.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feng D, Park O, Radaeva S, et al. Interleukin-22 ameliorates cerulein-induced pancreatitis in mice by inhibiting the autophagic pathway. Int J Biol Sci. 8:249–257. doi: 10.7150/ijbs.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Habtezion A, Kwan R, Akhtar E, et al. Panhematin provides a therapeutic benefit in experimental pancreatitis. Gut. 2011;60:671–679. doi: 10.1136/gut.2010.217208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakamichi I, Habtezion A, Zhong B, et al. Hemin-activated macrophages home to the pancreas and protect from acute pancreatitis via heme oxygenase-1 induction. J Clin Invest. 2005;115:3007–3014. doi: 10.1172/JCI24912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Forsmark CE. The early diagnosis of chronic pancreatitis. Clin Gastroenterol Hepatol. 2008;6:1291–1293. doi: 10.1016/j.cgh.2008.08.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.