Abstract
OBJECTIVE
To compare wound complication rates after skin closure with staples versus subcuticular suture in obese gynecology patients undergoing laparotomy through midline vertical incision.
METHODS
In this randomized controlled trial, women with a body mass index (BMI) ≥ 30 kg/m2 undergoing surgery by a gynecologic oncologist through midline vertical incision were randomized to skin closure with staples or subcuticular 4-0 monofilament suture. The primary outcome was the rate of wound complication, defined as the presence of a wound breakdown, or infection, within eight weeks postoperatively. Secondary outcomes included operative time, Stony Brook scar cometic score, and patient satisfaction. A sample size of 162 was planned to detect a 50% reduction in wound complications. At planned interim review (n=82), there was no significant difference in primary outcome.
RESULTS
Between 2013 and 2016, 163 women were analyzed, including 84 who received staples and 79 suture. Women who received staples were older (mean age 59 vs. 57 years), had lower mean BMI (37.3 vs 38.9 kg/m2), and fewer benign indications for surgery (22 vs. 27). There were no differences in wound complication rates between staple versus suture skin closure [28 (33%) vs. 25 (32%), relative risk (RR) 1.05, 95% confidence interval (CI) 0.68–1.64]. Women with staples reported worse median cosmetic scores (4/5 vs. 5/5, P<0.001), darker scar color (37 (49%) vs. 13 (18%), RR 2.69, 95%CI 1.57–4.63), and more skin marks [30 (40%) vs. 3 (4%), RR 9.47, 95% CI 3.02–29.65] compared to women with suture closure. There was no group difference regarding satisfaction with their scar. Stepwise multivariate analysis revealed BMI (OR 1.13, 95% CI 1.07–1.20), maximum postoperative glucose (OR 1.01, 95% CI 1.00–1.01), and cigarette smoking (OR 4.96, 95% CI 1.32–18.71) were correlates of wound complication.
CONCLUSION
Closure of midline vertical skin incisions with subcuticular suture does not reduce surgical site wound complications compared to staples in obese gynecology patients.
INTRODUCTION
The incidence of wound separations and infections in gynecology patients is as high as 37%.1,2 Patients with wound complications not only face a five-fold increased risk of reoperation compared to patients without wound issues, but they experience longer hospitalizations, five times higher readmission rates, and two-fold greater mortality.3–5 Overall, wound complications following laparotomy are estimated to cost an additional $4,000 per wound.2,3,6 These statistics are even more alarming in the context of the obesity epidemic in the United States and the known associations between increasing body mass index (BMI), subcutaneous tissue depth, and wound complications.2,7,8 Therefore, it is imperative to raise our surgical standards and investigate perioperative interventions to reduce wound complications.
Traditionally, stainless-steel staples have been an effective and efficient method of skin closure in gynecologic oncology cases where the majority of incisions are midline vertical and at least 20 centimeters (cms) in length1,9. Despite the paucity of clinical trials in gynecology, the obstetric literature demonstrates a decrease in wound complications and improvement in pain and cosmetic result with the use of suture.10–14
We aimed to better understand these discrepancies in the context of gynecologic surgeries for benign and oncologic indications. Therefore, we conducted a randomized controlled trial evaluating wound complication rates after skin closure with suture as compared to staples in obese women undergoing laparotomy through midline vertical incision. Secondary objectives included evaluating for differences in the incidence of wound infection, operative time, cosmetic result, and patient satisfaction scores.
MATERIALS AND METHODS
We conducted a single-center, randomized controlled trial evaluating complications in wounds closed with staples versus suture in obese women undergoing gynecologic surgery. All surgeries were performed under the supervision of faculty within the Division of Gynecologic Oncology. Women were eligible if they were aged 18–85 years with a BMI ≥ 30 kg/m2 undergoing a gynecologic procedure through midline vertical abdominal incision. Exclusion criteria included allergy to Monocryl® (poliglecaprone 25, a monofilament synthetic suture made from a copolymer of glycolide and epsilon-caprolactone; © Ethicon US) suture or stainless steel staples, history of abdominal or pelvic radiation, prior hernia repair with mesh or more than one hernia repair, concurrent pregnancy, incarceration, inability to provide informed consent, and non-English speaking. Women were also excluded if the current surgical procedure included a planned panniculectomy or hernia repair with mesh.
Eligible patients were approached and consented at either the preoperative outpatient visit or in the preoperative holding area on day of surgery. A computer-generated 1:1 simple randomization scheme was used. Skin closure groups were centrally assigned by our study coordinator on telephone verification of the correctness of inclusion criteria. Neither clinicians nor patients were masked to closure type. All study procedures were approved by the Human Research Protection Office of Washington University School of Medicine (WUSM) prior to participant recruitment (IRB #: 201304058). The Data and Safety Monitoring Committee met every six months to review complications and a report was generated and approved by the Quality Assurance and Safety Monitoring Committee at WUSM.
An established protocol for wound closure was followed.1 It included: fascial closure with #1 looped polydioxanone suture, placement of a subcutaneous drain, and reapproximation of Camper’s fascia with 3-0 plain gut suture. Despite evidence in the obstetric literature that subcutaneous drains increase the risk of postpartum cesarean wound complications and should not be used routinely15, there remains evidence to support to the use of a subcutaneous closed drainage system in obese gynecologic patients.16–18 Intraperitoneal drains were placed at the discretion of the attending surgeon. Women randomized to staples had their incision closed with 35 mm wide stainless steel staples using a Proximate Skin Stapler® (© Ethicon US). Staples were removed at an outpatient appointment 10–14 days postoperatively or sooner if warranted. Women randomized to the suture group had their skin incision closed with an absorbable 4-0 monofilament suture in a subcuticular fashion. Thin adhesive 3M™ Steri-Strips™ (© 3M US) were placed at the discretion of the surgeon. All wounds were dressed with a non-woven dressing strip in the operating room and subsequently removed on postoperative day two.
Chart review was performed for patient demographic and clinical information. All surgeons completed a wound assessment form and the Stony Brook Scar Evaluation Scale19 between four and eight weeks of surgery. This scale evaluates five factors: width, height, color, hatch marks/suture marks, and overall appearance. A higher score is considered a better cosmetic outcome. At this same outpatient visit, patients filled out a satisfaction survey about their scar, including general appearance, location, and comfort on a scale of one (worst) to five (best).
The primary outcome was wound complication rate within eight weeks postoperatively. Wound complication was defined as the presence of a wound breakdown, infection, or both. Surgical site infection (SSI) was based on the Centers for Disease Control and Prevention National Healthcare Safety network definitions.20 All SSIs in our study met criteria for superficial SSI, which was defined within 30 days after surgery and involved only skin and subcutaneous tissue of the incision and at least one of the following: 1) purulent drainage; 2) organisms isolated from a culture of fluid or tissue from the incision; 3) pain, swelling, redness, or heat; or 4) diagnosis made by a surgeon or attending physician. Secondary outcomes of interest included differences in operative time, cosmetic scores, and patient satisfaction between staple and suture skin closure.
We estimated the sample size for the trial assuming a baseline rate of 35%, on the basis of a prior study2 conducted at our institution and anticipated a 50% reduction in wound complication rate from 35% (staples) to 17.5% (suture). Therefore, a total of 162 women were needed in order to have a power of 80% with a one-tailed alpha of 0.05. This effect size was chosen based on level one evidence published in the obstetric literature which demonstrated a 60% decrease in wound complication rate when skin closure was performed with suture.10,12 An interim analysis using the O’Brien-Fleming approach was planned after enrollment of 82 total subjects using two-sample Z tests for proportions. An independent trial statistician performed the analysis for the data monitoring committee. The standardized Z-statistic from a two-sample Z test was −1.462, which was within the boundaries for the one-sided (−2.31, −0.49) and two-sided test (−2.80, 2.80). Therefore, no early stopping was indicated.
Demographic and clinical characteristics were summarized by descriptive statistics. Staples and suture group differences regarding demographics, clinical, surgical, and perioperative characteristics, as well as postoperative outcomes were analyzed using independent t-tests or Kruskal-Wallis tests for continuous variables and Fisher exact or chi-square tests for categorical variables, as appropriate. Univariate and multivariate logistic regressions were performed to examine risk factors that could be associated with the primary outcome (i.e., wound breakdown, infection, or both) including age, BMI (kg/m2), duration of surgery, surgery, estimated blood loss (ml), maximum inpatient glucose post-operative (mg/dl), skin closure type, race, cigarette smoking, diabetes mellitus, American Society of Anesthesiologist (ASA) physical status classification class, prior abdominal surgery, indication for surgery (benign vs. cancer), subcutaneous drain placement, and protocol compliance.
Stepwise selection was used in the multivariate logistic regression, where effect variables, “skin closure type” (i.e., staples vs. suture) and “subcutaneous drain placement” were forced to stay in every model during the selection process. This was intentionally done given our primary study objective was to assess impact of skin closure on wound complication. Furthermore, the impact of a subcutaneous drain to reduce midline vertical wound complications is unknown and although this was part of our wound protocol, we included this variable in our model to adjust for the unexpected rate of wound protocol deviations. For all the other potential risk factors not being forced to stay in the model, the entry and stay levels of the stepwise procedure were P=0.3 and P=0.15, respectively. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
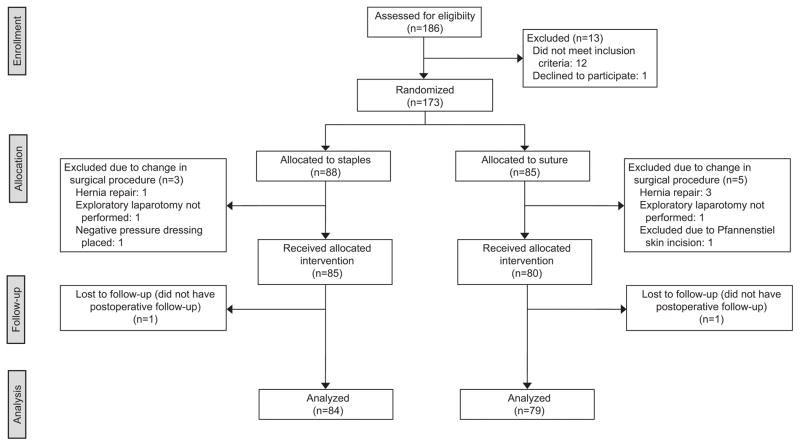
Between July 2013 and March 2016, 186 women were assessed for eligibility and 173 were enrolled in the study. Due to unanticipated intraoperative findings, eight women had a change in their surgical procedure which deemed them ineligible for study enrollment, and thus excluded from the final analysis. An interim analysis of the first 82 evaluable patients in our study did not show a significant difference in wound complication rate between staples versus suture. We also performed two sensitivity analyses excluding missing values as well as categorizing missing data as a positive composite outcome. The former did not change the composite complication variable from the core assessment. When we assumed missing data were a positive composite outcome for wound disruption or infection, the standardized Z-statistic from a two-sample Z test was −0.8102, which is within the boundaries for one-sided test (−2.31, −0.49) and two-sided test (−2.80, 2.80). Therefore, we did not stop trial enrollment at that time and in March 2016, we reached our target accrual. Of the 165 remaining women who received their allocated intervention, two patients were lost to follow-up, leaving 163 patients evaluable for analysis by intention-to-treat, 84 in the staple group and 79 in the suture group (Figure 1).
Figure 1.
Randomization and follow-up of study participants.
Demographic and clinical characteristics are presented in Table 1. The mean age for women in the staple and suture cohorts were 58.5 and 57.1 years, respectively. Most women had a BMI between 30–39.9 kg/m2, although women who received staples had a lower mean BMI than those closed with suture (37.3 vs 38.9 kg/m2). Overall, the majority of patients were white, had prior abdominal surgery, were not diabetic, and had a cancer diagnosis. Overall, the groups were well balanced with the exception of tobacco use, where women in the suture group were more likely to have never smoked [59 (57%) vs. 44 (43%), P=0.01]. Co-morbidities were self-reported, but overall high among the entire study population, with 115 (71%) having cardiac disease, 46 (28%) diabetes, and 47 (29%) pulmonary disease. Anesthesia assessment identified 77 (47%) of participants as having a severe systemic disease with or without a constant threat to life (ASA class III–IV).
Table 1.
Patient Demographics and Clinical Characteristics (N =163)
| Characteristic | Staples (n=84) n (%) |
Suture (n=79) n (%) |
P |
|---|---|---|---|
| Age (years) | 58.5 ± 13.2 | 57.1 ± 11.2 | 0.48 |
| BMI (kg/m2) | 0.44 | ||
| 30–39.9 | 60 (71) | 49 (62) | |
| 40–49.9 | 19 (23) | 24 (30) | |
| >= 50 | 5 (6) | 6 (8) | |
| Race | 0.44 | ||
| White | 68 (81) | 60 (76) | |
| Black | 16 (19) | 19 (24) | |
| Prior abdominal surgery | 51 (61) | 53 (67) | 0.40 |
| Tobacco use | 0.01 | ||
| Never | 44 (52) | 59 (75) | |
| Former | 33 (39) | 15 (19) | |
| Current | 7 (8) | 5 (6) | |
| Diabetes mellitus | 27 (32) | 19 (24) | 0.25 |
| Cardiovascular disease | 58 (50) | 57 (50) | 0.66 |
| Pulmonary disease | 20 (43) | 27 (57) | 0.14 |
| Prior malignancy | 7 (8) | 8 (10) | 0.69 |
| ASA class | 0.10 | ||
| 1–2 | 39 (46) | 47 (59) | |
| 3–4 | 45 (54) | 32 (41) | |
| Indications for surgery | 0.59 | ||
| Uterine cancer | 24 (29) | 23 (29) | |
| Ovarian/fallopian/peritoneal cancer | 24 (29) | 21 (27) | |
| Benign | 22 (26) | 27 (34) | |
| Other* | 11 (13) | 5 (6) | |
| Synchronous primary | 3 (4) | 3 (4) |
BMI = body mass index, ASA = American Society of Anesthesiologists
Data are mean ± standard deviation, or n (%) unless otherwise specified. No significant group difference was observed except for Tobacco use (P = 0.01).
Analyses were performed by Fisher exact test (if cell count was less than 5) or Chi-square test for categorical variables; and independent t-tests for continuous variables.
Includes borderline ovarian tumors (n=9), cervical cancer (n=2), unknown primary cancer (n=1), vaginal cancer (n=1), gastrointestinal tumors (n=3).
Surgical and perioperative outcomes are summarized in Table 2. Overall, the most common surgery type was cancer staging, followed by total abdominal hysterectomy with or without bilateral salpingo-oophorectomy, tumor cytoreduction, and adnexectomy alone. Skin incisions were almost exclusively done with cutting cautery, and on average 20 cm in length and 4 cm in depth with no differences between groups based on skin closure by suture versus staples. Prophylactic antibiotics were administered prior to skin incision in all but four women, including one clean procedure. Overall, wound protocol compliance was assessed in all study participants by reviewing the operative note and defined as having completed all steps in the pre-defined wound protocol as described above. Protocol compliance was similar between groups, 85% in those randomized to staples vs. 94% in suture group (P=0.06), with no differences in subcutaneous drain placement (P=0.10). Overall, staple closure was eight minutes faster than suture (P<0.001), however, there was no significant difference in the total duration of surgery between the two groups.
Table 2.
Surgical and Perioperative Characteristics (N =163)
| Characteristic | Staples (n=84) | Suture (n=79) | P |
|---|---|---|---|
| Continuous Variables (median (IQR)) | |||
| Incision closure time (minutes)* | 3 (2–4) | 11(9–15) | <.001 |
| Estimated blood loss (mL) | 300 (150–500) | 300 (200–500) | 0.61 |
| Duration of surgery (minutes) | 172.5 (136.5–230) | 173 (141–208) | 0.91 |
| Incision length (cm)* | 20.5 (17–24) | 19.5 (17–25) | 0.81 |
| Incision depth (cm)* | 4 (3.4–5.5) | 4 (3–6) | 0.98 |
| Maximum inpatient glucose level (mg/dL) | 168.5 (142–214) | 163 (140–218) | 0.89 |
| Minimum oxygen saturation within 24h postoperatively (%)* | 94 (93–95) | 94 (93–96) | 0.46 |
| Categorical Variables (n (%)) | |||
| Antibiotic prophylaxis given prior to skin Incision† | 0.15 | ||
| None | 3 (3.8) | 1 (1.4) | |
| Cephalosporin | 74 (93.7) | 63 (87.5) | |
| Ertapenem | 2 (2.5) | 6 (8.3) | |
| Piperacillin and tazobactam | 0 (0) | 2 (2.8) | |
| Surgery type | 0.18 | ||
| Adnexectomy alone | 5 (6) | 10 (13) | |
| TAH +/− BSO | 24 (29) | 19 (24) | |
| Cancer staging | 44 (52) | 33 (42) | |
| Tumor cytoreduction | 11 (13) | 17 (22) | |
| Bowel resection | 11 (13) | 9 (11) | 0.74 |
| Lysis of adhesions | 30 (36) | 21 (27) | 0.21 |
| Subcutaneous drain placement | 72 (86) | 74 (94) | 0.10 |
| Intraperitoneal drain placement | 7 (8) | 10 (13) | 0.37 |
IQR = interquartile range, TAH = total abdominal hysterectomy, BSO = bilateral salpingo-oophorectomy
Data are median (interquartile range), or n (%) unless otherwise specified. Missing values were excluded from the denominator of the percentages.
Analyses were performed by Fisher exact test (if cell count was less than 5) or Chi-square test for categorical variables; and independent t-tests for continuous variables.
Missing data for staples group only, n=81, except for incision closure time, n=78.
Both groups have missing data, n=79 for staples group and n=72 for suture group.
Overall, wound complications occurred in 53 (33%) women, with similar rates between skin closure with staples versus suture (33% vs. 32%, RR 1.05, 95% CI 0.68–1.64) (Table 3). Similarly, there was no difference in the incidence of either wound breakdown or wound infection. Of the wound separations assessed between 10–21 days postoperatively, there were no differences in median incision length [staples (n=18): 7 cm vs. suture (n=15): 4 cm], and a small, but significant difference in depth [staples (n=12): 4.3 cm vs. suture (n=9): 2 cm, P=0.04]. However, between four to eight weeks postoperatively, the median length of wound separation was larger in the staples group (n=18) compared to suture (n=11) (4.5 vs. 2.5 cm, respectively P=0.01). Of the 98 women diagnosed with a gynecologic cancer, 83 (85%) received adjuvant chemotherapy, 43 women closed with staples and 40 with suture. Three women total (staples: n=1, suture: n=2) experienced a delay in treatment due to prolonged wound healing.
Table 3.
Postoperative Outcomes (N =163)
| Outcome | Staples (n=84) | Suture (n=79) | Relative Risk (95% CI) |
|---|---|---|---|
| Primary composite outcome* | 28 (33) | 25 (32) | 1.05 (0.68–1.64) |
| Wound infection 10–21 d† | 5 (6) | 5 (6) | 0.95 (0.29–3.16) |
| Wound breakdown 10–21 d† | 19 (23) | 17 (22) | 1.06 (0.60–1.89) |
| Length (cm) (n, median (IQR) | 18, 7 cm (3–13) | 15, 4 cm (1–8) | |
| Depth (cm) (n, median (IQR) | 12, 4.3 cm (3–5.3) | 9, 2 cm (1–3) | |
| Wound breakdown 4–8 wks‡ | 21 (28) | 13 (19) | 1.51 (0.82–2.78) |
| Length (cm) (n, median (IQR) | 18, 4.5 cm (3–10) | 11, 2.5 cm (1–3) | |
| Adjuvant treatment received§ | 43 (52) | 40 (52) | 1.00 (0.74–1.34) |
| Delay of adjuvant treatment|| | 1 (2.3) | 2 (5) | 0.47 (0.04–4.93) |
CI = confidence interval
Data are n (%) unless otherwise specified. Missing values were excluded from the denominator of the percentages.
Analyses were performed by Fisher exact test (if cell count was less than 5) or Chi-square test for categorical variables; and independent t-tests for continuous variables.
Primary composite outcome = wound breakdown and surgical site infection.
Missing data in staple group only (n=83).
Missing data. Effective sample size per group: staples (n=75) and suture (n=70).
Missing data. Effective sample size per group: staples (n=83) and suture (n=77).
For delay of adjuvant treatment, n (%) and relative risk are calculated only on patients having adjuvant therapy administrated, n=43 for staples group and n=40 for suture group.
Patient-centered outcomes regarding scar cosmetics and satisfaction are reported in Table 4. Women with staple closure reported lower median cosmetic scores (4/5 vs. 5/5, P<0.001), darker scar color (49% vs. 18%, RR 2.69, 95% CI 1.57–4.63), and more hatch/suture marks (40% vs. 4%, RR 9.47, 95% CI 3.02–29.65) compared to women with suture closure. This did not translate into differences in median satisfaction scores of their scar appearance (suture 77% vs. staples 68%, P=0.11) nor in their satisfaction with the discomfort at the incision site (staples 71% vs. suture 77%, P=0.20).
Table 4.
Cosmetic Results and Patient Satisfaction of Scar
| Variable | Staples (n=84) | Suture (n=79) | Relative Risk (95% CI) |
|---|---|---|---|
| Continuous variables (n, median (IQR)) | |||
| Stony Brook Scar Evaluation score | 74, 4 (3–5) | 70, 5 (4–5) | |
| Satisfaction of appearance of scar (%) | 71, 68 (49–83) | 67, 77 (52–87) | |
| Satisfaction of location of scar (%) | 72, 77 (52–85) | 67, 78 (59–88) | |
| Satisfaction of discomfort at scar (%) | 73, 71 (51–83) | 67, 77 (52–86) | |
| Categorical variables (n (%)) | |||
| Stony Brook Scar width* | 0.95 (0.54–1.67) | ||
| >2 mm | 18 (24) | 18 (26) | |
| <=2 mm | 56 (76) | 52 (74) | |
| Stony Brook Scar height† | 2.60 (0.87–7.80) | ||
| Elevated or depressed | 11 (15) | 4 (6) | |
| Flat | 64 (85) | 67 (94) | |
| Stony Brook Scar color† | 2.69 (1.57–4.63) | ||
| Darker | 37 (49) | 13 (18) | |
| Same or lighter | 38 (51) | 58 (82) | |
| Stony Brook Scar hatch or suture marks† | 9.47 (3.02–29.65) | ||
| Present | 30 (40) | 3 (4) | |
| Absent | 45 (60) | 68 (96) | |
| Stony Brook Scar overall appearance‡ | 1.87 (0.17–20.16) | ||
| Poor | 2 (3) | 1 (1) | |
| Good | 74 (97) | 70 (99) |
IQR = interquartile range, CI = confidence interval, Stony Brook Scar Evaluation Scale Score17 [range, from 0 (worst) to 5 (best)] = Sum of width, height, color, hatch, and overall appearance, where a better outcome has a value of 1 and a worse outcome has a value of 0.
Data are n, median (interquartile range), or n (%). Missing values were excluded from the denominator of the percentages.
Analyses were performed by Fisher exact test (if cell count was less than 5) or Chi-square test for categorical variables; and Kruskal-Wallis test for continuous variables.
Scar width: Missing data. Effective sample size per group: staples (n=74) and suture (n=70).
Scar color: Missing data. Effective sample size per group: staples (n=75) and suture (n=71).
Overall appearance: Missing data. Effective sample size per group: staples (n=76) and suture (n=71).
Univariate logistic regression analyses found that BMI (P<0.001), duration of surgery (P=0.03), maximum postoperative glucose measured as inpatient (P=0.02), diabetes (P=0.03), and prior abdominal surgery (P=0.03) were significant correlates of wound complication. However, risk factors that remained in the final model after multivariate stepwise selection were BMI (P<0.001), maximum postoperative glucose (P=0.02), prior abdominal surgery (P=0.10), tobacco use (P=0.06), as well as the two factors that were forced to stay in every model during the selection process, skin closure type (P=0.35) and subcutaneous drain placement (P=0.54). Table 5 displays odd ratios (OR) and their 95% CIs estimated using univariate and multivariable logistic regression for the six risk factors that remained in the final multivariate stepwise selection model.
Table 5.
Univariate and Multivariate Logistic Regression Model Assessing Predictors of Wound Complications (N=163).
| Risk Factor* | N | Univariate OR (95% CI) | Multivariate aOR (95% CI) |
|---|---|---|---|
| Continuous Variables | |||
| BMI (kg/m2) | 163 | 1.12 (1.06–1.18) | 1.13 (1.07–1.20) |
| Maximum postoperative glucose (mg/dl)† | 163 | 1.01 (1.00–1.01) | 1.01 (1.00–1.01) |
| Categorical Variables | |||
| Skin closure | |||
| Staples | 84 | 1.08 (0.56–2.08) | 1.45 (0.67–3.17) |
| Suture | 79 | 1.0 | 1.0 |
| Subcutaneous drain placement | |||
| Yes | 146 | 4.02 (0.89–18.29) | 1.66 (0.326–8.449) |
| No | 17 | 1.0 | 1.0 |
| Tobacco use | |||
| Current | 12 | 2.43 (0.73–8.15) | 4.96 (1.32–18.71) |
| Former | 48 | 1.33 (0.64–2.77) | 1.18 (0.51–2.75) |
| Never | 103 | 1.0 | 1.0 |
| Prior abdominal surgery | |||
| Yes | 104 | 2.21 (1.06–4.60) | 1.99 (0.87–4.55) |
| No | 59 | 1.0 | 1.0 |
BMI = body mass index; OR = odds ratio; aOR = adjusted odds ratio; CI= confidence interval.
These risk factors were the ones that remained in the final model of multivariate stepwise selection, where factors “skin closure” and “subcutaneous drain placement” were forced to stay in every model during the selection process.
Measured during inpatient hospital stay by either fingerstick or metabolic panel.
DISCUSSION
Obese women undergoing gynecologic procedures through midline vertical incision experience high rates of wound complications that are unaffected by whether the wound is closed with staples or a subcuticular suture. Suture closure yields better cosmetic scores, lighter scar color, and fewer marks, but without a difference in women’s satisfaction with their scars.
Our study contributes to the existing literature on interventions to reduce SSI and wound complications, filling a gap in knowledge about wound care in gynecology patients. Previously, evidence-based practices for skin closure in gynecology have been extrapolated from the obstetrical literature. Two recent meta-analyses of randomized controlled trials11,14 both demonstrated a twofold increased risk of wound complications with staple closure in cesarean sections. In their pooled analysis, Tuuli et al reported a wound complication rate of 107/803 (13.4%) with staples and 45/684 (6.6%) with suture. Clay et al reported similar rates that favored suture 50/385 (13.0%) vs. 28/492 (5.7%; pooled OR, 2.11; 95% CI 1.29–3.48). The Cochrane review21 including these same studies concluded that there is “no conclusive evidence about how the skin should be closed after cesarean section.”
Our composite wound complication rate (32%) was higher than that in most other studies, although consistent with the 37% rate we reported previously.1 However, when broken down by individual outcomes of wound separation and infection, our 5% SSI rates are comparable to those reported in both the obstetric (1–15%)10,22, gynecologic (6%–7%),1,2,4,23 and even non-gynecologic surgical specialties such as orthopedics (1–3%).24 Our wound separation results also are in line with studies examining open abdominal surgery. Imamura and colleagues conducted a randomized controlled trial that showed no difference in SSI between staples and suture skin closure [27/201 (13.4%) vs 25/138 (12.6%), P=0.882)].25 Furthermore, the National Institute for Health and Clinical Excellence guidelines evaluated 11 studies of women undergoing a variety of surgical procedures and found no difference in wound complications between suture and staple closure.26
Our rate of wound separation does exceed that reported in obstetric studies, likely due to differences in surgical technique and patient characteristics including age, comorbidities, and median BMI. Most obstetrical trials are limited to transverse skin incisions and the benefits of suture closure seen with transverse incisions may not offset the increased complication rate contributed by lateral tension from abdominal wall fat for women with vertical midline incisions. Importantly, the mean BMI in our study (38 kg/m2) was the highest reported among any randomized controlled trials in the obstetric literature (average BMI 27–34 kg/m2).11,14,22
Particular strengths of this study include the randomized study design and broad inclusion criteria. This allowed for improved external validity, as did the use of Nosocomial Infections Surveillance System definitions20 and Stony Brook Scar Evaluation Scale19 to ensure objective ascertainment of our primary and secondary outcomes. Other study strengths relate to our methodology. We accounted for unexpected changes in the surgical procedure that appropriately excluded patients from eligibility and met our target sample size based on our power calculation.
This trial has several limitations. Generalizability of our results may be limited by its performance at a single, academic institution serving a high-risk, medically complicated referral population undergoing complex procedures. Thus, the prevalence of wound complications after hysterectomy at centers with less complicated patients and procedures may be lower. Our study also had limited racial and ethnic diversity. Lack of blinding among the participants and providers could potentially have introduced bias, but likely was non-directional. Protocol compliance, which was slightly higher in the suture group, is another opportunity to bias results away from the null, but likely inconsequential in this case especially given the low numbers of noncompliance and our intention-to-treat statistical approach. Lastly, our projected effect size of 50% reduction in wound complication may have been overestimated. Therefore, although we met our target sample size, the study might have been underpowered to detect a clinically significant difference in outcome. However, we did find significant differences in secondary outcomes. Further, given that wound complication rates differed by only 1% between the two groups, a larger trial is unlikely to have found clinically meaningful differences.
Future efforts to decrease the rate of wound complications, particularly wound separation need to build on our understanding from previous trials on retention sutures,27 wound protection devices,28 negative pressure dressings,29 and skin sealants.30 Optimizing operative techniques and protocols to reduce wound infection are essential to improving perioperative outcomes and should be supplemented with management of modifiable risk factors such as obesity, diabetes, and other contributing comorbidities.
Acknowledgments
SUPPORT:
Supported by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR000448 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Dr. Bradley Evanoff is the PI for the Clinical and Translational Science Award that supports all Washington University ITCS and Clinical Research Training Center activities (LMK, ARH). The research was also supported by a NIH/Paul Calabresi Career Development Award for Clinical Oncology (K12) 5K12CA132783-08 (APN). The Siteman Cancer Center is supported in part by an NCI Cancer Center Support Grant # P30 CA091842, Eberlein, PI.
The authors thank the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, MO., for the use of the Biostatistics Shared Resource, which provided statistical services.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
Each author has indicated that he or she has met the journal’s requirements for authorship.
CLINICAL TRIAL REGISTRATION: ClinicalTrials.gov, https://clinicaltrials.gov, NCT01977612.
References
- 1.Novetsky AP, Zighelboim I, Guntupalli SR, Ioffe YJ, Kizer NT, Hagemann AR, et al. A phase II trial of a surgical protocol to decrease the incidence of wound complications in obese gynecologic oncology patients. Gynecol Oncol. 2014;134(2):233–237. doi: 10.1016/j.ygyno.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nugent EK, Hoff JT, Gao F, Massad LS, Case A, Zighelboim I, et al. Wound complications after gynecologic cancer surgery. Gynecol Oncol. 2011;121(2):347–352. doi: 10.1016/j.ygyno.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 3.Kirkland KB, Briggs JP, Trivette SL, Wilkinson WE, Sexton DJ. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20(11):725–730. doi: 10.1086/501572. [DOI] [PubMed] [Google Scholar]
- 4.Mahdi H, Gojayev A, Buechel M, Knight J, SanMarco J, Lockhart D, et al. Surgical site infection in women undergoing surgery for gynecologic cancer. Int J Gynecol Cancer. 2014;24(4):779–786. doi: 10.1097/IGC.0000000000000126. [DOI] [PubMed] [Google Scholar]
- 5.Shepard J, Ward W, Milstone A, Carlson T, Frederick J, Hadhazy E, et al. Financial impact of surgical site infections on hospitals: the hospital management perspective. JAMA Surg. 2013;148(10):907–914. doi: 10.1001/jamasurg.2013.2246. [DOI] [PubMed] [Google Scholar]
- 6.Perencevich EN, Sands KE, Cosgrove SE, Guadagnoli E, Meara E, Platt R. Health and economic impact of surgical site infections diagnosed after hospital discharge. Emerg Infect Dis. 2003;9(2):196–203. doi: 10.3201/eid0902.020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schiller JS, Lucas JW, Ward BW, Peregoy JA. Summary health statistics for U.S. adults: National Health Interview Survey, 2010. Vital Health Stat. 2012;10(252):1–207. [PubMed] [Google Scholar]
- 8.Wall PD, Deucy EE, Glantz JC, Pressman EK. Vertical skin incisions and wound complications in the obese parturient. Obstet Gynecol. 2003;102(5 Pt 1):952–956. doi: 10.1016/s0029-7844(03)00861-5. [DOI] [PubMed] [Google Scholar]
- 9.Berek JS, Karam A, Cibula D. Surgical Techniques. In: Berek JS, Hacker NF, editors. Berek & Hacker’s Gynecologic Oncology. 6. Philadelophia (PA): Lippincott Williams & Wilkins; 2005. p. 787. [Google Scholar]
- 10.Basha SL, Rochon ML, Quinones JN, Coassolo KM, Rust OA, Smulian JC. Randomized controlled trial of wound complication rates of subcuticular suture vs staples for skin closure at cesarean delivery. Am J Obstet Gynecol. 2010;203(3):285e281–288. doi: 10.1016/j.ajog.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Clay FS, Walsh CA, Walsh SR. Staples vs subcuticular sutures for skin closure at cesarean delivery: a metaanalysis of randomized controlled trials. Am J Obstet Gynecol. 2011;204(5):378–383. doi: 10.1016/j.ajog.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 12.Figueroa D, Jauk VC, Szychowski JM, Garner R, Biggio JR, Andrews WW, et al. Surgical staples compared with subcuticular suture for skin closure after cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2013;121(1):33–38. doi: 10.1097/aog.0b013e31827a072c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frishman GN, Schwartz T, Hogan JW. Closure of Pfannenstiel skin incisions. Staples vs. subcuticular suture. J Reprod Med. 1997;42(10):627–630. [PubMed] [Google Scholar]
- 14.Tuuli MG, Rampersad RM, Carbone JF, Stamilio D, Macones GA, Odibo AO. Staples compared with subcuticular suture for skin closure after cesarean delivery: a systematic review and meta-analysis. Obstet Gynecol. 2011;117(3):682–690. doi: 10.1097/AOG.0b013e31820ad61e. [DOI] [PubMed] [Google Scholar]
- 15.American College of O, Gynecologists. ACOG Committee opinion no. 549: obesity in pregnancy. Obstet Gynecol. 2013;121(1):213–217. doi: 10.1097/01.aog.0000425667.10377.60. [DOI] [PubMed] [Google Scholar]
- 16.Gallup DC, Gallup DG, Nolan TE, Smith RP, Messing MF, Kline KL. Use of a subcutaneous closed drainage system and antibiotics in obese gynecologic patients. Am J Obstet Gynecol. 1996;175(2):358–361. doi: 10.1016/s0002-9378(96)70146-1. discussion 362. [DOI] [PubMed] [Google Scholar]
- 17.Inotsume-Kojima Y, Uchida T, Abe M, Doi T, Kanayama N. A combination of subcuticular sutures and a drain for skin closure reduces wound complications in obese women undergoing surgery using vertical incisions. J Hosp Infect. 2011;77(2):162–165. doi: 10.1016/j.jhin.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 18.Panici PB, Zullo MA, Casalino B, Angioli R, Muzii L. Subcutaneous drainage versus no drainage after minilaparotomy in gynecologic benign conditions: a randomized study. Am J Obstet Gynecol. 2003;188(1):71–75. doi: 10.1067/mob.2003.103. [DOI] [PubMed] [Google Scholar]
- 19.Singer AJ, Arora B, Dagum A, Valentine S, Hollander JE. Development and validation of a novel scar evaluation scale. Plast Reconstr Surg. 2007;120(7):1892–1897. doi: 10.1097/01.prs.0000287275.15511.10. [DOI] [PubMed] [Google Scholar]
- 20.Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol. 1992;13(10):606–608. [PubMed] [Google Scholar]
- 21.Mackeen AD, Berghella V, Larsen ML. Techniques and materials for skin closure in caesarean section. Cochrane Database Syst Rev. 2012;11:CD003577. doi: 10.1002/14651858.CD003577.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Tuuli MG, Liu J, Stout MJ, Martin S, Cahill AG, Odibo AO, et al. A Randomized Trial Comparing Skin Antiseptic Agents at Cesarean Delivery. N Engl J Med. 2016;374(7):647–655. doi: 10.1056/NEJMoa1511048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker JL, Piedmonte MR, Spirtos NM, Eisenkop SM, Schlaerth JB, Mannel RS, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27(32):5331–5336. doi: 10.1200/JCO.2009.22.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith TO, Sexton D, Mann C, Donell S. Sutures versus staples for skin closure in orthopaedic surgery: meta-analysis. BMJ. 2010;340:c1199. doi: 10.1136/bmj.c1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.mamura K, Adachi K, Sasaki R, Monma S, Shioiri S, Seyama Y, et al. Randomized Comparison of Subcuticular Sutures Versus Staples for Skin Closure After Open Abdominal Surgery: a Multicenter Open-Label Randomized Controlled Trial. J Gastrointest Surg. 2016;20(12):2083–2092. doi: 10.1007/s11605-016-3283-z. [DOI] [PubMed] [Google Scholar]
- 26.Leaper D, Burman-Roy S, Palanca A, Cullen K, Worster D, Gautam-Aitken E, et al. Prevention and treatment of surgical site infection: summary of NICE guidance. BMJ. 2008;337:a1924. doi: 10.1136/bmj.a1924. [DOI] [PubMed] [Google Scholar]
- 27.Soisson AP, Olt G, Soper JT, Berchuck A, Rodriguez G, Clarke-Pearson DL. Prevention of superficial wound separation with subcutaneous retention sutures. Gynecol Oncol. 1993;51(3):330–334. doi: 10.1006/gyno.1993.1299. [DOI] [PubMed] [Google Scholar]
- 28.Pinkney TD, Calvert M, Bartlett DC, Gheorghe A, Redman V, Dowswell G, et al. Impact of wound edge protection devices on surgical site infection after laparotomy: multicentre randomised controlled trial (ROSSINI Trial) BMJ. 2013;347:f4305. doi: 10.1136/bmj.f4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonds AM, Novick TK, Dietert JB, Araghizadeh FY, Olson CH. Incisional negative pressure wound therapy significantly reduces surgical site infection in open colorectal surgery. Dis Colon Rectum. 2013;56(12):1403–1408. doi: 10.1097/DCR.0b013e3182a39959. [DOI] [PubMed] [Google Scholar]
- 30.von Eckardstein AS, Lim CH, Dohmen PM, Pego-Fernandes PM, Cooper WA, Oslund SG, et al. A randomized trial of a skin sealant to reduce the risk of incision contamination in cardiac surgery. Ann Thorac Surg. 2011;92(2):632–637. doi: 10.1016/j.athoracsur.2011.03.132. [DOI] [PubMed] [Google Scholar]