Abstract
Mothers and their babies represent one of the closest dyadic units and thus provide a powerful paradigm to examine how affective states are shared, and result in, synchronized physiologic responses between two people. We recruited mothers and their 12- to 14-month-old infants (Ndyads = 98) to complete a lab study in which mothers were initially separated from their infants and assigned to either a low-arousal positive/relaxation condition, intended to elicit parasympathetic nervous system (PNS) reactivity, or a high-arousal negative/stress task, intended to elicit sympathetic nervous system (SNS) reactivity. Upon reunion, infants were placed either on their mothers’ laps (touch condition) or in a high chair next to the mother (no-touch condition). We then examined if the babies SNS and/or PNS responses changed from their baseline levels and how the dyads’ physiological responses – both PNS and SNS responses – synchronized over time as a function of mothers’ affect manipulation and touch condition. Three noteworthy findings were observed. Firstly, infants of mothers assigned to the relaxation task showed greater PNS increases and PNS covariation. Secondly, infants of mothers assigned to the stress task showed stronger SNS covariation with their mothers over time. Finally, infants who sat on their mothers’ laps (i.e., touch condition) showed stronger SNS covariation than those in the no-touch condition. Taken together, these results suggest that mothers’ affective states – low-arousal positive states as well as high-arousal negative states – can be “caught” by their infants, and that touch can play a critical role in stress contagion.
Keywords: Physiologic covariation, emotional synchrony, mother-infant dyads, affect transmission
While emotional experiences have long been conceptualized as based within the individual, there is growing recognition of the ways in which emotions are interpersonally generated, experienced, and regulated (Butler, 2015; Zaki & Williams, 2013). The transmission of affective states between people—affect contagion—is stronger among similar or close others and the function of affect contagion is theorized to augment social and behavioral coordination (Butler, 2011; Hatfield, Cacioppo, & Rapson, 1994; Mendes & West, 2017). Shared affective states are especially relevant to the parent-child relationship because young children develop self-regulation skills and establish healthy functioning, in part, through affective and behavioral synchronization with their parents (Feldman, 2012; Harrist & Waugh, 2002). The current study is one of only a few to date in which an experimental design is used to induce an affective state in one member of the dyad and then measure continuous sympathetic (SNS) and parasympathetic nervous system (PNS) responses to examine affective and physiologic activation and synchronization within parent-child dyads.
The current research extends previous work on shared affective states between mothers and infants in two important ways. In past work, we induced a high-arousal negative affective state (i.e., stress) in mothers and observed stress transmission to infants via greater infant SNS activation during reunion and stronger dyadic SNS covariation compared to mother-infant dyads in a non-stress condition (Waters, West, & Mendes, 2014). In the current work, we randomly assign mothers to experience either positive/relaxed affective states or negative/stress affective states while separated from their infants and then reunite the mother and infant and examine affect transmission from mothers to infants. We also examine how affect transmission occurs by exploring one possible mechanism of affect contagion in mother-infant dyads: physical touch.
Recently, there has been a call for research that moves beyond documenting whether contagion of affective states occurs, to how it occurs, in an effort to better understand the risks and benefits of shared affective states (Timmons et al., 2015). Understanding the process through which states are shared is particularly important in the developmental domain because parents play an integral role in the child’s developing self-regulatory abilities (Kopp, 1989). Moreover, touch provides a powerful mechanism through which affective states may be passed. Touch can convey discrete emotions between unfamiliar individuals (Hertenstein, Keltner, App, Bulleit, & Jaskolka, 2006). The touch of an intimate partner also buffers a person’s neurophysiological response to stress or pain, and fosters growth in premature infants by stimulating activity of the vagus nerve and gastric system (Coan, Schaefer, & Davidson, 2006; Diego et al., 2006; Feldman et al., 2010; Hertenstein, Holmes, McCullough, & Keltner, 2009). Together this work suggests that touch plays a significant role in the communication or transmission of affect as well as the modulation of physiologic responses.
To examine shared affective states between mothers and infants, we rely on dynamic changes in SNS and PNS responses measured from the dyad simultaneously; an approach that has a long history in psychological science (see Levenson & Gottman, 1983). Specifically, we measure moment-to-moment responses using electrocardiography (mother and infant) and impedance cardiography (just mother, see below) to obtain measures of SNS and PNS. These specific biological systems are well suited to examine shared affective states because these systems are sensitive and respond quickly (within seconds) to affective states, are continuous, unobtrusive, and dynamic, and the responses does not depend on verbal reporting or even conscious awareness (Mendes, 2016). Thus, measurement of the SNS/PNS enables researchers to capture fluctuations in affective states that may be otherwise inaccessible, particularly in preverbal infants (Porges, 1996).
Transmission of Affective States via PNS and SNS Activation
The PNS is responsible for supporting bodily functions of rest and restoration. In addition, polyvagal theory posits that PNS changes relate to the social engagement system (see Porges, 2007 for a review). For example, in the dyadic realm, coordination of PNS responses is associated with positive interactions in romantic couples and parent-child pairs (Helm, Sbarra, & Ferrer, 2014; Moore et al., 2009). However, this work has largely been correlational rather than experimental and thus does not provide insight into whether positive affective states are causally related to coordinated PNS responses in dyads (cf. Weisman, Zagoory-Sharon, & Feldman, 2012). We use PNS activation and covariation to investigate whether a mother’s low-arousal positive affective state can be transmitted to and embodied by her infant.
The SNS is responsible for supporting bodily functions during times of threat or stress, and these negative affective states can be transmitted via SNS responses between partners (Levenson & Ruef, 1993; Waters, et al., 2014). For example, in our past work, mothers randomly assigned to complete a stressful task and then reunited with their infants showed greater activation and stronger physiologic covariation of SNS responses than mothers who completed a non-stressful task when separated from their infants (Waters, et al, 2014).
Moving beyond documenting positive and negative affect contagion via PNS and SNS states, we also examine how these states are transmitted by focusing on a key interpersonal variable relevant to mother-infant interactions: touch. Arguably one of the most primary and powerful sensory experiences is touch. Studies of adult romantic pairs and parent-child pairs have found that warm, loving touch reduces neurophysiological reactivity to a stressor (Coan, Schaefer, & Davidson, 2006; Feldman, Singer, & Zagoory, 2010; Grewen, Anderson, Girdler, & Light, 2003). Moreover, transmission of negative emotions can be deliberately conveyed via touch between strangers (Hertenstein et al., 2009), but less work has examined how negative emotions might be transmitted physiologically through touch of close others. In our previous work with mothers and infants examining stress transmission, we did not manipulate the role of touch – all infants sat on their mothers’ laps during the study. In this work we directly manipulate touch between mothers and infants and examine the role of positive and negative emotion transmission.
Study Overview
In the current study we randomly assigned mothers to an affect manipulation that either involved watching a video designed to induce a relaxed, low arousal positive state or a demanding, negative social evaluation speech task designed to evoke high-arousal negative affect/stress. Based on past literature we expected the stress task to increase mothers’ SNS activation whereas the relaxing task would increase mothers PNS activation (e.g., Larsen, et al., 2007; Mendes, 2016; Porges, 2007). We confirmed the effectiveness of the maternal affect manipulations via changes in self-reported affect and physiologic responses.
We had five primary predictions guiding this work. First, we expected that infants would catch their mothers’ relaxed affective state, which would be evidenced by infants whose mother was in the positive affect/relaxed condition to have greater PNS activation during reunion. Second, we expected that infants whose mothers were in the negative affect/stress condition to have greater SNS activation. Third and fourth, we expected greater mother-infant PNS covariation following the relaxed condition and greater SNS covariation following the stress condition. Finally, we expected touch to moderate the activation and covariation. Specifically, that touch compared to no-touch would exacerbate SNS activation and covariation following stress and touch would exacerbate PNS activation and covariation following the relaxed condition.
Methods
Participants
Mothers (N =105; Mage = 33.77 years, SD = 5.55) and their 13-month olds (Mage = 13.19 months, SD = 1.25; 49% female) were recruited from the San Francisco Bay Area. Mothers were excluded if they had a BMI over 35, were hypertensive, had a pacemaker, took cardiac medications, or were pregnant. Three dyads attrited after completing online consent, but before arriving for the laboratory visit. Twenty-four dyads were assigned to the negative/stress+touch condition, 25 dyads to the negative/stress+no-touch condition, 25 dyads to the positive/relaxed condition, and 26 dyads to the positive/relaxed+no-touch condition. Four dyads attrited after beginning the laboratory visit due to infant distress. Dyads who began the lab visit (whether they completed the study or not) were compensated $75 for their participation.
Sixty-two percent of mothers were European American, 18% were Asian, 13% were Latina, 4% were African American, and 3% reported being multi-ethnic. Ninety-one percent of mothers were married or cohabiting with the infants’ father. Mothers were well-educated with 90% having at least a bachelor’s degree. There was a range of family income with 24.5% of participants earning $50,000 or less a year, 17% earning between $51,000 and $100,000 per year, 27% earning between $101,000 and $150,000 a year, and 30% earning at least $150,000 per year.
Procedures
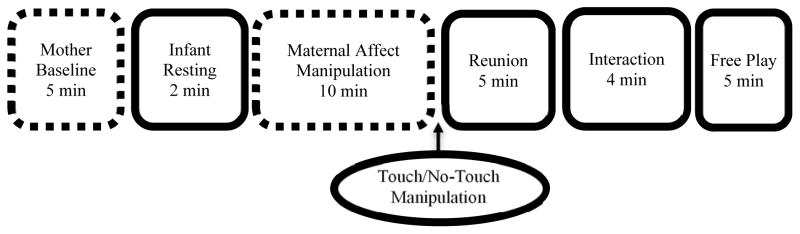
Upon arrival at the laboratory, each mother provided consent for audiovisual and physiological recording for herself and her infant (Figure 1 for overview) and completed a baseline affect measure (modified PANAS). The infant was entertained in a separate playroom while the experimenter attached sensors to the mother to measure SNS and PNS responses and the mother relaxed alone for a 5-minute period during which her resting/baseline physiologic responses were obtained.
Figure 1.
Hatched lines indicate mother was alone. Solid lines indicate mother and infant were together.
Then, the infant was brought to the mother and the experimenter attached sensors to measure physiologic responses of the infant. The experimenter played a recording of a common lullaby and instructed the mother to help the infant relax for a 2-minute episode during which the infant’s resting responses were obtained. This type of protocol is standard with young children to discourage movement during the recording (Alkon et al., 2003). Afterward, the infant returned to the playroom while the mother remained in the laboratory. For the four infants who became distressed during separation and could not be soothed within a few minutes, the maternal affect manipulation was cut short so that the infant could be returned to and soothed by the mother.
Mothers were randomly assigned to one of two length-matched affect manipulation conditions: low-arousal positive affect/relaxation task, in which a female experimenter showed mothers a 5- minute video of nature scenes accompanied by soothing music followed by a 5-minute video of images of family members engaged in positive interactions accompanied by Israel Kamakawiwo’Ole’s Somewhere Over the Rainbow (Human, Thorson, Woolley, & Mendes, 2016)1; or a high-arousal negative affect/stress task, in which mothers were instructed to give a 5-minute speech about her strengths and weaknesses as a person to a panel of two evaluators (one male, one female), which was followed by a 5-minute question and answer (Q&A) session. Throughout this 10- minute period, mothers received negative non-verbal feedback from the evaluators, including head-shaking, arm crossing, and frowning (Trier Social Stress Test [TSST]; Kirschbaum & Hellhammer, 1994). The participants were not aware of the details of either condition until after the baseline period was over and the experimenter then explained the (randomly assigned) upcoming task. Mothers were not told the purpose of the task or given an affect label (e.g., this is a stress or relaxation task); they were only instructed on how to complete the task. The experimenter obtained verbal consent to continue since the tasks were not described in the initial consent. Immediately after completing the tasks but before the infant was reunited with the mother, mothers completed the PANAS again.
Afterward, the infant rejoined the mother in the main laboratory room for a 5-minute reunion episode. This was the point at which the touch manipulation was introduced: half of the mothers received their infant directly into their laps (touch condition) whereas for the other half the infants were placed by the experimenter into a high chair next to the mothers who were instructed not to touch their infants (no-touch condition). We provided the (false) justification to not touch their child because touch would interfere with the physiologic signals. During the reunion, all mothers were provided age appropriate toys and instructed to play with their child naturally as they would at home. Following the reunion episode, infants underwent a mild social challenge in the form of the 4-minute interaction episode. A female who was familiar to the mother (the evaluator from the stress task or the assistant from the relaxation task) entered the room and proceeded to engage the mother in innocuous conversation (i.e., she asked pleasant questions about the infant’s development) for one minute. Then the experimenter attempted to engage the infant in toy play for three minutes. After this interaction with the experimenter concluded, the experimenter completed questions about the infants’ behavior towards them. Finally, mothers and infants were left alone in the room and engaged in a 5-minute free play episode in which they were again instructed to play naturally with some toys. Upon completion of the study, the sensors were detached from the mothers and infants and mothers were debriefed and compensated.
Measures
Maternal affect
We used the Positive and Negative Affect Schedule (PANAS; Watson, Clark & Tellegen, 1988) to assess mothers’ affect. Mothers rated their current affective state with 20 items on a 5-point scale from 1 (not at all) to 5 (a great deal). We calculated positive and negative affect scales for each time point (i.e., before and after the affect manipulation; αs ranged .86 to .91). Following our previous work to isolate high arousal, externalizing negative affect following the stress task (Waters, et al., 2014), we further differentiated negative affect scale items into an externalizing sub-scale consisting of three PANAS items: “hostile,” “irritable,” and “upset” (αs = .63 and .84 for pre and post, respectively). In addition, we added six new items to index low arousal positive affect/relaxation: “loved,” “calm,” “happy,” “relaxed, “close to loved ones,” and “warm” (αs = .91 and .92 for pre and post, respectively). These items were used as a manipulation check following the low-arousal positive affect/relaxation task.
Experimenters’ ratings of infants’ behavior
Upon completing the mother-infant interview, experimenters completed a questionnaire that queried the extent to which the infant smiled at the experimenter, how withdrawn (versus approach) the infant was toward them, how anxious, and how comfortable the infant appeared. Responses ranged on 1 to 5 Likert scale with 1 anchored at “not at all” and 5 “a great deal.” We reverse-coded withdrawn and anxious and formed a composite of infants’ comfort (α = .83).
Mother and infant ANS
We used impedance cardiography (ICG) and electrocardiography (ECG) on mothers to record SNS and PNS, which allowed us to calculate pre-ejection period (PEP) and respiratory sinus arrhythmia (RSA). PEP is a time-based measure that is calculated from the time of left ventricle contraction to the opening of the aortic valve and is considered a pure measure of SNS (no PNS influence). RSA (high-frequency heart rate variability) represents the change in heart period and partly reflects the influence of the cardiac vagal nerve and provides a reasonable measure of PNS.
In our pilot testing, we found that infants could not tolerate the mylar band electrode system that completely encircles the neck and torso used in impedance cardiography, so for infants we measured ECG only, which provided measures of inter-beat interval (IBI) (a less pure measure of SNS) and RSA. We used a modified lead II configuration of spot sensors placed on the infants’ torso to measure ECG. Data were sampled at 1000 Hz and integrated with a single Biopac MP150 unit with two ECG modules (one for each member of the dyad) and one NICO (impedance) module.
Physiologic measures were collected continuously on mothers starting at the baseline period through the end of the study. Measures were collected continuously on infants when they were with their mothers (i.e. baseline, and then continuously from the reunion to the end of the study). Data were scored in 30-second segments using Mindware IMP v 3.0.15 to calculate PEP for mothers, and Mindware HRV v 3.0.25 to calculate infant IBI, and RSA for both mothers and infants. We set the high frequency range to .24 and 1.04 for infants (Bar Haim, Marshall, & Fox, 2000). All data were visually inspected off-line by trained research assistants for artifacts and cleaned as needed. Segments in which less than 90% of the data were artifact-free were excluded from analyses.
Data analysis
Physiological reactivity scores were calculated by subtracting responses during the last 30-second segment of the baseline period from all subsequent 30-second segments. For maternal self-reported affect, pre-task ratings were subtracted from post-task ratings. In line with previous work, we focused on the first minute of the affect manipulation for physiologic reactivity in mothers and the first minute of interaction for physiological reactivity in infants using analysis of variance (ANOVA) for single time point data unless otherwise stated.
To measure mother-infant physiological covariation over the three episodes, we estimated the relationship between mothers’ physiological reactivity and infants’ physiological reactivity by regressing the infants’ physiologic score on the maternal score, within time-point (we chose mother as the outcome in order to adjust for the effect of mothers’ BMI on physiological reactivity). Covariation is estimated as the path from infants’ physiologic response to mothers’ physiologic response within the same time-point. We analyzed data using the MIXED procedure in SPSS to account for nonindependence in mothers’ physiologic states over time. Note that this procedure allows for missing data across the 28 30-second intervals of mother-infant interaction. For these models, we estimated the random effects of the intercept and of the infants’ physiologic state on the mothers’ state (which captures the random effect of physiological covariation), and the covariance between these two random effects (see Waters et al., 2014 for the same analysis procedure and online material for syntax and data from the previous study).
To estimate whether covariation strengthened or weakened over the three tasks overall and as a function of our manipulations, we included a continuous variable, episode (coded as −1, 0, 1, for reunion, interaction, and free-play, respectively) as a moderator of covariation. We note that using a continuous time variable in which each 30-second segment of physiological data is coded as one unit of time, (i.e., 1 through 10 reflects reunion, 11 through 18 is the interview- interaction, and 19 through 28 represent mother-infant free-play) instead of grouping segments of physiological data into their respective episodes yields comparable results.
We modeled linear changes in PNS covariation (i.e. the relationship between mother RSA and infant RSA reactivity) and SNS covariation (i.e. the relationship between mother PEP and infant IBI reactivity) in separate analyses. For each system, we examined the overall strength of covariation, the effect of maternal affect manipulation, touch manipulation, and whether covariation strengthened or weakened over the course of the three dyadic episodes. Thus, each model included the main effects of infant physiological state, episode, maternal affect condition, touch condition, and all possible interactions between these variables.
Results
Manipulation check
Self-reported affect
We began by confirming that mothers experienced the affect manipulation as intended by analyzing changes in mothers’ self-reported affect from before to after the affect induction. We observed significant differences between conditions for the PANAS positive affect scale, F(1, 97) = 8.96, p = .004, ηp 2 = .09. Relative to baseline affect, mothers who completed the negative/stress task showed decreases in positive affect (M = −2.43, SD = 6.82) compared to those who completed the positive/relaxation task (M = 1.63, SD = 6.6). No significant effects of condition were found for the PANAS negative affect scale, F(1, 97) = 0.59, p = .44, ηp 2 = .01. Similar to past work, there was a significant main effect for the externalizing negative affect subscale, F(1, 97) = 5.72, p = .017, ηp2 = .06, such that mothers in the negative/stress condition reported increases in externalizing negative affect (M = 0.76, SD = 2.4) compared to mothers in the positive/relaxation condition (M = −0.09, SD = 0.64). We also found a main effect positive affect subscale, F(1, 97) = 21.74, p < .001, ηp2 = .19, with mothers in the positive/relaxation condition reporting greater increases (Mchange = 2.32, SD = 3.31) than those in the negative/stress condition (Mchange = −0.94, SD = 3.61).
Mothers’ physiologic changes
We then examined the extent to which our affect manipulations changed maternal physiology. Using analysis of covariance with affect manipulation as the between-subject factor and BMI as the covariate, we found a main effect of maternal affect manipulation on mothers’ PEP reactivity, F(1, 92) = 21.48, p < .001, ηp2 = .19. Mothers completing the negative/stress task showed significantly more SNS activation than those in the positive/relaxation task (Table 1). We also found a significant effect of the affect manipulation on mothers’ RSA reactivity, F(1, 94) = 4.09, p = .046, ηp2 = .04. Mothers in the positive/relaxation condition exhibited greater parasympathetic activation while those in the negative/stress condition exhibited parasympathetic withdrawal (Table 1). These analyses provide evidence that negative/stress task engendered SNS reactivity, and our positive/relaxation task engendered PNS reactivity.
Table 1.
Descriptive statistics of mother physiological reactivity during the affect manipulation.
| Mother PEP reactivity | Mother RSA reactivity | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 95% CI | 95% CI | |||||||
| Affect manipulation | Mean | SD | Lower | Upper | Mean | SD | Lower | Upper |
| Relaxation | −0.41 | 6.33 | −1.7 | 0.88 | 0.17 | 0.91 | −0.01 | 0.35 |
| Stress | −11.39 | 14.06 | −14.25 | −8.53 | −0.19 | 1.03 | −0.4 | 0.02 |
Experimenters’ ratings of infants comfort
We examined the extent to which experimenters rated infants’ behavior during the parent-experimenter interaction. We observed a large main effect of maternal affect manipulation such that infants whose mother completed the relaxation task were rated as more comfortable by the experimenter (M = 2.125) than infants whose mother completed the stress task (M = 0.86), F (1, 91) = 12.17, Cohen’s d=.72, p < .001. The main effect for touch and the interaction were not significant. Although these appear to be compelling data, we do note that the experimenter who completed these ratings was aware of the maternal affect condition (indeed, they were instrumental in carrying out the manipulation) so there could be demand characteristics influencing this effect.
Infants’ physiologic reactivity by maternal affect manipulation
To examine whether infant physiological reactivity differed by maternal affect, touch, and the interaction, we examined infants’ RSA reactivity during the first minute of the interviewer-interaction with the mother and infant. We did not observe a main effect for maternal affect manipulation, F(1, 91) = 0.46, p = .50, ηp2 = .01, but there was a marginal main effect for the touch manipulation, F(1, 91) = 3.65, p = .06, ηp2 = .04, and a significant maternal affect X touch interaction, F(1, 91) = 6.37, p = .013, ηp2 = .07 (Table 2). Decomposing this interaction revealed that for those infants whose mothers completed the stress task, neither infants in the touch nor the no-touch condition showed a significant change in RSA, t(22) = 0.54, p = .60, 95% CI [−0.47, 0.80], t(23) = .09, p = .93, 95% CI [−.43, .46], respectively. For infants whose mothers completed the relaxation task, infant RSA reactivity increased more in the no-touch condition than in the touch condition, F(1, 91) = 12.25, p = .001, ηp2 = .21. Infants in the touch condition did not show change in RSA, t(23) = −1.23, p = .23, 95% CI [−0.74, 0.19], but those in the no-touch condition did, t(23) = 3.85, p = .001, 95% CI [0.36, 1.21]. Contrary to predictions, infants showed larger RSA increases in the relaxation + no-touch condition compared to the relaxation +touch condition. Thus infants whose mothers underwent a relaxation manipulation and who sat in a nearby high chair had PNS responses consistent with a more relaxed state than infants who sat on their mothers’ laps following the same affect manipulation.
Table 2.
Descriptive statistics of infant physiologic reactivity during the interaction episode.
| Infant IBI reactivity | Infant RSA reactivity | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 95% CI | 95% CI | |||||||
| Condition | Mean | SD | Lower | Upper | Mean | SD | Lower | Upper |
| Relax+Touch | −5.61 | 28.83 | −11.41 | 0.19 | −0.28 | 1.10 | −0.5 | −0.06 |
| Relax+No-Touch | 17.36 | 33.27 | 10.67 | 24.05 | 0.79 | 1.0 | 0.59 | 0.99 |
| Stress+Touch | −5.42 | 26.81 | −10.81 | −0.03 | 0.17 | 1.48 | −0.13 | 0.47 |
| Stress+No-touch | 13.94 | 32.36 | 7.43 | 20.45 | 0.02 | 1.05 | −0.19 | 0.23 |
Next we performed parallel analyses with infant IBI reactivity. We did not find a significant main effect for the maternal affect manipulation or for the maternal affect X touch manipulation interaction, F(1, 91) = 0.28, p = .60, ηp2 = .003, and F(1, 91) = 0.31, p = .58, ηp2 = .003, respectively. We did observe a significant main effect of touch manipulation, F(1, 91) = 13.09, p < .001, ηp2 = .13. As shown in Table 2 below, infants seated on their mothers’ laps showed greater SNS increases (i.e., decreased IBI reactivity) compared to those in the high chair. For dyads in which mothers experienced the negative/stress condition, infants who were placed on their mothers’ laps showed increased SNS activation relative to infants placed in the high chair, F(1, 91) = 4.64, p = .03, ηp2 = .10. Similarly, for dyads in which mothers experienced the relaxation condition infants who touched their mothers showed greater increases in SNS responses than those who did not touch their mothers, F(1, 91) = 4.23, p = .033, ηp2 = 09 (Table 2). These findings indicate that infants experienced sitting on mothers’ laps (i.e., the touch condition) as more physiologically arousing than sitting in a nearby high chair, regardless of mothers’ manipulated affect state.
In sum, we examined infants’ physiologic reactivity during the first minute of the interaction with the female interviewer who was responsible for either evaluating the mother in the stress condition or lead the mother through the relaxation condition. Importantly, the infant had no prior exposure to this female interviewer and the interviewer acted in a similar, polite manner regardless of the prior affect manipulation when in front of the infant. For mothers assigned to the relaxing, positive experience, their infants showed significant increases in RSA but only in the no-touch condition. When examining infants’ SNS activation (IBI changes), we found that mothers who completed the stressful, negative experience had infants who responded with greater SNS activation when they sat on their mothers’ lap (touch condition). Critically and consistent with the predictions, the no-touch condition did not result in an increase in SNS activation and instead showed a significant decrease in SNS responses from baseline consistent with infants having a non-activated physiologic response.
Mother-infant covariation prior to manipulations
Given the presumably close physical, affective, and perhaps physiologic, connection between mothers and infants, we could expect mother-infant dyads to exhibit physiological covariation naturally, regardless of our manipulations. To test this possibility, we conducted a separate set of analyses examining covariation (treating maternal physiology as the criterion and infant physiology as the predictor, and adjusting for mothers’ BMI) during the two minutes of baseline, prior to any manipulation. For baseline mother PEP-infant IBI covariation, we did not find evidence of covariation across the four baseline time points, t(250.42) = 0.59, p = .55, 95% CI [−.03, .05], nor did covariation increase over these four minutes, t(223.42) = 1.43, p = .15, 95% CI [−.004, .05]. For baseline RSA, there was no evidence of covariation across the four baseline time points, t(298.76) = 0.01, p = .99, 95% CI [−.15, .15], nor did RSA covariation increase over these four time points, t(241.56) = 0.84, p = .40, 95% CI [−.04, .10]. Thus, we did not observe any evidence that mothers and infants show synchronized physiological responses prior to manipulation, at least in this short time frame in a laboratory environment.
Mother-infant PNS covariation
We predicted that the relaxation condition would result in greater covariation of the PNS, as evidence by stronger RSA covariation values in this condition. We examined mother-infant RSA covariation from the reunion episode through the free play episode. The two-way infant RSA reactivity X touch interaction was not significant, t(78.05) = .50, p =.58, 95% CI [−.03, 0.06]. The two-way infant RSA reactivity X episode interact was also not significant, t(2090.18) = −.68, p =.50, 95% CI [−.05, 0.02]. These results indicate that we did not find moderating effects for touch manipulation or episode on mother-infant RSA covariation. The expected infant RSA reactivity X maternal affect interaction was significant, t(78.37) = 2.34, p = .02, 95% CI [0.01, 0.10]. Following the maternal negative/stress task, mother-infant RSA covariation was not different from zero, t(73.74) = −0.46, p = .65, 95% CI [−0.11, 0.07], but following maternal relaxation, mother-infant RSA covariation was positive and marginally significant from zero, t(86.70) = 1.83, p = .07, 95% CI [−0.01, 0.13]. These findings demonstrate that mothers in a relaxed affective state transmit their PNS responses to their infants while mothers in a high-arousal stress state did not.
Mother-infant SNS covariation
Similar to RSA covariation, we did not observe PEP/IBI covariation during the baseline episodes, prior to the manipulations. In previous work, we observed a significant SNS covariation following the maternal negative/stress task. Again replicating the earlier finding, mother PEP and infant IBI reactivity covaried positively from the reunion episode through the free play episode, t(82.87) = 3.05, p = .003, 95% CI [0.01, 0.04]. Next, we examined whether maternal affect manipulation, touch manipulation, or episode/time moderated SNS covariation. We did not observe significant 2- or 3-way interactions, but did observe a significant 4-way interaction of infant IBI reactivity X maternal affect X touch X episode, t(2077.12) = −2.43, p =.02, 95% CI [−0.02, −0.002]. We unpacked the four-way interaction separately for the two maternal affect conditions.
Maternal relaxation condition
Following the relaxation task, we did not find any significant interactions except for a marginal interaction between infant IBI reactivity and episode, t(2150.40) = 1.81, p = .07, 95% CI [−0.01, 0.02], indicating that covariation significantly increased from the reunion episode to free play episode. However, covariation was not significantly different from zero for reunion, t(124.52) = 0.34, p = .73, 95% CI [−.02, .03], or interaction, t(81.88) = 1.21, p = .23, 95% CI [−.008, .03], and was marginally different from zero at free play, t(108.05) = 1.88, p = .062, 95% CI [−0.001, 0.05].
Maternal negative/stress condition
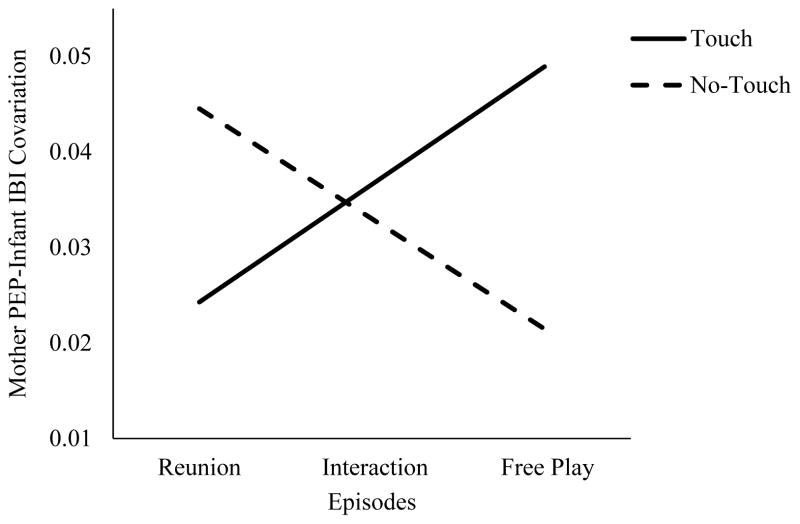
Among dyads for whom mothers were assigned to the stress task, the infant IBI reactivity X touch manipulation X episode interaction term was significant, t(1992.73) = 2.07, p = .04, 95% CI [0.001, 0.02). Thus, strength of SNS covariation varied as a function of both touch and episode. We deconstructed this finding by examining the strength of SNS covariation at each episode, first within the stress+touch condition, followed by the stress+no-touch condition. In the stress+touch condition, SNS covariation was not significantly different from zero at the reunion, t(133.82) = 1.31, p = .19, 95% CI [−0.001; 0.06] but increased over time with significant effects during the interaction, t(87.95) = 2.20, p = .03, 95% CI [0.004, 0.07], and the free play, t(144.45) = 2.54, p = .012, 95% CI [0.01, 0.09]. These findings suggest that SNS covariation increased over the three episodes among dyads whose mothers completed the negative/stress task and infants were in the mothers’ arms during the remainder of the study (Figure 2).
Figure 2.
Mother PEP-infant IBI covariation over the three dyadic episodes following the maternal stress task moderated by touch manipulation.
In contrast, when examining dyads whose mothers were in the stress condition but the infant was in the high chair (no-touch), SNS covariation was significantly different from zero at the reunion and interaction episodes, t(116.35) = 2.60, p = .01, 95% CI [0.01, 0.08]; t(79.18) = 2.13, p = .04, 95% CI [0.002, 0.06], respectively. However, by the end of the study, during the free play episode, covariation was not significantly different from zero, t(109.11) = 1.25, p = .21, 95% CI [−0.012; 0.05]. These findings suggest that SNS covariation decreased over the three episodes (Figure 2). Taken together these results suggest that while stress transmission occurred for both the stress+touch and stress+no-touch conditions, they demonstrated opposite temporal trajectories. Touch strengthened the effect of SNS covariation over time, whereas lack of touch weakened SNS covariation.
Discussion
We experimentally manipulated mothers’ affective states and mother-infant touch to examine transmission of positive and negative affective states through physiological covariation in mother-infant dyads. Regarding PNS responses, infants whose mothers experienced a low-arousal relaxation condition exhibited significant increases in RSA reactivity, suggesting a calmer, more “socially engaged” state, but contrary to our expectations this finding was only observed in the no-touch condition and not in the touch condition. We also found evidence of stronger mother-infant PNS covariation following the relaxation condition relative to the stress condition regardless of whether the mother and infant were touching or not. In contrast, regarding SNS reactivity, infants who were held by mothers who had experienced a stress task showed SNS covariation with their infants. The strength of SNS covariation increased over time in the touch condition; mother-infant dyads in the no-touch condition showed weaker SNS covariation over time.
An emerging body of research focuses on the associations between the PNS responses of social partners (Crowell et al., 2014; Helm, Sbarra, & Ferrer, 2014; Moore & Calkins, 2004) and this work has examined the tendency for partners’ PNS responses to become correlated or synchronized during interactions. Our findings align with and extend this work with an experimental manipulation of an affective state which influences PNS responses. By inducing PNS activation using a relaxing, positive video that induced a sense of calm and social warmth in mothers while they were separated from their infants, we were able to build a case for causality for mother-infant PNS covariation. Nevertheless, this covariation is not conclusive evidence for the transmission of maternal affective state to her infant. According to polyvagal theory, an increase in RSA (as we induced in the relaxation task) is associated with activation of a social engagement system that facilitates eye contact with a social partner as well as attention to the human voice, facial expressions, and gestures (Porges, 2007). Thus, mothers in a state of low-arousal positive affect may be better able to attune to and thus synchronize with their infants’ affective and physiologic states than mothers in a state of high-arousal negative affect. Regardless of directionality, elevated RSA induced by low-arousal relaxation task may be a means by which close partners co-regulate and improve each other’s emotional states.
We found that mother-infant PNS covariation was robust to whether dyads touched or not. While contrary to our hypotheses, this finding is consistent with studies of romantic pairs in which RSA synchrony can be achieved through face-to-face orientation without touch (see Helm, Sbarra, & Ferrer, 2014). Touch is arguably more central to the mother-infant relationship than the adult romantic relationship, but our results suggest that low-arousal positive affect may be transmitted through other avenues (e.g., bodily posture, tone of voice). Tests of these possible mechanisms await future research.
Because embodied stress can have negative effects on health and functioning, it is important to understand possible mechanisms of this process. In previous work we found that infants catch their mothers’ negative stress through covariation of SNS responses (Waters, et al., 2014) and we replicated this effect in the current work. We have now examined stress contagion in well over 100 mother-infant dyads, and the question we attempt to address here is how mother-infant stress contagion might be disrupted or attenuated. We found that infants whose mothers did not touch them showed significantly weaker stress contagion, suggesting that touch may be one pathway through which stress is transmitted from mother to infant. We are confident that SNS activation was transmitted from mother to infant because the infants in our study were not distressed when they rejoined their mothers, but were in a generally calm, positive state when they were placed either into their mothers’ arms or into a high chair nearby. Indeed distressed infants in any condition resulted in an ending of the study.
The moderating effect of touch on stress contagion has implications for the parent-child relationship. Maintaining proximity to the parent is one of the primary functions of the infant’s attachment bond. Close proximity facilitates physical contact, which in turn seems to facilitate stress transmission between parent and child. This may be highly adaptive in the context of acute danger, but when a parent’s stress response is not valuable information regarding the safety of the immediate environment, there may be a straightforward way for parents to buffer infants from their stress. By engaging with their young children without holding or physically touching them, stressed parents relate to their child through the face and voice which are potentially more readily down regulated than the bodily cues that are available to the child through touch. Future studies are needed to test such pathways as facial expression, gaze, vocal tone to further unpack this possible behavioral intervention for how stressed parents interact with their children.
We also found that the strength of SNS covariation increased over time in the touch condition, a phenomenon we had observed in earlier work, but not in the no-touch condition. This does not mean necessarily that negative/stress+touch dyads remained in a highly SNS activated state over time, but that these mothers’ and infants’ physiologic responses became more and more tightly synchronized the longer they were together. Thus as mothers’ SNS reactivity returned to baseline over time, their infants’ physiologic reactivity followed suit. For dyads in the negative/stress+no-touch condition, mothers’ and infants’ SNS responses were unrelated to each other by the end of the free play episode. While research with close pairs as well as strangers finds that physiologically synchronized pairs often exhibit greater behavioral coordination than unsynchronized pairs, it is yet unknown what the implications might be in the context of a stressed parent and her child.
There are several limitations to the current study. The maternal affect manipulations involved a high-arousal negative social evaluation task designed to evoke strong SNS activation and a low-arousal positive video task designed to evoke PNS activation. We did not examine a condition in which positive affect was accompanied by strong SNS activation because our previous work found that this maternal affective state was a weaker stress transmission than a negative high arousal state (Waters, et al, 2014). For the no-touch manipulation, infants were placed in a high chair alongside of their seated mothers. This configuration was meant to parallel the arrangement of the touch condition as closely as possible, but mothers were likely to spend more of the interaction period face-to-face with their infants in the no-touch condition than the touch condition. We expected that touch would immediately increase infants’ SNS reactivity especially following the maternal negative/stress task. While this was the case, touch also increased infant SNS reactivity and decreased PNS reactivity following the maternal relaxation task. This finding was unexpected, but aligns with the results of a recent study in which pleasant stroking with a brush increased infants’ heart rate (Fairhurst, Loken, & Grossman, 2014). This suggests that touch, including a mother’s touch, may be somewhat physiologically arousing for an infant, regardless of the mother’s affective state. Finally, our community sample consisted largely of lower risk families and this limits generalizability of our findings to higher risk populations.
In sum, our findings demonstrate support for the phenomenon of mother-infant affect contagion and establish physical contact, or lack thereof, as a means by which stress contagion is modulated. Thus, we demonstrate not only how acute parental psychological stress gets into their children’s bodies, but also what can be done to exacerbate or attenuate affect transmission. This has important implications for chronically stressed families and warrants extension to such populations.
Acknowledgments
This research was supported by grants from the National Science Foundation (BCS 1430799), National Institute of Mental Health T32 (MH019391) and National Institute of Aging (R24AG048024).
Footnotes
Relaxation videos can be viewed at http://wendyberrymendes.com/cms/emotion-health-and-psychophysiology-lab/research-materials.html
Some of the data in this manuscript were presented at the International Congress for Infant Studies (2015) and the Society for Affective Science (2015).
References
- Alkon A, Goldstein LH, Smider N, Essex MJ, Kupfer DJ, Boyce WT. Developmental and contextual influences on autonomic reactivity in young children. Developmental Psychobiology. 2003;42:64–78. doi: 10.1002/dev.10082. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Marshall PJ, Fox NA. Developmental changes in heart period and high frequency heart period variability from 4 months to 4 years of age. Developmental Psychobiology. 2000;37:44–56. doi: 10.1002/1098-2302(200007)37:1<44::aid-dev6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Bratslavsky E, Finkenauer C, Vohs KD. Bad is stronger than good. Review of General Psychology. 2001;5:323–370. doi: 10.1037/1089-2680.5.4.323. [DOI] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Autonomic determinism: The modes of autonomic control, doctrine of autonomic space, and the laws of autonomic constraint. Psychological Review. 1991;98:459–487. doi: 10.1037/0033-295x.98.4.459. [DOI] [PubMed] [Google Scholar]
- Butler EA. Interpersonal affect dynamics. It takes two (and time) to tango. Emotion Review. 2015 doi: 10.1177/1754073915590622. [DOI] [Google Scholar]
- Butler EA. Temporal interpersonal emotion systems: The “TIES” that form relationships. Personality and Social Psychology Review. 2011;15:367–393. doi: 10.1177/1088868311411164. [DOI] [PubMed] [Google Scholar]
- Coan JA, Schaefer JS, Davidson RJ. Lending a hand: Social regulation of the neural response to threat. Psychological Science. 2006;17:1032–1039. doi: 10.1111/j.1467-9280.2006.01832.x. [DOI] [PubMed] [Google Scholar]
- Crowell SE, Baucom BR, Yaptangco M, Bride D, Hsiao R, McCauley E, Beauchaine TP. Emotion dysregulation and dyadic conflict in depressed and typical adolescents: Evaluating concordance across psychophysiological and observational measures. Biological Psychology. 2014;98:50–58. doi: 10.1016/j.biopsycho.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cwir D, Carr PB, Walton GM, Spencer SJ. Your heart makes my heart move: Cues of social connectedness cause shared emotions and physiological states among strangers. Journal of Experimental Social Psychology. 2011;47:661–664. [Google Scholar]
- Davis M, Suveg C. Focusing on the positive: A review of the role of child positive affect in developmental psychopathology. Clinical Child and Family Psychology Review. 2014;17:97–124. doi: 10.1007/s10567-013-01620y. [DOI] [PubMed] [Google Scholar]
- Diego MA, Field T, Hernandez-Reif M, Deeds O, Ascencio A, Bergert G. Preterm infant massage elicits consistent increases in vagal activity and gastric mobility that are associated with greater weight gain. Acta Pediatrica. 2006;21:1588–1591. doi: 10.1111/j.1651-2227.2007.00476.x. [DOI] [PubMed] [Google Scholar]
- Fairhurst MT, Loken L, Grossmann T. Physiological and behavioral responses reveal 9-month-old infants’ sensitivity to pleasant touch. Psychological Science. 2014;25:1124–1131. doi: 10.1177/0956797614527114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. Parent-infant synchrony: A biobehavioral model of mutual influences in the formation of affiliative bonds. Monographs of the Society for Research in Child Development. 2012;77:42–51. doi: 10.1111/j.1540-5834.2011.00660.x. [DOI] [Google Scholar]
- Feldman R, Singer M, Zagoory O. Touch attenuates infants’ physiological reactivity. Developmental Science. 2010;13:271–278. doi: 10.1111/j.1467-7687.2009.00890.x. [DOI] [PubMed] [Google Scholar]
- Ferrer E, Helm JL. Dynamical systems modeling of physiological coregulation in dyadic interactions. International Journal of Psychophysiology. 2013;88:296–308. doi: 10.1016/j.ijpsycho.20120.10.013. [DOI] [PubMed] [Google Scholar]
- Grewen KM, Anderson BJ, Girdler SS, Light KC. Warm partner contact is related to lower cardiovascular reactivity. Behavioral Medicine. 2003;29:123–130. doi: 10.1080/08964280309596065. [DOI] [PubMed] [Google Scholar]
- Harrist AW, Waugh RM. Dyadic synchrony: Its structure and function in children’s development. Developmental Review. 2002;22:555–592. [Google Scholar]
- Hatfield E, Cacioppo JT, Rapson RL. Emotional contagion. Current Directions in Psychological Science. 1994;2:96–99. [Google Scholar]
- Helm JL, Sbarra DA, Ferrer E. Coregulation of respiratory sinus arrhythmia in adult romantic partners. Emotion. 2014;3:522–531. doi: 10.1037/a0035960. [DOI] [PubMed] [Google Scholar]
- Hertenstein MJ, Holmes R, McCullough M, Keltner D. The communication of emotion via touch. Emotion. 2009;9:566–573. doi: 10.1037/a0016108. [DOI] [PubMed] [Google Scholar]
- Hertenstein MJ, Keltner D, App B, Bulleit B, Jaskolka A. Touch communicates distinct emotions. Emotion. 2006;6:528–533. doi: 10.1037/1528-3542.6.3.528. [DOI] [PubMed] [Google Scholar]
- Human LJ, Thorson KR, Woolley J, Mendes WB. When two positives make a negative: Subtractive effects of pairing positive psychological and pharmacological manipulations on emotions, social behavior, and physiology. Hormones and Behavior (pending minor revisions) [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology. 1994;19:313–333. doi: 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Kopp CB. Regulation of distress and negative emotions: A developmental view. Developmental Psychology. 1989;25:343–354. doi: 10.1037/0012-1649.25.3.343. [DOI] [Google Scholar]
- Levenson RW, Gottman JM. Marital interaction: Physiological linkage and affective exchange. Journal of Personality and Social Psychology. 1983;45:587–597. doi: 10.1037//0022-3514.45.3.587. [DOI] [PubMed] [Google Scholar]
- Lindsey EW, Cremeens PR, Colwell MJ, Caldera YM. The structure of parent-child dyadic synchrony in toddlerhood and children’s communication competence and self-control. Social Development. 2009;18:375–396. doi: 10.1111/j.1467-9507.2008.00489.x. [DOI] [Google Scholar]
- Mendes WB. Emotion and the autonomic nervous system. In: Barrett LF, Lewis M, Haviland-Jones J, editors. Handbook of Emotions. 4. New York, NY: Guilford Publications, Inc; 2016. pp. 166–181. [Google Scholar]
- Mendes WB, West TV. Catching affect: Physiological covariation in dyads and groups. Current Opinion in Psychology 2017 [Google Scholar]
- Moore GA, Calkins SD. Infants’ vagal regulation in the still-face paradigm is related to dyadic coordination of mother-infant interaction. Developmental Psychology. 2004;40:1068–1080. doi: 10.1037/0012-1649.40.6.1068. [DOI] [PubMed] [Google Scholar]
- Moore GA, Hill-Soderlund AL, Propper CB, Calkins SD, Mill-Koonce WR, Cox MJ. Mother-infant vagal regulation in the face-to-face still-face paradigm is moderated by maternal sensitivity. Child Development. 2009;80:209–223. doi: 10.1111/j.1467-8624.2008.01255.x. [DOI] [PubMed] [Google Scholar]
- Papp LM, Pendry P, Adam EK. Mother-adolescent physiological synchrony in naturalistic settings: Within-family cortisol associations and moderators. Journal of Family Psychology. 2009;23:882–894. doi: 10.1037/a0017147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry NB, Calkins SD, Nelson JA, Leerkes EM, Marcovitch S. Mothers’ responses to children’s negative emotions and child emotion regulation: The moderating role of vagal suppression. Developmental Psychobiology. 2012;54:503–513. doi: 10.1002/dev.20608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW. Physiological regulation in high-risk infants: A model for assessment and potential intervention. Development and Psychophathology. 1996;8:43–58. doi: 10.1017/s0954579400006969. [DOI] [Google Scholar]
- Timmons AC, Margolin G, Saxbe DE. Physiological linkage in couples and its implications for individual and interpersonal functioning: A literature review. Journal of Family Psychology. 2015;29:720–731. doi: 10.1037/fam0000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters SF, West TV, Mendes WB. Stress contagion: Physiological covariation between mothers and infants. Psychological Science. 2014;25:934–942. doi: 10.1177/0956797613518352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Weisman O, Zagoory-Sharon O, Feldman R. Oxytocin administration to parent enhances infant physiological and behavioral readiness for social engagement. Biological Psychiatry. 2012;72:982–989. doi: 10.1016/j.biopsych.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Zaki J, Williams WC. Interpersonal emotion regulation. Emotion. 2013;13:803–810. doi: 10.1037/a0033839. [DOI] [PubMed] [Google Scholar]