Summary
Community-based integrated tuberculosis(TB)/human immunodeficiency virus (HIV) case finding demonstrates high yield for TB and HIV; a high proportion with drug resistance, without prior treatment; and the majority of tuberculosis in HIV-negative individuals, potentially representing a reservoir that is perpetuating the TB epidemic.
Keywords: community, HIV, integration, intensive case finding, tuberculosis
Abstract
Background
Intensive case finding is endorsed for tuberculosis (TB) control in high-risk populations. Novel case-finding strategies are needed in hard-to-reach rural populations with high prevalence of TB and human immunodeficiency virus (HIV).
Methods
We performed community-based integrated HIV and TB intensive case finding in a rural South African subdistrict from March 2010 to June 2012. We offered TB symptom screening, sputum collection for microbiologic diagnosis, rapid fingerstick HIV testing, and phlebotomy for CD4 cell count. We recorded number of cases detected and calculated population-level rates and number needed to screen (NNS) for drug-susceptible and -resistant TB.
Results
Among 5615 persons screened for TB at 322 community sites, 91.2% accepted concurrent HIV testing, identifying 510 (9.9%) HIV-positive individuals with median CD4 count of 382 cells/mm3 (interquartile range = 260–552). Tuberculosis symptoms were reported by 2049 (36.4%), and sputum was provided by 1033 (18.4%). Forty-one (4.0%) cases of microbiologically confirmed TB were detected for an overall case notification rate of 730/100000 (NNS = 137); 11 (28.6%) were multidrug-resistant or extensively drug-resistant TB. Only 5 (12.2%) TB cases were HIV positive compared with an HIV coinfection rate of 64% among contemporaneously registered TB cases (P = .001).
Conclusion
Community-based integrated intensive case finding is feasible and is high yield for drug-susceptible and -resistant TB and HIV in rural South Africa. Human immunodeficiency virus–negative tuberculosis predominated in this community sample, suggesting a distinct TB epidemiology compared with cases diagnosed in healthcare facilities. Increasing HIV/TB integrated community-based efforts and other strategies directed at both HIV-positive and HIV-negative tuberculosis may contribute to TB elimination in high TB/HIV burden regions.
Tuberculosis (TB) remains a global public health scourge with increasing global incidence. This is particularly challenging in South Africa where the reported TB incidence was 834 per 100000 persons in 2015 [1]. Rising TB incidence and mortality affect people living with human immunodeficiency virus (HIV) disproportionately; high TB mortality rates are attributed at least in part to late presentation to care, diagnostic delays, and lack of integrated TB and HIV services [2, 3]. The incorporation of HIV services throughout the TB care continuum from screening to treatment completion has been recognized as essential to improving outcomes [4, 5].
The World Health Organization has endorsed the “3Is” strategy to curb the TB epidemic among people living with HIV: (1) intensive case finding (ICF); (2) infection control; and (3) isoniazid preventive therapy [6, 7]. With respect to ICF, TB screening is recommended among high-risk groups, those attending congregate settings, and contacts of known TB patients. Intensive case finding in healthcare facilities in high HIV-prevalent regions among people living with HIV who are not yet diagnosed or others considered at high risk has resulted in high TB yield [8]. Although the World Health Organization does not recommend population-level TB screening, efforts directed at specific regions based on local epidemic characteristics may be appropriate [9]. This approach may be justified in hard-to-reach rural populations where the prevalence of both TB and HIV is high [10]. Furthermore, community-based studies in several resource-limited settings have shown impressively high TB yield using various screening strategies [11–13].
The optimal strategies for community-level TB screening will vary by region and available resources [9, 10, 12] but should be guided by evidence affirming this approach. Although ICF strategies targeting households may be conducive to individual counseling and reaching expanded populations, congregate settings in rural areas where community members gather may provide opportunities for greater efficiency in resource-constrained settings with widely dispersed populations with inadequate transportation for both public health workers and patients who may have difficulty accessing healthcare services. Similarly, despite the availability of an array of TB diagnostic tools, the most effective diagnostic strategy will depend on available resources. Because acid fast bacilli (AFB) smear as a screening tool is highly insensitive, particularly in people living with HIV, and provides no information about drug susceptibility (DST), either culture with DST or GeneXpert are necessary in areas where multidrug resistant or extensively drug resistant (MDR/XDR) TB prevalence is high.
Despite evidence confirming TB/HIV treatment effectiveness in optimizing treatment outcomes in sub-Saharan Africa where prevalence of both conditions is high [2, 14, 15], strategies that integrate HIV/TB screening may likely prove efficacious but have not been empirically tested. If found to be effective, such strategies as community-based ICF should facilitate early case detection and avoid late presentation to care for both diseases, interrupt community transmission, and reach potentially hidden populations within the community who might otherwise avoid or have limited access to established healthcare systems [12, 13, 16–18]. We therefore report on the yield of a novel community-based integrated TB/HIV ICF strategy (CBICF) in a highly prevalent HIV, TB, and MDR/XDR TB rural district in KwaZulu-Natal province, South Africa.
METHODS
We conducted this community-based intensive case-finding strategy in the rural subdistrict of Msinga, KwaZulu-Natal, South Africa, which is comprised of 2000 square kilometers and has a population of 180000 traditional Zulu people [19]. The region is characterized by an extremely high HIV antenatal prevalence rate (>30%) and high rates of drug-susceptible TB (1100/100000) and was the site of the initial uncovering of an epidemic of MDR/XDR TB [20]. Approximately 60%–70% of newly diagnosed TB patients are HIV coinfected [21]. Msinga is the poorest medical subdistrict in South Africa with high levels of poverty (68%), low literacy, and high unemployment (85%) [22]. The population is widely dispersed, living in isolated family compounds of traditional Zulu huts, often without ventilation, electricity (61%), and clean water (69%) [19]. Transportation is impeded primarily because of the rugged terrain, and most roads are unpaved, and access requires vehicles with high ground clearance. The provincial district hospital, 16 primary care clinics, and 3 mobile clinics provide healthcare for the region. Tuberculosis specimens may be collected at primary care clinics, but all diagnostics, including chest radiography, are only available at the district hospital.
From March 2010 to June 2012, a CBICF team consisting of health educators, nurses, and HIV counselors attended community-based congregate settings, including taxi ranks, municipality events, home-based care events, pension pay points (social grant distribution sites), and prisons to provide integrated TB/HIV screening services. Secondary school visits ceased when the Department of Education placed a moratorium on school-based testing in 2011. The team provided health education on a variety of topics, including HIV transmission, symptoms, and treatment; TB symptoms, diagnosis, and treatment; condom use; sexually transmitted infections; pregnancy; and prenatal care. The team announced the availability of free voluntary HIV testing and TB screening. For those who voluntarily sought services, staff members administered a brief survey that included demographic characteristics, previous HIV testing, previous TB treatment, and current TB symptoms (cough, fever, night sweats, weight loss, chest pain, hemoptysis). We deployed a 2-step (screen and confirmation) rapid HIV testing strategy, and phlebotomy was offered for CD4 testing if positive [23, 24]. Individuals with cough >2 weeks or >2 other symptoms were requested to produce 2 sputum specimens to be tested using AFB smear and culture with DST [25]. Cough of any duration was introduced as a criterion at the end of the first year [26]. Blood and sputum specimens were transported on the same day to the district hospital where CD4 processing and Ziehl-Neelsen were performed (auramine smear became available in early 2012). If only 1 sputum specimen was provided, it was preferentially sent for culture. For individuals undergoing CD4 testing, staff provided results and counseling either by cell phone or in person, with referral for antiretroviral therapy (ART) based on South African national guidelines [27]. Sputum culture was sent to the provincial TB referral laboratory in Durban for culture and drug susceptibility testing. We notified the local TB directly observed therapy—short course (DOTS) office of positive AFB smears and culture results; patients were traced and notified if sputum results were positive and referred for drug-susceptible or drug-resistant TB therapy as appropriate. Community members with symptoms who were not able to produce sputum were referred to their local primary care clinic for further evaluation.
Demographic characteristics were described using percentages and medians with interquartile ranges. We recorded number of cases found and population level rates and number needed to screen (NNS) to find 1 active TB case by using total community population as the denominator for both. Comparison of CBICF results with Department of Health statistics, obtained from medical record review, was performed with χ2 analysis. For all analyses, a P value < .05 was considered statistically significant. All statistical analysis was performed using SAS 9.3 (SAS Institute).
Ethical approval was obtained from the institutional review boards at the University of KwaZulu-Natal and Yale University School of Medicine.
RESULTS
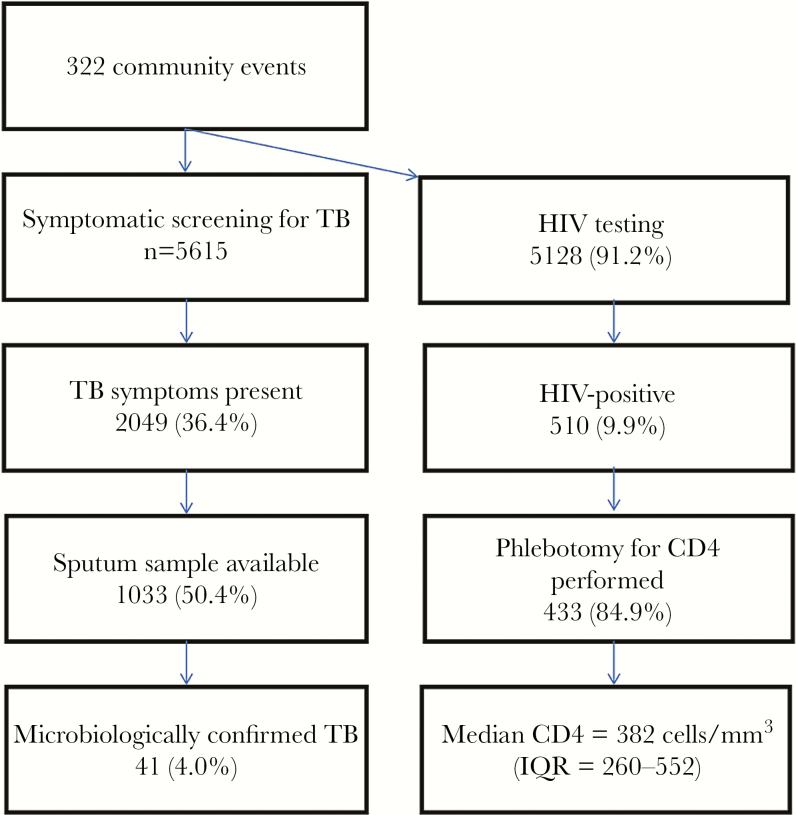
We conducted CBICF at 322 community events over 28 months with 5615 individuals undergoing TB symptom screening; 5128 (91.3%) also accepted concurrent rapid HIV testing (Figure 1). Among the congregate settings (Table 1), screening sites included municipality events (n = 2121; 37.7%), home-based care events (n = 1208; 21.5%), pension pay points where monthly social stipends were provided (n = 1179; 21.0%), secondary schools (n = 353; 6.3%), taxi ranks (n = 313; 5.6%), health fairs (n = 254; 4.5%), and prisons (n = 189; (3.4%). Among 140 community members in year 1 who underwent screening, 129 (92%) reported a preference for testing in community settings instead of healthcare settings, and 13 (9.3%) reported preferring to test in private where they could not be seen by other community members.
Figure 1.
Flow diagram of community-based integrated tuberculosis and human immunodeficiency virus intensive case finding, March 2010–June 2012. Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range; TB, tuberculosis.
Table 1.
Tuberculosis Community-Based Intensive Case Finding by Community Site
| Congregate Site | Visits per Site | Number Screened | Screeners With TB Symptoms (n = 1995) | No. (%) TB Suspects (Sputum Submitted) (n = 1033) | Number Microbiolog- ically Confirmed (n = 41) | NNS |
|---|---|---|---|---|---|---|
| Secondary schools | 8 | 353 | 76 | 45 (12.8) | 1 | 353 |
| Pension pay points | 84 | 1179 | 465 | 205 (17.4) | 5 | 236 |
| Home-based care events | 83 | 1208 | 439 | 266 (22) | 11 | 110 |
| Municipality events | 100 | 2119 | 783 | 403 (19) | 16 | 132 |
| Health fairs | 17 | 254 | 88 | 31 (12.2) | 1 | 254 |
| Taxi ranks | 23 | 313 | 70 | 35 (11.2) | 2 | 157 |
| Prisons | 7 | 189 | 74 | 48 (25.4) | 5 | 38 |
| Total | 322 | 5615 | 1995 | 1033 | 41 | 137 |
Abbreviations: NNS, number needed to screen; TB, tuberculosis.
Among those screened, 69% were female with a median age of 41 years (interquartile range [IQR] = 22–56). Tuberculosis symptoms were reported by 2049 (36.4%); all attempted to provide sputum, but of those with symptoms, only 1033 (50.4%) were able to submit sputum for microbiological evaluation. Among these, 561 (54.3%) submitted only 1 sputum specimen, which was sent for culture. Sputum from 16 (1.5%) AFB smear-positive individuals had available confirmatory cultures, and 12 were positive. Contamination affected 2 sputum cultures and 6 DST specimens. Community-based ICF detected 41 (4.0%) cases of microbiologically confirmed TB for an overall case notification rate of 730 per 100000 persons (Table 2). All cases were traced and referred for treatment. The NNS to yield 1 TB case was 137. Among TB cases, 19 (46%) were female; median age was 45 years (IQR = 27–57). Only 6 (14.6%) of these cases self-reported prior history of TB treatment.
Table 2.
Intensive Case Finding Yield by Human Immunodeficiency Virus Status
| Yield | Total | HIV Status | |
|---|---|---|---|
| Seronegative | Seropositive | ||
| No. screened (%) | 5615 | 5105 (90.9) | 510 (9.1) |
| No. and proportion with TB symptoms (sputum submitted, %) | 1033 | 917 (88.8) | 116 (11.2) |
| Microbiologically confirmed TB cases, No. (%) | 41 | 36 (87.8) | 5 (12.2) |
| Drug susceptible | 30 | 25 | 5 |
| MDR TBa | 7 | 7 | 0 |
| Pre-XDR TBa | 2 | 2 | 0 |
| XDR TBa | 2 | 2 | 0 |
| TB case notification rate | |||
| Overall | 730/100000 | 641/100000 | 89/100000 |
| Drug-susceptible TB | 464/100000 | 386/100000 | 77/100000 |
| Drug-resistant TB | 196/100000 | 196/100000 | … |
| Number needed to screen | |||
| Overall | 137 | 156 | 1123 |
| Drug-susceptible TB | 216 | 259 | 1294 |
| Drug-resistant TB | 510 | 510 | … |
aMDR TB is tuberculosis that is resistant to isoniazid and rifampin. Pre-XDR TB is tuberculosis that is resistant to isoniazid, rifampin, and either a quinolone or an injectable agent. XDR TB is tuberculosis that is resistant to isoniazid, rifampin, quinolone, and injectibale agent.
Abbreviations: HIV, human immunodeficiency virus; MDR, multidrug resistant; TB, tuberculosis; XDR, extensively drug resistant.
Among the 41 TB cases, 11 (26.8%) were found to have drug-resistant TB, for a case notification rate of 196 per 100000 persons and NNS of 510 (Table 2); 7 (63.4%) of these were MDR TB, 2 (18.2%) were pre-XDR TB, and 2 (18.2%) were XDR TB cases. Among the 11 MDR/XDR TB cases, only 1 (9%) reported previous TB treatment, 5 years earlier. After reporting of culture results, all 11 patients with drug-resistant TB were found to be alive on tracing.
Among the various community congregate settings, home-based events and prisons, followed by municipality events, yielded the greatest proportion of individuals with TB symptoms who were able to submit a sputum specimen (Table 1). Although the number of microbiologically confirmed cases with Mycobacterium tuberculosis (Mtb) was greatest at home-based events and municipality events, the NNS was greatest at prisons.
Five hundred ten (9.9%) community members were found to be HIV infected or self-identified themselves as previously known to be HIV-positive. Most (n = 433; 84.9%) underwent phlebotomy for CD4 count testing; the median CD4 cell count was 382 cells/mm3 (IQR = 260–552). Among those with TB symptoms (n = 1033), 116 (11.2%) were HIV-positive (Table 2).
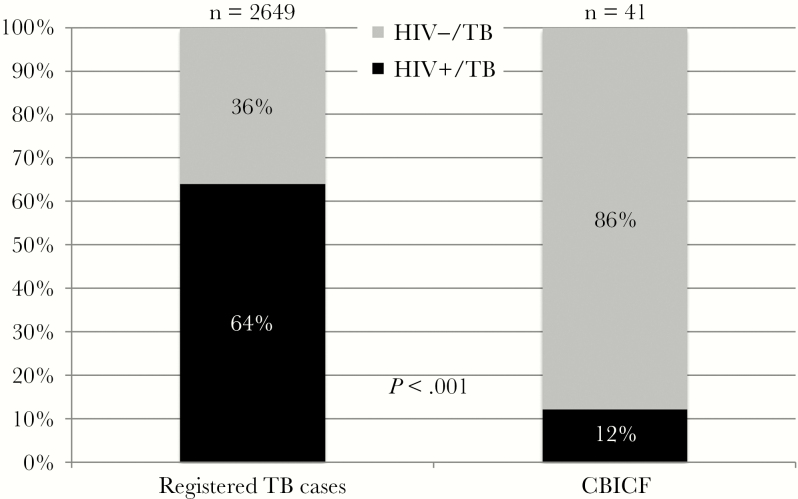
Of 41 cases of TB detected through CBICF, only 5 (12.2%) were HIV coinfected. This is in contrast with 64% HIV coinfection of registered TB cases in Msinga during the same period (P < .001) (Figure 2). Two patients found to have MDR TB had refused HIV testing; none of the other patients identified with drug-resistant TB were HIV coinfected, in contrast with Msinga registered cases where 80%–90% are HIV coinfected [20, 28, 29]. The case notification rate, at 641 per 100000 for HIV-negative TB cases with an NNS of 156 (Table 2), was 7-fold higher than that of HIV-positive TB cases, with a rate of 89 per 100000 persons and NNS of 1123.
Figure 2.
Human immunodeficiency virus–associated tuberculosis cases among officially registered cases, compared with community-based intensive case finding cases, March 2010–June 2012, in Msinga, Kwazulu-Natal. Abbreviations: CBICF, community-based intensive case finding; HIV, human immunodeficiency virus; TB, tuberculosis.
DISCUSSION
In this article, we have described a novel application of the WHO-endorsed ICF strategy to the community setting in a rural area with high prevalence for HIV, TB, and MDR and XDR TB [20]. We have demonstrated that using a community-based approach to case finding activities that integrates both TB and HIV screening is feasible and acceptable to community members in rural settings. Notably, this article shows that the CBICF strategy produces a high yield for both drug-susceptible and drug-resistant TB in the community and that the epidemiology of TB in this community sample is primarily HIV-negative, distinct from the predominantly HIV-positive epidemiology seen in healthcare facilities where most notified cases are diagnosed and where more symptomatic patients are likely to seek treatment.
The data presented here have potentially important implications for TB/HIV policy and practice, particularly in resource-limited settings where a substantial proportion of patients live in hard-to-reach rural settings. Case finding for TB and HIV in an integrated manner at the community congregate gathering level may efficiently diagnose TB cases at an earlier stage of disease, as evidenced here when all community members with MDR/XDR TB were traced and found to be alive after culture results became available; this is distinctly different from previous reports from the same site among hospitalized patients where at least 50% had died within 30 days of obtaining sputum, long before culture results were received [14]. Furthermore, as demonstrated by mathematical modeling [30], CBICF may interrupt transmission and contribute to declines in epidemic trajectories, especially if TB cases initiate TB treatment and those coinfected with HIV are successfully linked to HIV care, initiated on ART, and retained in care. These epidemiologic findings need verification in future studies to inform potential interventions; if confirmed, CBICF may represent a complimentary strategy to existing TB elimination efforts.
The high TB case notification rate (730/100000) described here is particularly notable given that TB diagnosis in this sample required microbiological confirmation. Countrywide, in South Africa, TB cases diagnosed by sputum AFB and/or culture represent approximately 40%–50% of all notified TB cases, and the remainder are defined by clinical criteria alone [31]. The yield in our CBICF sample thus represents a minimum estimate of TB cases in the community compared with conventional case detection and notification practice.
This CBICF strategy differs from other case-finding strategies by focusing on congregate settings in the community to facilitate screening efficiency. Previous studies have evaluated household-level ICF screening in urban and peri-urban settings, which also produce high yield [13, 16]. In 1 of these studies, an AFB smear–only strategy yielded a case rate of 832 per 100000 persons. In another study, use of AFB smear and culture yielded a rate of 870 per 100000 persons. Had we used AFB smear only, our case notification rate would have been 247 per 100000 persons, which is substantially lower than by using culture. Furthermore, household and community congregate setting strategies, such as the one we report in which different congregate settings within the community were used, may target different populations within the community and may be complimentary. Important among the congregate screening settings is the high yield of TB in prisoners, which is consistent with the international literature [32, 33]. Additional effectiveness studies comparing and combining household and congregate settings are needed to determine the optimal community-based approach, although these will likely differ by region [13].
Unexpectedly, the proportion of HIV coinfection among microbiologically diagnosed TB cases in this community-derived sample was significantly lower than that of overall registered cases in the region. The epidemiology of drug-susceptible and drug-resistant TB and HIV in this community sample appears to be distinct from that reported by the national TB program, where the majority of cases are diagnosed in healthcare facilities. Although HIV prevalence in the community is high, the great majority of community members with microbiologically confirmed TB cases in our study were HIV-negative. We speculate that those with active drug-susceptible and drug-resistant tuberculosis monoinfection may represent a reservoir of TB in the community that is contributing to the TB epidemic propagation. Those who are HIV-negative and immunocompetent are likely to have slower development and longer duration of symptoms and higher bacillary burden compared with HIV-infected patients [34], whereas those with HIV/TB coinfection are more likely to progress more quickly and seek medical attention, thereby reducing total duration of infectiousness, an important component of epidemic propagation. The lower NNS for HIV-negative community members and the improved performance of rapid diagnostics such as AFB microscopy and, importantly, GeneXpert make this population a particularly attractive screening target. Among the HIV-positive community members in this sample, symptoms and signs of active TB may be different at the relatively high observed median CD4 count found here than observed in TB/HIV–coinfected patients with lower CD4 cell counts presenting to healthcare facilities.
Findings here contribute to a growing body of literature that suggests that community-level screening for TB, including in rural settings, may require different screening algorithms than those used in healthcare facilities [26, 35], consistent with the pre-HIV–era TB literature.
Of great concern is the high case notification rate of drug-resistant tuberculosis identified using the CBICF strategy, primarily among HIV-negative individuals and those without any prior history of treated tuberculosis. Such findings point to primary transmission of drug-resistant TB beyond healthcare settings, particularly primary MDR/XDR TB, as predicted previously by modeling studies and recently confirmed in empiric studies, with potentially disastrous consequences [30, 36, 37]. From a clinical perspective, the finding that all patients were alive by the time sputum culture results were available is reassuring and unlike earlier studies of drug-resistant TB where most patients, largely HIV coinfected, died before receipt of cultures results [20]. Unlike other studies in the region, however, HIV coinfection was low among identified drug-resistant TB patients in our community sample compared with the vast majority of MDR/XDR TB patients in KwaZulu-Natal [14, 15, 28, 29]. Earlier community-based diagnosis may provide an opportunity for interventions that may improve survival in this population and also decrease transmission of drug-resistant organisms [15]. Although modeling has confirmed the impact on reducing incidence and mortality from TB and HIV [30], these findings require confirmation in other regions. Furthermore, a recent study has demonstrated the cost effectiveness of the integrated TB/HIV CBICF strategy in rural resource-limited regions [38].
Despite the number of key findings from this study, several limitations remain. First, this study is solely focused on innovative public health and community-based strategies to simultaneously identify those with TB and HIV in rural settings; thus, clinical diagnostics such as chest radiographs that facilitate TB diagnosis were not available and beyond the scope of this evaluation. Future clinical evaluations are needed to assess disease severity and determine the impact of earlier case detection on disease outcomes for HIV and TB to fully evaluate the utility of the community-based screening strategy. Second, the results of any clinical evaluation and any additional community members who were referred to their local clinic for further evaluation were beyond the scope of this study. Additionally, the study was performed before the availability of GeneXpert in our region, and thus we are unable to comment on how its availability might have influenced the study results. Fourth, we recognize potential sampling bias in our strategy. This CBICF strategy did not formally screen a statistically predetermined population-based representative sample but rather provided a convenience community-derived sample available at public congregate settings in this widely dispersed rural population. Although our sample is similar to the age and sex distribution in the district [19], it may not be fully representative of the entire community because our screening sites might underrepresent undiagnosed community members who are sick with or at highest risk for HIV or TB and would not be present at the community sites. Our sample was not restricted to those with traditional risk factors for TB, including those who might be older, contacts of TB cases, or those with HIV. Furthermore, we recognize that the restrictions on testing in schools reduced our access to adolescent populations, although the markedly lower incidence of TB in adolescents perhaps makes this a less important demographic for TB testing. Lastly, although we used standardized surveys about past HIV and TB history, we could not confirm whether someone was previously treated for TB and relied on patient’s self-report of prior treatment as we were unable to match individuals with official TB registries. Last, sputum was collected only from those who reported symptoms and were able to expectorate sputum, and thus our findings do not reflect a true population-based estimate of TB in our rural community setting.
Notwithstanding these limitations, important findings emerged that have the potential to inform future expanded community-based efforts and mathematical modeling studies to assess the impact of CBICF strategies on a larger scale and under an array of different assumptions. Specifically, we demonstrated that integrated TB and HIV CBICF is feasible and acceptable in rural impoverished settings and demonstrates extraordinarily high yield for drug susceptible and drug resistant TB and for HIV; that primary MDR/XDR TB is occurring in the community and in the absence of HIV coinfection; and that in this rural community sample, HIV-negative TB predominates, suggesting a reservoir of chronic TB disease that contributes to perpetuation of the TB epidemic. Integration of TB/HIV screening at the community level needs to be investigated more fully; ICF efforts to identify individuals with both HIV-positive and HIV-negative tuberculosis may provide an important contribution toward the elimination of TB.
Acknowledgments
The authors are grateful for the assistance from and partnership with the Church of Scotland Hospital and the KwaZulu Natal Department of Health. We particularly acknowledge the important contributions of the research team at Philanjalo NGO.
Disclaimer. The funders had no role in study design, analysis, or reporting.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (K23 AI089260); Fogarty International Clinical Research Program (R24TWOO7988); United States Agency for International Development/University Research Corporation (674-C-00-09-00121-00); President’s Emergency Plan (PEPFAR) for AIDS Relief (U62 CCU 223540); Gilead Foundation (157201); Irene Diamond Fund (R05130); and the Doris Duke Charitable Foundation (2010073).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Global Tuberculosis Report 2016. Geneva, Switzerland: World Health Organization; 2016. [Google Scholar]
- 2. Abdool Karim SS, Naidoo K, Grobler A et al. . Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362:697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lessells RJ, Swaminathan S, Godfrey-Faussett P. HIV treatment cascade in tuberculosis patients. Curr Opin HIV AIDS. 2015;10:439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Legido-Quigley H, Montgomery CM, Khan P et al. . Integrating tuberculosis and HIV services in low- and middle-income countries: a systematic review. Trop Med Int Health. 2013;18:199–211. [DOI] [PubMed] [Google Scholar]
- 5. Harries AD, Lawn SD, Getahun H et al. . HIV and tuberculosis–science and implementation to turn the tide and reduce deaths. J Int AIDS Soc. 2012;15:17396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization. WHO Three I’s Meeting: Intensified Case Finding (ICF), Isoniazid Preventive Therapy (IPT) and TB Infection Control (IC) for People Living With HIV. Geneva, Switzerland: WHO; 2008. [Google Scholar]
- 7. World Health Organization. Guidelines for Intensified Tuberculosis Case-Finding and Isoniazid Preventive Therapy for People Living With HIV in Resource Constrained Settings. Geneva, Switzerland: WHO; 2010. [Google Scholar]
- 8. Shenoi SV, Brooks RP, Catterick K et al. . “Cough officer” nurses in a general medical clinic successfully detect drug-susceptible and -resistant tuberculosis. Public Health Action. 2013;3:46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization. Systematic Screening for Active Tuberculosis: Principles and Recommendations. Geneva, Switzerland: WHO; 2013. [PubMed] [Google Scholar]
- 10. Kranzer K, Houben RM, Glynn JR et al. . Yield of HIV-associated tuberculosis during intensified case finding in resource-limited settings: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Corbett EL, Bandason T, Duong T et al. . Comparison of two active case-finding strategies for community-based diagnosis of symptomatic smear-positive tuberculosis and control of infectious tuberculosis in Harare, Zimbabwe (DETECTB): a cluster-randomised trial. Lancet. 2010;376:1244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lorent N, Choun K, Thai S et al. . Community-based active tuberculosis case finding in poor urban settlements of Phnom Penh, Cambodia: a feasible and effective strategy. PLoS One. 2014;9:e92754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ayles H, Muyoyeta M, Du Toit E et al. ; ZAMSTAR team Effect of household and community interventions on the burden of tuberculosis in southern Africa: the ZAMSTAR community-randomised trial. Lancet. 2013;382:1183–94. [DOI] [PubMed] [Google Scholar]
- 14. Gandhi NR, Shah NS, Andrews JR et al. ; Tugela Ferry Care and Research (TF CARES) Collaboration HIV coinfection in multidrug- and extensively drug-resistant tuberculosis results in high early mortality. Am J Respir Crit Care Med. 2010;181:80–6. [DOI] [PubMed] [Google Scholar]
- 15. Shenoi SV, Brooks RP, Barbour R et al. . Survival from XDR-TB is associated with modifiable clinical characteristics in rural South Africa. PLoS One. 2012;7:e31786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ayles H, Schaap A, Nota A et al. ; Peter Godfrey-Faussett for the ZAMSTAR Study Team Prevalence of tuberculosis, HIV and respiratory symptoms in two Zambian communities: implications for tuberculosis control in the era of HIV. PLoS One. 2009;4:e5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Claassens M, van Schalkwyk C, den Haan L et al. . High prevalence of tuberculosis and insufficient case detection in two communities in the Western Cape, South Africa. PLoS One. 2013;8:e58689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sekandi JN, List J, Luzze H et al. . Yield of undetected tuberculosis and human immunodeficiency virus coinfection from active case finding in urban Uganda. Int J Tuberc Lung Dis. 2014;18:13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Republic of South Africa Department of Health. Umzinyathi District Profile. KwaZulu Natal, South Africa: KwaZulu Natal Department of Health; 2012. [Google Scholar]
- 20. Gandhi NR, Moll A, Sturm AW et al. . Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368:1575–80. [DOI] [PubMed] [Google Scholar]
- 21. Massyn N, Peer N, English R et al. . District Health Barometer 2015/16. Durban, South Africa: Health Systems Trust; 2016. [Google Scholar]
- 22. District Health Barometer 2010/2011 Health Systems Trust; 2011. http://www.hst.org.za/sites/default/files/DHB_Datafile_19Dec2011.xlsx. Accessed 1 February 2013.
- 23. Kompala T, Moll AP, Mtungwa N et al. . Impact of nurse-delivered community-based CD4 services on facilitating pre-ART care in rural South Africa. BMC Health Serv Res. 2016;16:374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Upadhya D, Moll AP, Brooks RP et al. . What motivates use of community-based human immunodeficiency virus testing in rural South Africa? Int J STD AIDS. 2016;27:662–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shah NS, Moodley P, Babaria P et al. . Rapid diagnosis of tuberculosis and multidrug resistance by the microscopic-observation drug-susceptibility assay. Am J Respir Crit Care Med. 2011;183:1427–33. [DOI] [PubMed] [Google Scholar]
- 26. Cain KP, McCarthy KD, Heilig CM et al. . An algorithm for tuberculosis screening and diagnosis in people with HIV. N Engl J Med. 2010;362:707–16. [DOI] [PubMed] [Google Scholar]
- 27. Republic of South Africa Department of Health. South African National Antiretroviral Treatment Guidelines. Pretoria: National Department of Health, 2004. [Google Scholar]
- 28. O’Donnell MR, Padayatchi N, Master I et al. . Improved early results for patients with extensively drug-resistant tuberculosis and HIV in South Africa. Int J Tuberc Lung Dis. 2009;13:855–61. [PMC free article] [PubMed] [Google Scholar]
- 29. Pietersen E, Ignatius E, Streicher EM et al. . Long-term outcomes of patients with extensively drug-resistant tuberculosis in South Africa: a cohort study. Lancet. 2014;383:1230–9. [DOI] [PubMed] [Google Scholar]
- 30. Gilbert JA, Long EF, Brooks RP et al. . Integrating community-based interventions to reverse the convergent TB/HIV epidemics in Rural South Africa. PLoS One. 2015;10:e0126267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. World Health Organization. TB Burden Estimates. Available at:http://www.who.int/tb/country/data/download/en/. Accessed 24 September 2014. [Google Scholar]
- 32. Altice FL, Azbel L, Stone J et al. . The perfect storm: incarceration and the high-risk environment perpetuating transmission of HIV, hepatitis C virus, and tuberculosis in Eastern Europe and Central Asia. Lancet. 2016;388:1228–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dolan K, Wirtz AL, Moazen B et al. . Global burden of HIV, viral hepatitis, and tuberculosis in prisoners and detainees. Lancet. 2016;388:1089–102. [DOI] [PubMed] [Google Scholar]
- 34. Sterling TR, Pham PA, Chaisson RE. HIV infection-related tuberculosis: clinical manifestations and treatment. Clin Infect Dis. 2010;50(suppl 3):S223–30. [DOI] [PubMed] [Google Scholar]
- 35. Getahun H, Kittikraisak W, Heilig CM et al. . Development of a standardized screening rule for tuberculosis in people living with HIV in resource-constrained settings: individual participant data meta-analysis of observational studies. PLoS Med. 2011;8:e1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Basu S, Friedland G, Medlock J et al. . Averting epidemics of extensively drug-resistant tuberculosis. Proc Natl Acad Sci U S A. 2009;106:7672–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shah NS, Auld SC, Brust JC et al. . Transmission of extensively drug-resistant tuberculosis in South Africa. N Engl J Med. 2017;376:243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gilbert JA, Shenoi SV, Moll AP et al. . Cost-effectiveness of community-based TB/HIV screening and linkage to care in rural South Africa. PLoS One. 2016;11:e0165614. [DOI] [PMC free article] [PubMed] [Google Scholar]