Abstract
Context
Inflammatory signaling may play an important role in the pathogenesis of pulmonary arterial hypertension (PAH).
Objective
To assess the incidence of PAH in patients with mild and severe psoriasis compared with their respective controls.
Design
From January 2004 to November 2012, we performed a retrospective cohort study of patients with psoriasis in the Kaiser Permanente Southern California Health Plan. Patients with an International Classification of Diseases, Ninth Revision Clinical Modification diagnostic code for psoriasis (696.1) or psoriatic arthritis (696.0) without a prior diagnosis of primary PAH (416.0) or secondary PAH (416.8) were eligible for inclusion. Patients who had never received a diagnosis of psoriasis were frequency-matched by age, sex, and race to form the control cohorts.
Main Outcome Measures
Incidence of PAH in patients with psoriasis compared with matched controls.
Results
There were 10,115 patients with mild psoriasis, 3821 with severe psoriasis, and 69,360 matched controls. On multivariable analysis, there was a significantly increased risk of PAH developing in the severe psoriasis cohort vs their controls (hazard ratio = 1.46, 95% confidence interval = 1.09–1.94).
Conclusion
The systemic inflammatory process underlying psoriasis may be a cause for an increased risk of PAH, but there are numerous secondary causes of PAH, some of which were not accounted for in our study. Further prospective, randomized controlled trials are necessary to establish psoriasis as a risk factor for PAH.
INTRODUCTION
Psoriasis is a chronic, immune-mediated, inflammatory skin disorder affecting 1% to 3% of the population.1 As a multisystem disease, psoriasis is also known to cause an inflammatory arthritis in 6% to 42% of patients.2 Other comorbidities known to be associated with psoriasis include obesity, metabolic syndrome, Type 2 diabetes mellitus, and coronary artery disease.1 In fact, an elevated risk of myocardial infarction in patients with psoriasis has been repeatedly documented.3–5
Elucidating cardiovascular disease in psoriasis patients has therefore been an area of active research. Gunes et al6 performed transthoracic echocardiography (TTE) in 47 patients with psoriasis and 20 healthy controls to evaluate heart disease in psoriasis. They found that mild pulmonary hypertension was significantly higher among patients with psoriasis (31.9% vs 0%, p = 0.003). Causes of primary pulmonary arterial hypertension (PAH) include idiopathic, familial, associated with other disorders, associated with substantial venous or capillary involvement, and persistent pulmonary hypertension of the newborn. Secondary causes of PAH include left-sided heart disease, lung respiratory disease, and chronic thrombotic or embolic disease.7 We performed a retrospective cohort study of Kaiser Permanente (KP) Southern California (KPSC) members to assess the incidence of PAH in patients with psoriasis.
METHODS
Study Design
From January 2004 to November 2012, a retrospective cohort study was performed from members of the KPSC Health Plan. Four cohorts were evaluated, including mild psoriasis (no phototherapy, oral therapy, or biologic therapy), control for mild psoriasis, severe psoriasis (any use of phototherapy, oral therapy, or biologic therapy), and control for severe psoriasis. The diagnosis of PAH by International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic code during the study period was the primary outcome under consideration. The institutional review board of KPSC approved the study protocol.
Study Population and Data Source
Patients were recruited from KPSC, a large integrated health maintenance organization with approximately 3.6 million members as of November 2012. The demographics of KPSC members are representative of the Southern California population. The data source used was KP HealthConnect, the electronic medical record database of KPSC hospitals, clinics, and pharmacies. Members of KPSC receive nearly all their covered health care at KPSC facilities except for emergency medical care at non-Health Plan facilities. More than 92% of members have prescription drug benefits and use KPSC pharmacies for medications.
Inclusion/Exclusion Criteria
Inclusion criteria for patients were an ICD-9-CM diagnostic code for psoriasis (696.1) or psoriatic arthritis (696.0), received on 3 different dates from January 2004 to June 2012. The third psoriasis diagnosis date was considered the index date. Patients must have been enrolled in the KPSC Health Plan for at least 1 year before the index date and must have had at least 1 medical encounter per year. The severe psoriasis cohort included patients who had received the following therapies during the study period: methotrexate, cyclosporine, acitretin, etanercept, adalimumab, infliximab, ustekinumab, efalizumab, alefacept, psoralen and ultraviolet A phototherapy, or ultraviolet B phototherapy. The mild psoriasis cohort was composed of patients who had not received any of these therapies.
Any patient with a prior diagnosis of primary PAH (ICD-9 CM Code 416.0) or secondary PAH (ICD-9-CM Code 416.8) was excluded from the study. For the control cohorts, patients who had never received a diagnosis of psoriasis were frequency-matched by age, sex, and race to each patient in the mild and severe psoriasis cohorts, thus creating 2 control groups.
Outcomes and Follow-up
The study outcome was the new-onset diagnosis of primary or secondary PAH (416.0 or 416.8). An independent chart review was performed for all 221 patients with psoriasis who had a diagnosis of PAH to verify the presence of pulmonary hypertension on TTE. Follow-up continued until the last visit before the end of the study in November 2012 unless any of the following prior events occurred: 1) diagnosis of PAH, 2) death during the study period, or 3) disenrollment from KPSC.
Confounding Variables
Within 12 months before the index date, we collected information on patient age, sex, obesity, smoking history, and comorbidities that may be potential risk factors for PAH, including hypertension (401.9), Type 2 diabetes mellitus (250.x1, 250.x3), and dyslipidemia (272.4). We also analyzed the use of antihypertensive medications, lipid-lowering drugs, statins, β-blockers, and diabetic medications as potential confounders in our study.
Statistical Analysis
All statistical analyses were performed using SAS Enterprise Guide Version 4.3 (SAS Institute Inc, Cary, NC); all p values are 2-sided, and p < 0.05 was considered statistically significant. Percentages and continuous variables were summarized as mean and standard deviation, and categorical variables were summarized as counts. Associations between psoriasis and various covariates were tested using the χ2 test for categorical variables and the t-test for continuous variables. All statistical tests were 2-tailed.
The incidence of PAH in the mild and severe psoriasis cohorts was compared with the incidence of PAH in the respective control group using an unadjusted Cox proportional hazards model. The incidence rates were then adjusted for age, sex, diabetes, dyslipidemia, hypertension, diabetes therapy, statin therapy, hypertension therapy, and β-blocker therapy. Obesity and smoking were not included in the model because they were insufficiently recorded. Each dichotomous variable in the model was checked for proportionality while adjusting for the other covariates in the model by examining diagnostic log-log survival plots.
RESULTS
Baseline Characteristics
There were 10,115 patients in the mild psoriasis cohort and 50,309 patients in the matched control group. The average age of the mild psoriasis cohort was 57.1 years compared with the average age of 57.6 years in the control cohort. The men-to-women proportion (47.2:52.8) was also equivalently matched. Ethnicity was comparable, except for a notably higher proportion of black patients in the control group (11.6% vs 4.8%). Compared with controls, the mild psoriasis cohort had a higher prevalence of hypertension (53.2% vs 49.3%), dyslipidemia (52.2% vs 48.6%), and diabetes (20.5% vs 17.6%), as well as use of the medications used to manage these comorbidities (Table 1).
Table 1.
Baseline characteristics of cohortsa
| Characteristic | Mild | Severe | ||||
|---|---|---|---|---|---|---|
| Controls (N = 50,309) | Psoriasis (N = 10,115) | Mild psoriasis vs control, p value | Controls (N = 19,051) | Psoriasis (N = 3821) | Severe psoriasis vs control, p value | |
| Age at diagnosis, years | ||||||
| Mean (SD) | 57.6 (14.84) | 57.1 (14.86) | 0.011 | 52.9 (13.67) | 52.4 (13.68) | 0.058 |
| Median (IQR) | 58 (48–68) | 58 (47–68) | 54 (44–62) | 53 (44–62) | ||
| Age category, years | ||||||
| < 45 | 9552 (19.0) | 1987 (19.6) | 0.17 | 4905 (25.7) | 1023 (26.8) | 0.462 |
| 45–54 | 10,378 (20.6) | 2133 (21.1) | 5186 (27.2) | 1048 (27.4) | ||
| 55–64 | 13,527 (26.9) | 2700 (26.7) | 5150 (27) | 1016 (26.6) | ||
| ≥ 65 | 16,852 (33.5) | 3295 (32.6) | 3810 (20) | 734 (19.2) | ||
| Sexb | ||||||
| Women | 26,559 (52.8) | 5343 (52.8) | 9441 (49.6) | 1894 (49.6) | ||
| Men | 23,750 (47.2) | 4772 (47.2) | 9610 (50.4) | 1927 (50.4) | ||
| Ethnicity | ||||||
| Missing | 1912 (3.8) | 362 (3.6) | < 0.001 | 763 (4.0) | 129 (3.4) | < 0.001 |
| White | 23,369 (46.5) | 5829 (57.6) | 8568 (45.0) | 2025 (54.8) | ||
| Black | 5606 (11.6) | 467 (4.6) | 2196 (12) | 206 (5.6) | ||
| Hispanic | 13,516 (27.9) | 2348 (23.2) | 5326 (29.1) | 906 (24.5) | ||
| Asian/Pacific Islander | 4858 (10) | 911 (9.0) | 1783 (9.7) | 489 (13.2) | ||
| Other | 1048 (2.2) | 198 (2.0) | 415 (2.3) | 66 (1.8) | ||
| Hypertension | ||||||
| No | 25,495 (50.7) | 4729 (46.8) | < 0.001 | 11,467 (60.2) | 1976 (51.7) | < 0.001 |
| Yes | 24,814 (49.3) | 5386 (53.2) | 7584 (39.8) | 1845 (48.3) | ||
| Dyslipidemia | ||||||
| No | 25,858 (51.4) | 4833 (47.8) | < 0.001 | 11,875 (62.3) | 2322 (60.8) | 0.069 |
| Yes | 24,451 (48.6) | 5282 (52.2) | 7176 (37.7) | 1499 (39.2) | ||
| Diabetes | ||||||
| No | 41,465 (82.4) | 8043 (79.5) | < 0.001 | 16,335 (85.7) | 3129 (81.9) | < 0.001 |
| Yes | 8844 (17.6) | 2072 (20.5) | 2716 (14.3) | 692 (18.1) | ||
| Lipid medication | ||||||
| No | 31,015 (61.6) | 5945 (58.8) | < 0.001 | 13,550 (71.1) | 2612 (68.4) | < 0.001 |
| Yes | 19,294 (38.4) | 4170 (41.2) | 5501 (28.9) | 1209 (31.6) | ||
| Hypertension medication | ||||||
| No | 22,889 (45.5) | 4228 (41.8) | < 0.001 | 10,402 (54.6) | 1809 (47.3) | < 0.001 |
| Yes | 27,420 (54.5) | 5887 (58.2) | 8649 (45.4) | 2012 (52.7) | ||
| Statin medication | ||||||
| No | 31,059 (61.7) | 5951 (58.8) | < 0.001 | 13,557 (71.2) | 2618 (68.5) | < 0.001 |
| Yes | 19,250 (38.3) | 4164 (41.2) | 5494 (28.8) | 1203 (31.5) | ||
| Beta-blocker medication | ||||||
| No | 35,669 (70.9) | 6836 (67.6) | < 0.001 | 14,769 (76.9) | 2755 (71.7) | < 0.001 |
| Yes | 14,640 (29.1) | 3279 (32.4) | 4436 (23.1) | 1086 (28.3) | ||
| Diabetes medication | ||||||
| No | 42,759 (85) | 8315 (82.2) | < 0.001 | 16,682 (87.6) | 3184 (83.3) | 0.0001 |
| Yes | 7550 (15) | 1800 (17.8) | 2369 (12.4) | 637 (16.7) | ||
Data are number (percentage) unless otherwise indicated.
Study groups were matched by sex, so it is not appropriate to calculate a p value for the comparison.
IQR = interquartile range; SD = standard deviation.
In the severe psoriasis cohort, 3821 patients were matched to 19,051 control patients. The average age of the severe psoriasis cohort was 52.4 years compared with an average age of 52.9 among controls. Sex was matched appropriately as well (men:women, 50.4:49.6). There was a higher proportion of black (12% vs 5.6%) and Hispanic (29.1% vs 24.5%) patients in the control group. Similar to the mild psoriasis cohort, the severe psoriasis cohort had a higher prevalence of hypertension (48.3% vs 39.8%), dyslipidemia (39.2% vs 37.7%), diabetes (18.1% vs 14.3%), and concomitant medication use compared with controls (see Table 1).
Incidence and Risk of Pulmonary Arterial Hypertension
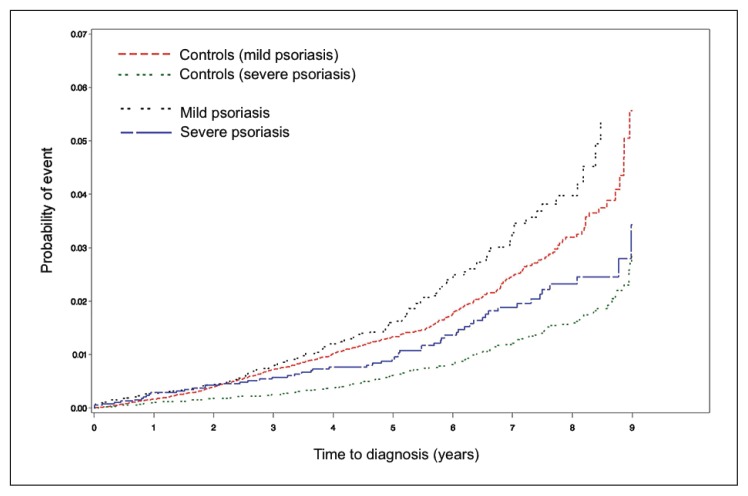
There were 221 patients with mild and severe psoriasis with a new-onset diagnosis of primary or secondary PAH. All these patients demonstrated elevated pulmonary arterial pressures on TTE; 31 patients also received right-sided heart catheterization. Only 6 of the 221 patients with psoriasis received a diagnosis of primary PAH (416.0), now known as idiopathic PAH. The incidence per 1000 person-years of PAH in the mild psoriasis cohort vs control was 4.4 and 3.5; the incidence in the severe psoriasis cohort vs control was 3.1 and 2.0 (Table 2). Kaplan-Meier cumulative incidence plots demonstrated an increased probability of PAH in the mild psoriasis cohort vs control as well as the severe psoriasis cohort vs control (Figure 1). On multivariable analysis, patients with severe psoriasis were at increased risk of PAH compared with their respective controls (hazard ratio [HR] = 1.46, 95% confidence interval [CI] = 1.09–1.94). Patients with mild psoriasis were not at significantly increased risk of PAH (p = 0.058).
Table 2.
Incidence rates of cohorts
| Characteristic | Mild | Severe | ||
|---|---|---|---|---|
| Controls (N = 50,309) | Psoriasis (N = 10,115) | Controls (N = 19,051) | Psoriasis (N = 3821) | |
| No. of person years | 179,355 | 36,005 | 96,386 | 19,369 |
| New PAH cases, no. (%) | 626 (1.2) | 159 (1.6) | 191 (1.0) | 61 (1.6) |
| Incidence per 1000 person-years | 3.5 | 4.4 | 2.0 | 3.1 |
| Follow-up time, years | ||||
| Mean (SD) | 3.6 (2.00) | 3.6 (2.00) | 5.0 (2.2) | 5.1 (2.21) |
| Median | 3.5 | 3.5 | 5.3 | 5.3 |
| Q1–Q3a | 2.0–4.9 | 2.0–4.9 | 3.4–6.9 | 3.5–7.0 |
Q2 = median.
PAH = pulmonary arterial hypertension; Q = quartile points; SD = standard deviation.
Figure 1.
Kaplan-Meier curve for cumulative incidence of pulmonary arterial hypertension (PAH).
Cumulative incidence of PAH developing is greater in patients with mild psoriasis vs their controls as well as patients with severe psoriasis vs their controls.
In our analysis of hypertension, dyslipidemia, and Type 2 diabetes mellitus, hypertension was the only comorbidity associated with a significantly increased risk of PAH (HR 1.32, 95% CI = 1.06–1.64). Other factors associated with an increased risk included older age (≥ 65 years vs < 45 years: HR = 8.27, 95% CI = 5.48–12.47), hypertension medication (yes vs no: HR = 1.39, 95% CI = 1.07–1.81), β-blocker medication (yes vs no: HR = 1.99, 95% CI = 1.72–2.31), and diabetes medication (yes vs no: HR = 1.81, 95% CI = 1.43–2.29; Table 3). Obesity and smoking were not included in our multivariable analysis because they were insufficiently recorded.
Table 3.
Univariate and multivariable Cox proportional hazard regression models of risk of pulmonary arterial hypertension in patients with mild and severe psoriasis compared with controls
| Characteristic | Hazard ratio (95% confidence interval) | p value |
|---|---|---|
| Psoriasis (unadjusted) | ||
| Severe psoriasis vs control | 1.55 (1.16–2.06) | 0.0031 |
| Mild psoriasis vs control | 1.25 (1.05–1.49) | 0.0109 |
| Severe psoriasis vs mild psoriasis | 0.59 (0.43–0.79) | 0.0004 |
| Psoriasis (adjusted) | ||
| Severe psoriasis vs control | 1.46 (1.09–1.94) | 0.0106 |
| Mild psoriasis vs control | 1.18 (0.99–1.41) | 0.0580 |
| Severe psoriasis vs mild psoriasis | 0.83 (0.62–1.12) | 0.2280 |
| Age category, years | ||
| 45–54 vs < 45 | 1.68 (1.07–2.66) | 0.0251 |
| 55–64 vs < 45 | 3.49 (2.30–5.31) | < 0.001 |
| ≥ 65 vs < 45 | 8.27 (5.48–12.47) | < 0.001 |
| Other | ||
| Men vs Women | 0.88 (0.78–1.00) | 0.0457 |
| Hypertension (yes vs no) | 1.32 (1.06–1.64) | 0.0142 |
| Dyslipidemia (yes vs no) | 1.02 (0.86–1.21) | 0.8473 |
| Diabetes (yes vs no) | 1.08 (0.85–1.36) | 0.5270 |
| Lipid medication (yes vs no) | 2.25 (0.56–9.11) | 0.2540 |
| Hypertension medication (yes vs no) | 1.39 (1.07–1.81) | 0.0127 |
| Statin (yes vs no) | 0.47 (0.12–1.89) | 0.2874 |
| Beta-blocker (yes vs no) | 1.99 (1.72–2.31) | < .0001 |
| Diabetes medication (yes vs no) | 1.81 (1.43–2.29) | < .0001 |
DISCUSSION
Through this retrospective cohort study, we found that patients with severe psoriasis are at increased risk of development of PAH. This elevated risk was not seen in patients with mild psoriasis. Of the comorbidities we analyzed, we found that hypertension was independently associated with a higher risk of PAH as well. Our results substantiate the findings by Gunes et al6 of a higher prevalence of PAH in patients with psoriasis, and to our knowledge provide the first study to demonstrate an elevated risk of PAH in patients with psoriasis.
The pathogenesis of PAH has been shown to involve endothelial damage and remodeling of the pulmonary arterial vasculature. Dysfunction of voltage-gated potassium (K+) channels in pulmonary arterial smooth-muscle cells (PASMCs) triggers vasoconstriction and PASMC proliferation, causing vascular medial hypertrophy. Endothelial damage is also present in PAH, which reduces the production of vasodilator substances and predisposes to in situ thrombosis.8 Additionally, recent studies are discovering a nuclear factor-κB (NF-κB) inflammatory signaling pathway in PAH.9 All of these elements combine to cause increased pulmonary vascular resistance, pulmonary hypertension, and ultimately, progressive right heart failure.8
Broadly related to the pathogenesis of PAH, the systemic inflammatory process underlying psoriasis has been known to cause endothelial dysfunction,6,10 and platelet activation in psoriasis has been show to promote a prothrombotic state.6,11 Likewise, NF-κB has also been shown to be an important inflammatory mediator in the pathogenesis of psoriasis.12,13 However, as NF-κB is important in numerous chronic inflammatory diseases such as rheumatoid arthritis, asthma, and inflammatory bowel disease, it is not specific to psoriasis.14
Another link between psoriasis and PAH is the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). Inflammatory dendritic cells in psoriasis have been shown to express TRAIL, which activates keratinocytes to express CCL20, an important chemokine in the pathogenesis of psoriasis.15 Serum levels of TRAIL are also significantly higher in patients with psoriatic arthritis.16 In PAH, a recent study demonstrated that TRAIL is highly expressed in the PASMCs of patients with PAH, and blockade or genetic deletion of TRAIL in rodent models prevented the development of PAH.17
We acknowledge limitations to our study. The grouping of psoriasis severity based on treatment may have misclassified some patients. However, dermatologists in community practice do not commonly use standardized measurements such as body surface area or the Psoriasis Area and Severity Index. Furthermore, a difference between psoriasis with and without psoriatic arthritis could not be reliably made using ICD-9-CM coding. Although the gold standard for diagnosing PAH is through right-sided cardiac catheterization, most PAH cases were diagnosed with TTE alone. Yet, it is recognized that TTE is the most useful initial screening modality for PAH.18
Also, we did not include all possible confounders in our multivariable analysis, including obesity and smoking (both known to be associated with psoriasis), as well as other diseases associated with PAH, such as chronic obstructive pulmonary disease, connective tissue diseases, human immunodeficiency virus infection, portal hypertension, congenital heart diseases, schistosomiasis, and chronic hemolytic anemia.19 Furthermore, our analysis did not account for the potential difference in the number of medical encounters per year between patients with psoriasis and their controls. It is possible that increased number of medical visits in patients with psoriasis increased their likelihood of undergoing studies such as TTE, which would not have been used in a comparable control patient with fewer medical encounters. Given the limitations of a retrospective analysis and the absence of controlled variables, it is possible that our results could be attributed to one or more of these other factors.
Another important consideration is the cardiovascular association with psoriasis. A greater proportion of cardiology patients undergoing TTE for various indications may have psoriasis as well. This raises the possibility that an increased number of patients with psoriasis with asymptomatic PAH received a diagnosis, increasing the incidence of PAH despite a lack of clinical significance. Moreover, the control groups in our study had a higher prevalence of black and Hispanic patients, who at the population level are known to have decreased access to health care.20 This may have further biased our study toward finding an elevated incidence of PAH in the study groups.
CONCLUSION
We performed a retrospective cohort study from the KPSC Health Plan, which possesses a large and stable membership, accurate diagnosis and documentation, substantial longitudinal follow-up, and comprehensive health care coverage. We believe that the inflammatory-mediated pathway underlying psoriasis and PAH may explain the association between these 2 diseases. In a similar study in the KP Northern California Health Plan,21 99 patients with a confirmed diagnosis of psoriatic arthritis were compared with matched control subjects and were found not to have a significant increased risk of atherothrombotic disease but did have an increased prevalence of systemic hypertension and heart failure. It is important to note that these retrospective studies establish possible associations, but further studies, including long-term prospective trials and/or registries, are necessary to establish psoriasis as a risk factor for PAH.
The Prince in a Kingdom
The heart, like the prince in a kingdom, in whose hands lie the chief and highest authority, rules over all, it is the … foundation from which all power is derived, on which all power depends in the animal body.
— William Harvey, 1578–1657, English physician, first known physician to describe the systemic circulation and properties of blood being pumped to the brain and body by the heart
Acknowledgments
We would like to acknowledge Judith D Bebchuk, ScD, with the Department of Research and Evaluation, Kaiser Permanente Southern California, for performing the statistical analyses.
Kathleen Louden, ELS, of Louden Health Communications provided editorial assistance.
Footnotes
Disclosure Statement
Dr Wu is an Investigator for AbbVie, North Chicago, IL; Amgen, Thousand Oaks, CA; Eli Lilly and Co, Indianapolis, IN; Janssen Pharmaceuticals, Titusville, NJ; Novartis Corp, Basel, Switzerland; and Regeneron, Tarrytown, NY. The author(s) have no other conflicts of interest to disclose.
References
- 1.Al-Mutairi N, Al-Farag S, Al-Mutairi A, Al-Shiltawy M. Comorbidities associated with psoriasis: An experiencee from the Middle East. J Dermatol. 2010 Feb;37(2):146–55. doi: 10.1111/j.1346-8138.2009.00777.x. DOI: https://doi.org/10.1111/j.1346-8138.2009.00777.x. [DOI] [PubMed] [Google Scholar]
- 2.Gladman DD, Antoni C, Mease P, Clegg DO, Nash P. Psoriatic arthritis: Epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005 Mar;64(Suppl 2):ii14–7. doi: 10.1136/ard.2004.032482. DOI: https://doi.org/10.1136/ard.2004.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006 Oct 11;296(14):1735–41. doi: 10.1001/jama.296.14.1735. DOI: https://doi.org/10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 4.Lin HW, Wang KH, Lin HC, Lin HC. Increased risk of acute myocardial infarction in patients with psoriasis: A 5-year population-based study in Taiwan. J Am Acad Dermatol. 2011 Mar;64(3):495–501. doi: 10.1016/j.jaad.2010.01.050. DOI: https://doi.org/10.1016/j.jaad.2010.01.050. [DOI] [PubMed] [Google Scholar]
- 5.Xiao J, Chen LH, Tu YT, Deng XH, Tao J. Prevalence of myocardial infarction in patients with psoriasis in central China. J Eur Acad Dermatol Venereol. 2009 Nov;23(11):1311–5. doi: 10.1111/j.1468-3083.2009.03318.x. DOI: https://doi.org/10.1111/j.1468-3083.2009.03318.x. [DOI] [PubMed] [Google Scholar]
- 6.Gunes Y, Tuncer M, Calka O, et al. Increased frequency of pulmonary hypertension in psoriasis patients. Arch Dermatol Res. 2008 Sep;300(8):435–40. doi: 10.1007/s00403-008-0859-9. DOI: https://doi.org/10.1007/s00403-008-0859-9. [DOI] [PubMed] [Google Scholar]
- 7.Galiè N, Torbicki A, Barst R, et al. Task Force. Guidelines on diagnosis and treatment of pulmonary arterial hypertension. The Task Force on Diagnosis and Treatment of Pulmonary Arterial Hypertension of the European Society of Cardiology. Eur Heart J. 2004 Dec;25(24):2243–78. doi: 10.1016/j.ehj.2004.09.014. DOI: https://doi.org/10.1016/j.ehj.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Firth AL, Mandel J, Yuan JX. Idiopathic pulmonary arterial hypertension. Dis Model Mech. 2010 May-Jun;3(5–6):268–73. doi: 10.1242/dmm.003616. DOI: https://doi.org/10.1242/dmm.003616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hosokawa S, Haraguchi G, Sasaki A, et al. Pathophysiological roles of nuclear factor kappaB (NF-kB) in pulmonary arterial hypertension: Effects of synthetic selective NF-kB inhibitor IMD-0354. Cardiovasc Res. 2013 Jul 1;99(1):35–43. doi: 10.1093/cvr/cvt105. DOI: https://doi.org/10.1093/cvr/cvt105. [DOI] [PubMed] [Google Scholar]
- 10.Kremers HM, McEvoy MT, Dann FJ, Gabriel SE. Heart disease in psoriasis. J Am Acad Dermatol. 2007 Aug;57(2):347–54. doi: 10.1016/j.jaad.2007.02.007. DOI: https://doi.org/10.1016/j.jaad.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Marongiu F, Sorano GG, Bibbò C, et al. Abnormalities of blood coagulation and fibrinolysis in psoriasis. Dermatology. 1994;189(1):32–7. doi: 10.1159/000246755. DOI: https://doi.org/10.1159/000246755. [DOI] [PubMed] [Google Scholar]
- 12.Goldminz AM, Au SC, Kim N, Gottlieb AB, Lizzul PF. NF-κB: An essential transcription factor in psoriasis. J Dermatol Sci. 2013 Feb;69(2):89–94. doi: 10.1016/j.jdermsci.2012.11.002. DOI: https://doi.org/10.1016/j.jdermsci.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Moorchung N, Kulaar JS, Chatterjee M, Vasudevan B, Tripathi T, Dutta V. Role of NF-κB in the pathogenesis of psoriasis elucidated by its staining in skin biopsy specimens. Int J Dermatol. 2014 May;53(5):570–4. doi: 10.1111/ijd.12050. DOI: https://doi.org/10.1111/ijd.12050. [DOI] [PubMed] [Google Scholar]
- 14.Barnes PJ, Karin M. Nuclear factor-κB: A pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997 Apr 10;336(15):1066–71. doi: 10.1056/NEJM199704103361506. DOI: https://doi.org/10.1056/nejm199704103361506. [DOI] [PubMed] [Google Scholar]
- 15.Zaba LC, Fuentes-Duculan J, Eungdamrong NJ, et al. Identification of TNF-related apoptosis-inducing ligand and other molecules that distinguish inflammatory from resident dendritic cells in patients with psoriasis. J Allergy Clin Immunol. 2010 Jun;125(6):1261–1268.e9. doi: 10.1016/j.jaci.2010.03.018. DOI: https://doi.org/10.1016/j.jaci.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofbauer LC, Schoppet M, Christ M, Teichmann J, Lange U. Tumour necrosis factor-related apoptosis-inducing ligand and osteoprotegerin serum levels in psoriatic arthritis. Rheumatology (Oxford) 2006 Oct;45(10):1218–22. doi: 10.1093/rheumatology/kel108. DOI: https://doi.org/10.1093/rheumatology/kel108. [DOI] [PubMed] [Google Scholar]
- 17.Hameed AG, Arnold ND, Chamberlain J, et al. Inhibition of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) reverses experimental pulmonary hypertension. J Exp Med. 2010 Oct 22;209(11):1919–35. doi: 10.1084/jem.20112716. DOI: https://doi.org/10.1084/jem.20112716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nauser TD, Stites SW. Diagnosis and treatment of pulmonary hypertension. Am Fam Physician. 2001 May 1;63(9):1789–98. [PubMed] [Google Scholar]
- 19.Souza R, Jardim C, Humbert M. Idiopathic pulmonary arterial hypertension. Semin Respir Crit Care Med. 2013 Oct;34(5):560–7. doi: 10.1055/s-0033-1355439. DOI: https://doi.org/10.1055/s-0033-1355439. [DOI] [PubMed] [Google Scholar]
- 20.Egede LE. Race, ethnicity, culture, and disparities in health care. J Gen Intern Med. 2006 Jun;21(6):667–9. doi: 10.1111/j.1525-1497.2006.0512.x. DOI: https://doi.org/10.1111/j.1525-1497.2006.0512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kondratiouk S, Udaltsova N, Klatsky AL. Associations of psoriatic arthritis and cardiovascular conditions in a large population. Perm J. 2008 Fall;12(4):4–8. doi: 10.7812/tpp/07-141. DOI: https://doi.org/10.7812/tpp/07-141. [DOI] [PMC free article] [PubMed] [Google Scholar]