Abstract
Plant expression systems have been developed to produce anti-cancer vaccines. Plants have several advantages as bioreactors for the production of subunit vaccines: they are considered safe, and may be used to produce recombinant proteins at low production cost. However, several technical issues hinder large-scale production of anti-cancer vaccines in plants. The present review covers design strategies to enhance the immunogenicity and therapeutic potency of anti-cancer vaccines, methods to increase vaccine-expressing plant biomass, and challenges facing the production of anti-cancer vaccines in plants. Specifically, the issues such as low expression levels and plant-specific glycosylation are described, along with their potential solutions.
Keywords: Cancer, Vaccine, Glycosylation, Plant expression system, Subunit vaccine, Recombinant protein
INTRODUCTION
Advances in molecular biology and immunology have led to the successful development of recombinant subunit vaccines, which effectively use antigenic epitopes and proteins to prevent infectious diseases, unlike conventional vaccines made of killed or attenuated pathogens (Rappuoli, 2007). Vaccines that can express and process recombinant proteins similar to those of the naïve pathogenic organisms have been produced in bacterial, insect, mammalian, and yeast cells (Monsurrò et al., 2002; Radford et al., 2002; Fazlalipour et al., 2015; Qiao et al., 2015). However, the practical value of these expression systems is limited, as they require a large volume of media for biomass production and require purification steps, which can increase the overall production cost. Plant expression systems for vaccine production, in which plants are used as bioreactors, may be a promising alternative that can produce vaccines at low cost. Production in plants also prevents the risk of pathogen contamination associated with the use of conventional or recombinant subunit vaccines produced from mammalian systems (Ma et al., 2005; Park et al., 2015; Kim et al., 2016).
The first plant-derived vaccine was produced in tobacco plants transformed with the Streptococcus mutans surface protein antigen A in 1990 (Curtiss and Cardineau, 1997). Since then, antigenic proteins of viruses, bacteria, and enteric and non-enteric pathogens, as well as tumor-associated antigens, have been produced in a wide range of plant species using stable or transient expression systems (Kurokawa et al., 2013; Lee et al., 2013; Lim et al., 2015). Immunization with plant-derived antigenic proteins such as vaccines may someday prevent millions of people from getting infectious diseases. Furthermore, the idea of vaccination against cancer has led to a potentially novel strategy to produce tumor-associated antigens (TAAs) in plant systems (Lu et al., 2012). Many anti-cancer vaccines expressed in plants have been studied and even approved for clinical trials (Tacket, 2009).
However, for the large-scale production of anti-cancer vaccines in plants to be viable, it is necessary to understand the advantages and disadvantages of these systems. The present review covers issues associated with the production of anti-cancer vaccines in plants.
IN VITRO AND IN VIVO PLANT PRODUCTION SYSTEMS
Vaccines can be produced using in vivo and in vitro plant systems. Whole plant expression platforms, including stable transgenic and transient plant systems, are in vivo systems. In these systems, environmental cultivation conditions such as temperature, light, water, and nutrients in the air and soil should be properly controlled, as they affect vaccine protein production levels and their functionality (Jamal et al., 2009). Although transgenic or transiently expressing plants are generally cultivated in containment greenhouses or chamber rooms, plants can be grown under variable microenvironment conditions that may enable pathogen infections, with consequences for plant biomass, transgene expression, and quality control of plant-derived vaccines (Jamal et al., 2009). Thus, several institutes, including the Center for Molecular Biotechnology (Fraunhofer USA, Newark, DE, USA) and the Biodesign Institute at Arizona State University (Tempe, AZ, USA), as well as companies, such as Kentucky BioProcessing (Owensboro, KY, USA) and Medicago (Quebec, Canada), use greenhouse complexes in which internal conditions are controlled and external pollutants and insects are excluded, consistent with Current Good Manufacturing Practice standards, as well as employing recombinant protein purification.
In in vitro systems, microenvironment conditions can be precisely controlled without the risk of pathogenic contamination. These systems culture plant cells and organs in aseptic conditions. In plant cell culture systems, dedifferentiated cells can be cultivated in callus form, using callus culture or initiating the single cells obtained from fragmentation of a callus into cell suspension culture. In general, cells divide more rapidly in cell suspension cultures in liquid media than in callus cultures on agar (Evans et al., 2003). Examples of the products of in vitro systems include many recombinant proteins that are vaccine candidates, such as the Hepatitis B surface antigen (Smith et al., 2002) in Glycine max and Nicotiana tabacum, human granulocyte-macrophage colony stimulating factor (hGM-CSF) in N. tabacum (Lee et al., 2004; Hong et al., 2006), viral protein 1 (VP1) epitope of foot-and-mouth disease virus (FMDV) in Nicotiana benthamiana (Zhang et al., 2010), recombinant glucocerebrosidase in Daucus carota (Shaaltiel et al., 2007), and human interleukin (IL)-2 and IL-4 in N. tabacum (Magnuson et al., 1998). Bright Yellow-2 (BY-2) and NT1 tobacco lines, which lack the ability to regenerate, are often used in plant cell suspension cultures (Mayo et al., 2006; Vasilev et al., 2013).
The affinity column purification process for recombinant therapeutic proteins expressed from tobacco leaves can remove alkaloids (Ko et al., 2004). In addition, low-alkaloid tobacco plant varieties can be used in the production of recombinant vaccine proteins (Rymerson et al., 2003). However, there are still safety concerns related to alkaloid contamination of tobacco cell-based oral vaccines. Edible oral vaccines based on rice seed thus offer a safe, simple, and cost-effective approach (Takaiwa, 2007; Kuo et al., 2013; Kurokawa et al., 2013). Furthermore, suspension rice cell cultures have other advantages, such as precisely controlled environments, faster increase in cell biomass, and lack of alkaloid contaminants in orally administered therapeutic proteins (Huang and McDonald, 2012; Kuo et al., 2013).
These suspension cultures can even secrete recombinant proteins out of the cell into the culture medium when the signal peptide is fused to the proteins, making the cell lysis step unnecessary for extraction (Liu et al., 2012). Carrot cells have been used for in vitro suspension-based production of diverse recombinant therapeutic proteins (Shaaltiel et al., 2007), building on the successful establishment of a carrot cell suspension culture in 1970 (Nishi and Sugano, 1970). The recombinant human enzyme glucocerebrosidase has been successfully produced in a carrot cell suspension culture (Shaaltiel et al., 2007). The carrot cell-derived glucocerebrosidase was developed by the Israeli biotechnology company, approved as a drug for humans, and licensed in the United States. The plant cell-derived is considered an alternative therapeutic option to production in the Chinese hamster ovary cell system to treat Gaucher’s disease.
Two types of plant organs, shoots and roots, can be cultivated in vitro (Paz-Maldonado and González-Ramírez, 2014). Hairy root culture systems have been established in many plant species. Hairy roots can be indefinitely propagated in liquid medium with stable morphologies; however, hairy root growth is somewhat slower than growth in shoot organ culture and suspension cell culture (Rigano and Walmsley, 2005). The hairy root culture system was originally applied to produce secondary phytometabolites, which are considered natural biopharmaceuticals and include antimicrobial flavonoids, Ginkgolide A, L-3,4-Dihydroxyphenylalanine (L-Dopa), and saponin in plants (Li et al., 1998; Hwang et al., 1999; Hussain et al., 2012). Agrobacterium rhizogenes-mediated transformation can generate transgenic hairy root expression systems, which can be applied to produce recombinant therapeutic proteins (Tepfer and Casse-Delbart, 1987; Sharp and Doran, 2001; Gaume et al., 2003; Medina-Bolivar et al., 2003). Therefore, the hairy root culture-based production system is more genetically stable than cell suspension culture (Rao and Ravishankar, 2002). The hairy root organ culture system is thus a reliable technology for use with A. rhizogenes-mediated transformation. This expression system should be used when media and containment environmental space are relatively cheap.
Although the cell suspension culture system has important advantages, there are still several challenges to be overcome, such as improvement of protein yield and production cost effectiveness and humanization of the glycosylation patterns of proteins. For recombinant protein yield, the factors of strong expression promoter, codon choice, and subcellular localization (Jamal et al., 2009; Lee et al., 2013) have been optimized. For glycosylation humanization, studies have explored the removal of glycosylation reactions for xylosyltransferase and fucosyltransferase by knock-out/knock-down approaches using RNAi technology (Loos and Steinkellner, 2014) and the Clustered regularly interspaced short palindromic repeats associated protein-9 nuclease (CRISPR/Cas9) system for plant genome editing, as well as introduction of human glycosylation enzymes such as sialic acid transferases and galactosyltransferase using transient and stable transformations (Strasser et al., 2007; Dicker et al., 2016).
PLANT EXPRESSION SYSTEMS FOR VACCINE PRODUCTION
Subunit vaccines can be produced by plant transformation methods using either stable transformation or transient expression. In the stable transformation system, vaccine genes are inserted into plant genomes for stable transgene expression, whereas in the transient expression system, genes are expressed in the cytoplasm without stable genomic insertion (i.e., the plant is not genetically modified). The expression of recombinant vaccine proteins has been achieved primarily using stable gene transformation. Agrobacterium-mediated transformation is the main tool to transfer the transfer DNA (T-DNA) region, which contains the gene expression cassette consisting of promoter, recombinant vaccine protein gene of interest, and terminator, into the plant genomic DNA. This technique has allowed the stable transformation of many plant species to express recombinant anti-cancer vaccine proteins (Brodzik et al., 2008; Lu et al., 2012; Lim et al., 2015; Kang et al., 2016; Kim et al., 2016).
Transgenic plants with stable gene expression enable rapid biomass increases and consequent production of recombinant vaccine proteins with low cost inputs (e.g., soil, water, sunlight, fertilizers). Stable gene insertion in plant genomes can transfer recombinant protein genetic traits to the next generation via the seed. Seed storage is a huge advantage of stable transgenic plant systems. Seeds with high protein content can stably accumulate seed storage proteins containing recombinant vaccine proteins in an intact form when the proteins are localized to the endoplasmic reticulum subcellular organelles in soybean (Maruyama et al., 2014). In addition, corn has been considered a promising transgenic plant species for production of recombinant therapeutic proteins because it produces large volumes of seeds that may be stored at low cost. Genetically stable plant lines can be used to generate seed banks for large-scale commercial production. Another advantage of stable transgenic plant expression systems is that changing plant biomass production levels is easily achieved by controlling the size of the seedling cultivation field. Furthermore, cross-fertilization between transgenic plants expressing two different recombinant proteins can generate a sibling plant to produce multiple genes, such as monoclonal antibodies containing heavy and light chains and multimeric complex proteins for enhancing immune responses (Jamal et al., 2012).
Chloroplast transformation is an attractive alternative in which the gene of interest is inserted not into the nuclear genomic DNA but into the chloroplast DNA. This DNA is maternally inherited and is not transmitted through pollen. Hence, in transplastomic plants obtained from chloroplast transformation, gene flow does not occur (Daniell et al., 1998; Ruf et al., 2001). Furthermore, transplastomic plants have no gene silencing or positional effects due to site-specific transgene integration. However, post-translational modification in chloroplasts differs from that in eukaryotes, in which glycosylation occurs; instead, it is more analogous to that in prokaryotes, which do not perform glycosylation. Therefore, chloroplast transformation is not an appropriate approach for the production of therapeutic proteins that require glycosylation for biological activities. In addition, although some plant species, such as tobacco, lettuce (Lactuca sativa), and cotton (Gossypium hirsutum), can be transformed through chloroplast transformation, few plant species are suitable for plastid transformation compared with the number that are suitable for genomic transformation (Meyers et al., 2010).
Stable transgenic plants have several limitations. First, it is time-consuming to establish transgenicity; in addition, these plants have low expression capability. They also have low production efficiency of recombinant proteins compared with transient expression plant systems and plastid transgenic plants.
There are several approaches to improve recombinant vaccine production efficiency in stable transgenic plants. Ideal plant species for stable transformation contain high levels of total soluble proteins (Song et al., 2015), grow easily and rapidly, and produce large amounts of seeds in a short period of time. Tobacco possesses these qualities and has been regarded as an ideal plant system for recombinant protein production (Song et al., 2015). If the roots are left intact, the tobacco plant can regrow from the stem after the upper portion of the plant has been harvested, making it unnecessary to plant new seedlings (Kim et al., 2016) and saving cultivation time. The entire biomass of this plant evenly expresses a recombinant colorectal cancer vaccine candidate, including the leaves and stem (Lim et al., 2015), and it grows rapidly to 1.2–1.8 m in height with 20–35 leaves (30–75 cm length and 25–45 cm width). In addition, tobacco plants express the systematically recombinant colorectal cancer vaccine protein gastrointestinal carcinoma antigen fused to the immunoglobulin fragment crystallizable region (GA733-Fc) in the whole plant, including the leaves and the stem, throughout the growth period until floral expression (Lim et al., 2015). To improve production efficiency, biomass harvesting time and harvesting of specific plant tissues should be optimized (Lim et al., 2015).
Transient expression can be achieved by agroinfiltration of Agrobacterium tumefaciens, carrying plant expression binary vectors or plant viral vectors into intercellular spaces instead of integrating them into the plant genome. This technique results in the transient production of recombinant vaccine proteins and is comparable to microbial or mammalian in vitro expression systems. Transient expression using Agrobacterium infiltration technology has mainly been applied to the production of vaccines and antibodies that are ready for commercialization. The best plant species for transient expression is N. benthamiana, which is susceptible to diverse plant viruses (Sheludko et al., 2007; Goodin et al., 2008; Conley et al., 2011) and is amenable to systemic infiltration by Agrobacterium.
Agroinfiltration is induced with both syringe and vacuum methods. The syringe infiltration method is simple and cost effective and does not require any special equipment. However, syringe infiltration must be conducted on individual leaves unless Agrobacterium or viral expression vectors are systematically spread to the whole plant. It is time consuming and inefficient when an industrial amount of plant biomass needs to be infiltrated. However, this method can be often utilized as a quick test tool to confirm transgene expression before stable transformation is carried out. In contrast, vacuum infiltration is more reliable for scaled-up production of recombinant proteins in plants.
In general, transient gene expression offers the advantages of higher protein accumulation levels and faster production processes over stable transgenic expression. Several transient expression approaches have therefore been developed for use in large-scale production. Two deconstructed viral vectors, one based on a tobacco mosaic virus RNA replicon system and the other derived from the bean yellow dwarf virus DNA replicon system (geminiviral vectors), have been established (Matzeit et al., 1991; Liu et al., 1998; Zhang and Mason, 2006; Goodin et al., 2008; Lico et al., 2008). In the vector system, the so-called proviral expression vectors carrying genes of interest do not harbor essential viral functions for replication of wild-type virus (Marillonnet et al., 2004). This deconstructed viral vector platform can express multimeric vaccine and antibody proteins within 2 weeks at 5 g of protein per kg of fresh plant biomass. It is a versatile technology that is suitable for the production of seasonal and pandemic flu vaccines, as well as of recombinant proteins for patient-specific immunotherapy, which must be generated quickly for application (Klimyuk et al., 2014). Current Good Manufacturing Process-compliant facilities for large-scale manufacturing of recombinant antigens and antibodies in N. benthamiana using a tobacco mosaic virus RNA replicon system technology are being constructed and run worldwide (Kentucky BioProcessing; iBio Inc., Newark, DE, USA; Bio-Manguinhos/Fiocruz, RJ, Brazil; Fraunhofer USA). Viral vector expression systems can avoid issues associated with genetically modified plants, as viral vector-derived recombinants can be obtained immediately after simple virus transfection. This system does not require entire transformation processes, such as transformation, in vitro regeneration, rooting induction, or plant rehabilitation as in in vivo culture. It requires only the cloning of the plant viral vector carrying the gene of interest and a plant host. However, compatibility between the virus and plant host is required, which limits applicable plant hosts.
EFFECT OF DEVELOPMENTAL AND ENVIRONMENTAL FACTORS ON VACCINE PROTEIN EXPRESSION
Plant growth conditions and harvest times, as well as tissue positions, affect protein expression levels and glycosylation structures of cancer vaccine proteins in plant (Gomord et al., 2005; Lim et al., 2015). When recombinant cancer vaccines are produced in plants, these diverse factors should be optimized for quantity and quality control.
To improve recombinant vaccine production efficiency in stable transgenic plants, it is important to choose ideal plant species for stable transformation and to control environmental factors that affect plant health conditions. Biomass harvesting time and specific plant tissue are other important factors that should be optimized to improve the efficiency of recombinant vaccine protein production. Plant biomass should be harvested before flowering to avoid transgene flow (Jensen et al., 2004; Wang and Ge, 2006; Spangenberg et al., 2012). Colorectal cancer vaccine protein levels in leaves and stems harvested after flower fertilization were lower than those in plant material harvested before the blossoming period. The highest level of expression of a colorectal cancer vaccine protein was observed in the 12 weeks after transplanting from the in vitro plant seedlings (Lim et al., 2015).
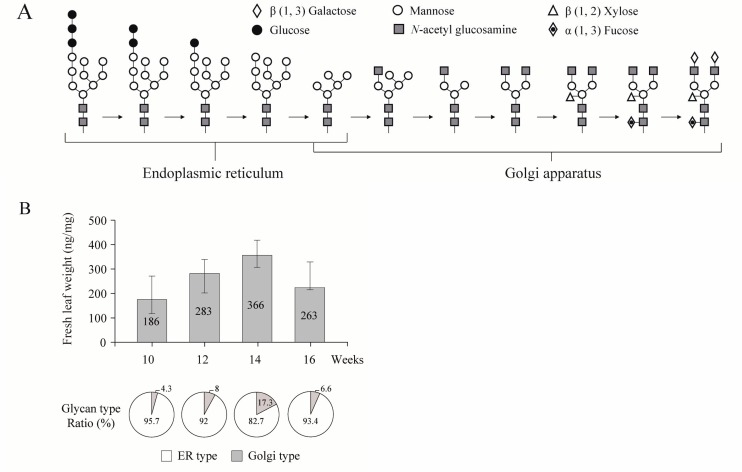
Glycosylation, which is essential for proper biological activities of the recombinant vaccine proteins, can be affected by the photosynthetic ability of plant cells, as the glucose obtained from photosynthesis is an essential element in the N-glycosylation pathway (Jamal et al., 2009). Blue light (440 nm) and far red light (660 nm) are selectively applied as an energy source for photosynthesis to enable plant growth (Singh et al., 2015). Environmental factors such as temperature, soil nutrition, salinity, and drought stress all affect glycosylation, as well as protein qualities and quantities (Jamal et al., 2009; Kang et al., 2016). Glycosylation patterns in the anti-cancer vaccine GA733-FcK in tobacco vary at the developmental stages (Lim et al., 2015). The GA733-FcK protein harbors the endoplasmic reticulum retention motif KDEL (Lys-Asp-Glu-Leu); theoretically, proteins with KDEL are oligomannose-glycosylated in plants. However, these glycosylation patterns are plant specific, in contrast with oligomannose-type glycosylation patterns (Lim et al., 2015). In particular, when the plants are in full bloom, the glycosylation pattern is slightly shifted from the oligomannose-type glycoforms (glycoforms of endoplasmic reticulum-retained protein) to plant-specific glycoforms (glycoforms of secreted protein) (Fig. 1). The glycosylation rate of the colorectal cancer vaccine protein GA733-Fc fused with the KDEL (GA733-FcK) is much lower in yellow leaves, which contain low numbers of chloroplasts for photosynthesis, than in green leaves, which contain high numbers of chloroplasts, indicating the importance of photosynthesis for the glycosylation of recombinant cancer vaccines in plants (Lim et al., 2015). Furthermore, glycosylation patterns are plant tissue-specific (Lim et al., 2015). The plant leaves growing from the upper stem produced more recombinant glycoproteins of the oligomannose type than did leaves attached to the lower portion of the stem (Donaldson et al., 1999).
Fig. 1.
Plant N-glycosylation pathway and its profile of cancer vaccine expressed in plants. (A) Glycosylation processing pathway in plant (modified from Jamal et al., 2009). (B) The comparative amount of plant-derived colorectal cancer antigen GA733-FcK protein expression level and the ratio of Golgi/endoplasmic reticulum-type glycans along with growth periods (modified from Lim et al., 2015).
CANCER VACCINES IN PLANTS
The production of cancer vaccines in plant expression systems must consider several specific factors. First, in order to successfully prevent and cure diseases, proper vaccine candidates should be chosen and designed to induce potent immune responses against these diseases. Second, it is essential to choose proper antigenic proteins that the immune system can target.
Immune responses to cancer vaccines include mucosal and systemic challenges to a vaccine after its direct application to mucosal surfaces or parenteral injection, respectively (Rigano and Walmsley, 2005). Direct application of a vaccine to mucosal sites that can induce a quick mucosal immune response, including protective humoral, cell-mediated, and later systemic immune responses, may be the best choice in some enteric and respiratory diseases (Rigano and Walmsley, 2005). Rapid advancements in the fields of molecular immunology and cancer biology have improved our understanding of the role of the immune system in cancer and the development of therapeutic and preventive vaccines. Therapeutic cancer vaccines, which are administered to current cancer patients, stimulate the protective ability of the immune system to specifically recognize, attack, and kill tumor cells (Cripps et al., 2001). Cancer preventive vaccines are administered to healthy people to prevent cancer from developing (Bachmann and Jennings, 2010).
During the body’s immune response to cancer, antigen-presenting cells display antigens that are complexed with major histocompatibility complexes (MHC II and I) on their surfaces, which are recognized by T-lymphocytes, and which eventually stimulate these T-lymphocytes (CD4+ or CD8+) to become mature helper and cytotoxic T cells, respectively. The mature helper T cells recruit and prime cytotoxic T lymphocytes to kill tumor cells (Frazer et al., 2007). Anti-cancer vaccines produced in plants must be oriented to proper antigenic proteins that the immune system can target. Tumor antigenic proteins may be classified into two types: tumor-specific antigens (TSAs) and TAAs. TSAs are specifically expressed on the tumor cells and trigger better immune responses than do TAAs. However, it is difficult to identify TSAs as vaccine candidates, as they are very uncommon. TAAs are expressed on both tumor and normal cells and stimulate a weaker immune response than do TAAs; however, they are commonly identified on tumor cells.
Many anti-cancer vaccines have been expressed in plants (Table 1). Non-Hodgkin’s lymphoma (Zhang et al., 2009), colorectal cancer (Bendandi et al., 2010; Lim et al., 2015), and cervical cancer (Smith et al., 2002; Verch et al., 2004) have been targeted. For non-Hodgkin’s lymphoma, individualized (patient-specific) recombinant idiotype vaccines against follicular B cell lymphoma were produced in N. benthamiana leaves using the transient plant expression system (Zhang et al., 2009; Pineo et al., 2013). This approach allowed rapid production and recovery of idiotypic single-chain antibodies (scFv) derived from each patient’s tumor and immunization with their own individual therapeutic antigen. Variation in glycan structures on the antigen do not impair immunogenicity or affect vaccine safety.
Table 1.
Cancer vaccines expressed in plants
| Target/Antigen/Strategy | Host plant | Transformation platform | Immunogenicity/efficacy (development status) | References |
|---|---|---|---|---|
| Tumor-associated colorectal cancer antigen: Fused to Fc high mannose type glycan | N. tabacum | Stable | Induced anti-cancer IgGs (pre-clinical) | Brodzik et al., 2006; Lu et al., 2012; Lim et al., 2015 |
| Prostatic acid phosphatase (PAP) antigen: Fused to IgM Fc | N. tabacum | Stable | Induced anti-PAP IgGs (pre-clinical) | Kang et al., 2016 |
| Tumor-associated colorectal cancer antigen | N. benthamiana | Transient | Serum in vaccinated mice inhibited colorectal tumor in nude mice (pre-clinical) | Verch et al., 2004 |
| Human papilloma virus 16 L1/L2 chimaeras | N. benthamiana | Transient | Not tested | Pineo et al., 2013 |
| Non-Hodgkin’s lymphoma (NHL) | N. benthamiana | Transient | Induced immune responses (Phase II) | McCormick et al., 2011 |
| E7 oncoprotein from human papilloma virus 16 | N. benthamiana | Transient | Induced E7-specific IgG and prevented tumor development | Plchova et al., 2011 |
| Her2 protein | N. benthamiana | Transient | Induced anti-Her2 antibody including trastuzumab-like activities | Chotprakaikiat et al., 2016 |
| Plant virus particle-based cancer immunotherapy | N. benthamiana | Transient | Activated TLR7 and Induced high levels of protective antibody | Massa et al., 2007; Jobsri et al., 2015; Peruzzi and Chiocca, 2016 |
| MUC1-based plant vaccine for breast cancer | N. benthamiana | Transient | Induced anti-MUC1 serum antibodies | Pinkhasov et al., 2011 |
Another transient expression system was applied to produce the colorectal cancer antigen GA733 using tobacco mosaic virus plant viral vector (McCormick et al., 2008; Bendandi et al., 2010). Plant-derived GA773 stimulated humoral immune responses in mice; however, sera from mice injected with the plant-derived version did not show sufficient Antibody-dependent cell-mediated cytotoxicity (ADCC) and Complement Dependent Cytotoxicity (CDC) activity compared with the response to mammalian-derived GA733 (Shivprasad et al., 1999). In contrast, sera from mice injected with GA733 that was transiently expressed in Swiss chard (Beta vulgaris var. cicla) and low alkaloid tobacco (N. tabacum var. LAMD609) showed inhibition of growth of SW948 human colorectal cancer cells that were xenografted on to nude mice (Brodzik et al., 2008). The GA733 expression level was 5 mg per kg of fresh plant leaf tissue, which is not sufficient for commercialization; therefore, GA733 was fused to the immunoglobulin Fc fragment to enhance protein stability and create a better yield from the plant (Staib et al., 2001). Indeed, the expression level of the GA733 fused to Fc was 10-fold higher than that without the Fc (Brodzik et al., 2008). In addition, the Fc fused to vaccines can facilitate easier purification by protein-A or G affinity chromatography (Lu et al., 2012; Lim et al., 2015; Park et al., 2015).
The constant region Fc fragment can enhance the immune response as a result of an increased Fc receptor-mediated uptake by antigen-presenting cells, such as dendritic cells, on which Fc receptors exist (Andrianov et al., 2010; Lu et al., 2012). The antigen-antibody complexes can assemble to become multimerized peptide antigens in plants, eventually enhancing immune responses (Pleass, 2009). The antigens were fused to the C-terminus of the heavy chain of the antibody and expressed with light chains in transgenic plants (Bhoo et al., 2011). The antigen-antibody fusion proteins are assembled to form virus-like multimeric structures, which are more immunogenic than the antigen itself (Chargelegue et al., 2005).
CONCLUSIONS
Production of anti-cancer vaccines in plant systems has several advantages over animal-based systems, including lower upstream process costs, reduced risk of mammalian pathogen contamination, and ease of scalability (Table 2). Nevertheless, to fully commercialize plant systems for anti-cancer vaccine production, two major concerns should be eliminated: low expression of recombinant vaccine proteins and unwanted plant-specific glycosylation. Advance in plant biotechnology, glycoengineering, and molecular immunology can overcome such drawbacks in plant systems for recombinant vaccine production. Thus, plants can be considered to be a potential alternative to current mammalian based vaccine production system.
Table 2.
Advantages of various systems for expressing recombinant proteins (modified from Raskin et al., 2002)
| Host | Advantages of recombinant protein expression in various systems | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Speed | Operating cost | Capital cost | Glycosylation | Multimeric assembly | Folding | Safety | Scalability | |
| Bacteria | + + + | + + | + + | + | + | + | + + | + |
| Yeast | + + | + + | + | + | + | + | + + | + + |
| Insect cell culture | + + + | + + | + | + | + + | + | + | + + |
| Plant | + + | + + + | + + + | + + | + + | + + | + + + | + + + |
| Mammalian cell culture | + | + | + | + + | + + | + + + | + | + |
| Transgenic animal | + | + | + + | + + + | + + + | + + | + | + + |
Acknowledgments
This research was supported by a grant (Code# PJ0111102016) from the Korean Rural Development Administration, National Research Foundation of Korea Grant funded by the Korean Government (MEST) (NRF-2014R1-A2A1A11052922).
Footnotes
CONFLICT OF INTEREST
The authors confirm that they have no conflicts of interest.
REFERENCES
- Andrianov V, Brodzik R, Spitsin S, Bandurska K, McManus H, Koprowski H, Golovkin M. Production of recombinant anthrax toxin receptor (ATR/CMG2) fused with human Fc in planta. Protein Expr Purif. 2010;70:158–162. doi: 10.1016/j.pep.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol. 2010;10:787–796. doi: 10.1038/nri2868. [DOI] [PubMed] [Google Scholar]
- Bendandi M, Marillonnet S, Kandzia R, Thieme F, Nickstadt A, Herz S, Fröde R, Inogés S, López-Dìaz de Cerio A, Soria E, Villanueva H, Vancanneyt G, McCormick A, Tusé D, Lenz J, Butler-Ransohoff JE, Klimyuk V, Gleba Y. Rapid, high-yield production in plants of individualized idiotype vaccines for non-Hodgkin’s lymphoma. Ann Oncol. 2010;21:2420–2427. doi: 10.1093/annonc/mdq256. [DOI] [PubMed] [Google Scholar]
- Bhoo SH, Lai H, Ma J, Arntzen CJ, Chen Q, Mason HS. Expression of an immunogenic Ebola immune complex in Nicotiana benthamiana. Plant Biotechnol J. 2006;9:807–816. doi: 10.1111/j.1467-7652.2011.00593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodzik R, Glogowska M, Bandurska K, Okulicz M, Deka D, Ko K, van der Linden J, Leusen JH, Pogrebnyak N, Golovkin M, Steplewski Z, Koprowski H. Plant-derived anti-Lewis Y mAb exhibits biological activities for efficient immunotherapy against human cancer cells. Proc Nati Acad Sci USA. 2006;103:8804–8809. doi: 10.1073/pnas.0603043103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodzik R, Spitsin S, Golovkin M, Bandurska K, Portocarrero C, Okulicz M, Steplewski Z, Koprowski H. Plant-derived EpCAM antigen induces protective anti-cancer response. Cancer Immunol Immunother. 2008;57:317–323. doi: 10.1007/s00262-007-0366-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chargelegue D, Drake PM, Obregon P, Prada A, Fairweather N, Ma JK. Highly immunogenic and protective recombinant vaccine candidate expressed in transgenic plants. Infect Immun. 2005;73:5915–5922. doi: 10.1128/IAI.73.9.5915-5922.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotprakaikiat W, Allen A, Bui-Minh D, Harden E, Jobsri J, Cavallo F, Gleba Y, Stevenson FK, Ottensmeier C, Klimyuk V, Savelyeva N. A plant-expressed conjugate vaccine breaks CD4+ tolerance and induces potent immunity against metastatic Her2+ breast cancer. Oncoimmunology. 2016;22:e1166323. doi: 10.1080/2162402X.2016.1166323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley AJ, Zhu H, Le LC, Jevnikar AM, Lee BH, Brandle JE, Menassa R. Recombinant protein production in a variety of Nicotiana hosts: a comparative analysis. Plant Biotechnol J. 2011;9:434–444. doi: 10.1111/j.1467-7652.2010.00563.x. [DOI] [PubMed] [Google Scholar]
- Cripps AW, Kyd JM, Foxwell AR. Vaccines and mucosal immunisation. Vaccine. 2001;19:2513–2515. doi: 10.1016/S0264-410X(00)00481-3. [DOI] [PubMed] [Google Scholar]
- Curtiss R, III, Cardineau GA, inventors. Washington University assignee Oral immunization by transgenic plants. 1997. Oct 21, United States patent US 5,679880.
- Daniell H, Datta R, Varma S, Gray S, Lee SB. Containment of herbicide resistance through genetic engineering of the chloroplast genome. Nat Biotechnol. 1998;16:345–348. doi: 10.1038/nbt0498-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicker M, Tschofen M, Maresch D, König J, Juarez P, Orzaez D, Altmann F, Steinkellner H, Strasser R. Transient glyco-engineering to produce recombinant IgA1 with defined N- and O-glycans in plants. Front Plant Sci. 2016;7:18. doi: 10.3389/fpls.2016.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson M, Wood HA, Kulakosky PC, Shuler ML. Glycosylation of a recombinant protein in the Tn5B1-4 insect cell line: influence of ammonia, time of harvest, temperature, and dissolved oxygen. Biotechnol Bioeng. 1999;63:255–262. doi: 10.1002/(SICI)1097-0290(19990505)63:3<255::AID-BIT1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Evans DE, Coleman JOD, Kearns A. Plant Cell Culture. Tayler & Francis Group; London: 2003. [Google Scholar]
- Fazlalipour M, Keyvani H, Monavari SH, Mollaie HR. Expression, purification and immunogenic description of a hepatitis C virus recombinant coreE1E2 protein expressed by yeast pichiapastoris. Jundishapur J Microbiol. 2015;8:e17157. doi: 10.5812/jjm.8(4)2015.17157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer IH, Lowy DR, Schiller JT. Prevention of cancer through immunization: Prospects and challenges for the 21st century. Eur J Immunol. 2007;37:S148–S155. doi: 10.1002/eji.200737820. [DOI] [PubMed] [Google Scholar]
- Gaume A, Komarnytsky S, Borisjuk N, Raskin I. Rhizosecretion of recombinant proteins from plant hairy roots. Plant Cell Rep. 2003;21:1188–1193. doi: 10.1007/s00299-003-0660-3. [DOI] [PubMed] [Google Scholar]
- Gomord V, Chamberlain P, Jefferis R, Faye L. Biopharmaceutical production in plants: problems, solutions and opportunities. Trends Biotechnol. 2005;23:559–565. doi: 10.1016/j.tibtech.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Goodin MM, Zaitlin D, Naidu RA, Lommel SA. Nicotianabenthamiana: its history and future as a model for plant-pathogen interactions. Mol Plant Microbe Interact. 2008;21:1015–1026. doi: 10.1094/MPMI-21-8-1015. [DOI] [PubMed] [Google Scholar]
- Hong SY, Kwon TH, Jang YS, Kim SH, Yang MS. Production of bioactive human granulocyte-colony stimulating factor in transgenic rice cell suspension cultures. Protein Expr Purif. 2006;47:68–73. doi: 10.1016/j.pep.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Huang TK, McDonald KA. Bioreactor systems for in vitro production of foreign proteins using plant cell cultures. Biotechnol Adv. 2012;30:398–409. doi: 10.1016/j.biotechadv.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Hussain MS, Fareed S, Ansari S, Rahman MA, Ahmad IZ, Saeed M. Current approaches toward production of secondary plant metabolites. J Pharm Bioallied Sci. 2012;4:10–20. doi: 10.4103/0975-7406.92725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SJ, Kim KS, Byo BK, Hwang B. Saponin production by hairy root cultures of Panax ginseng CA Meyer: Influence of PGR and Polyamines. Biotechnol Bioprecess Eng. 1999;4:309–312. doi: 10.1007/BF02933759. [DOI] [Google Scholar]
- Jamal A, Ko K, Kim HS, Choo YK, Joung H, Ko K. Role of genetic factors and environmental conditions in recombinant protein production for molecular farming. Biotechnol Adv. 2009;27:914–923. doi: 10.1016/j.biotechadv.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Jamal A, Lee JH, Lee KJ, Oh DB, Kim DS, Lee KK, Choo YK, Hwang KA, Ko K. Chimerism of Multiple Monoclonal Antibodies Expressed in a Single Plant. Hortic Environ Biotechnol. 2012;53:544–551. doi: 10.1007/s13580-012-0153-9. [DOI] [Google Scholar]
- Jensen CS, Salchert K, Gao C, Andersen C, Didion T, Nielsen KK. Floral inhibition in red fescue (Festucarubra L.) through expression of a heterologous flowering repressor from Lolium. Mol Breed. 2004;13:37–48. doi: 10.1023/B:MOLB.0000012327.47625.23. [DOI] [Google Scholar]
- Jobsri J, Allen A, Rajagopal D, Shipton M, Kanyuka K, Lomonossoff GP, Ottensmeier C, Diebold SS, Stevenson FK, Savelyeva N. Plant virus particles carrying tumour antigen activate TLR7 and Induce high levels of protective antibody. PLoS ONE. 2015;10:e0118096. doi: 10.1371/journal.pone.0118096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Shin YK, Park SW, Ko K. Effect of Nitrogen Deficiency on Recombinant Protein Production and Dimerization and Growth in Transgenic Plants. Hortic Environ Biotechnol. 2016;57:299–307. doi: 10.1007/s13580-016-0045-5. [DOI] [Google Scholar]
- Kim DS, Song I, Kim J, Kim DS, Ko K. Plant recycling for molecular biofarming to produce recombinant anti-cancer mAb. Front Plant Sci. 2016;7:1037. doi: 10.3389/fpls.2016.01037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimyuk V, Pogue G, Herz S, Butler J, Haydon H. Production of recombinant antigens and antibodies in Nicotiana benthamiana using ‘magnifection’ technology: GMP-compliant facilities for small- and large-scale manufacturing. Curr Top Microbiol Immunol. 2014;375:127–154. doi: 10.1007/82_2012_212. [DOI] [PubMed] [Google Scholar]
- Ko K, Wei X, Crooks PA, Koprowski H. Elimination of alkaloids from plant-derived human monoclonal antibody. J. Immunol. Methods. 2004;286:79–85. doi: 10.1016/j.jim.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Kuo YC, Tan CC, Ku JT, Hsu WC, Su SC, Lu CA, Huang LF. Improving pharmaceutical protein production in Oryza sativa. Int J Mol Sci. 2013;14:8719–8739. doi: 10.3390/ijms14058719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa S, Nakamura R, Mejima M, Kozuka-Hata H, Kuroda M, Takeyama N, Oyama M, Satoh S, Kiyono H, Masumura T, Teshima R, Yuki Y. MucoRice-cholera toxin B-subunit, a rice-based oral cholera vaccine, down-regulates the expression of α-amylase/trypsin inhibitor-like protein family as major rice allergens. J Proteome Res. 2013;12:3372–3382. doi: 10.1021/pr4002146. [DOI] [PubMed] [Google Scholar]
- Lee JH, Park DY, Lee KJ, Kim YK, So YK, Ryu JS, Oh SH, Han YS, Ko K, Choo YK, Park SJ, Brodzik R, Lee KK, Oh DB, Hwang KA, Koprowski H, Lee YS, Ko K. Intracellular reprogramming of expression, glycosylation, and function of a plant-derived antiviral therapeutic monoclonal antibody. PLoS ONE. 2013;8:e68772. doi: 10.1371/journal.pone.0068772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Kim YH, Roh YS, Myoung HJ, Lee KY, Kim DI. Bioreactor operation for transgenic Nicotiana tabacum cell cultures and continuous production of recombinant human granulocyte-macrophage colony-stimulating factor by perfusion culture. Enzyme Microb Technol. 2004;35:663–671. doi: 10.1016/j.enzmictec.2004.08.019. [DOI] [Google Scholar]
- Li W, Asada Y, Yoshikawa T. Antimicrobial flavonoids from Glycyrrhizaglabra hairy root cultures. Planta Med. 1998;64:746–747. doi: 10.1055/s-2006-957571. [DOI] [PubMed] [Google Scholar]
- Lico C, Chen Q, Santi L. Viral vectors for production of recombinant proteins in plants. J Cell Physiol. 2008;216:366–377. doi: 10.1002/jcp.21423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim CY, Lee KJ, Oh DB, Ko K. Effect of the developmental stage and tissue position on the expression and glycosylation of recombinant glycoprotein GA733-FcK in transgenic plants. Front Plant Sci. 2015;5:778. doi: 10.3389/fpls.2014.00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Davies JW, Stanley J. Mutational analysis of bean yellow dwarf virus, a geminivirus of the genus Mastrevirus that is adapted to dicotyledonous plants. J Gen Virol. 1998;79:2265–2274. doi: 10.1099/0022-1317-79-9-2265. [DOI] [PubMed] [Google Scholar]
- Liu YK, Huang LF, Ho SL, Liao CY, Liu HY, Lai YH, Yu SM, Lu CA. Production of mouse granulocyte-macrophage colony-stimulating factor by gateway technology and transgenic rice cell culture. Biotechnol Bioeng. 2012;109:1239–1247. doi: 10.1002/bit.24394. [DOI] [PubMed] [Google Scholar]
- Loos A, Steinkellner H. Plant glyco-biotechnology on the way to synthetic biology. Front Plant Sci. 2014;5:523. doi: 10.3389/fpls.2014.00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Lee KJ, Shao Y, Lee JH, So Y, Choo YK, Oh DB, Hwang KA, Oh SH, Han YS, Ko K. Expression of GA733-Fc fusion protein as a vaccine candidate for colorectal cancer in transgenic plants. J Biomed Biotechnol. 2012;2012:364240. doi: 10.1155/2012/364240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JK, Chikwamba R, Sparrow P, Fischer R, Mahoney R, Twyman RM. Plant-derived pharmaceuticals-the road forward. Trends Plant Sci. 2005;10:580–585. doi: 10.1016/j.tplants.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Magnuson NS, Linzmaier PM, Reeves R, An G, HayGlass K, Lee JM. Secretion of biologically active human interleukin-2 and interleukin-4 from genetically modified tobacco cells in suspension culture. Protein Expr Purif. 1998;13:45–52. doi: 10.1006/prep.1998.0872. [DOI] [PubMed] [Google Scholar]
- Marillonnet S, Giritch A, Gils M, Kandzia R, Klimyuk V, Gleba Y. In planta engineering of viral RNA replicons: efficient assembly by recombination of DNA modules delivered by Agrobacterium. Proc Natl Acad Sci USA. 2004;101:6852–6857. doi: 10.1073/pnas.0400149101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama D, Sugiyama T, Endo T, Nishikawa S. Multiple BiP genes of Arabidopsis thaliana are required for male gametogenesis and pollen competitiveness. Plant Cell Physiol. 2014;55:801–810. doi: 10.1093/pcp/pcu018. [DOI] [PubMed] [Google Scholar]
- Massa S, Franconi R, Brandi R, Muller A, Mett V, Yusibov V, Venuti A. Anti-cancer activity of plant-produced HPV16 E7 vaccine. Vaccine. 2007;25:3018–3021. doi: 10.1016/j.vaccine.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Matzeit V, Schaefer S, Kammann M, Schalk HJ, Schell J, Gronenborn B. Wheat dwarf virus vectors replicate and express foreign genes in cells of monocotyledonous plants. Plant Cell. 1991;3:247–258. doi: 10.1105/tpc.3.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo KJ, Gonzales BJ, Mason HS. Genetic transformation of tobacco NT1 cells with Agrobacterium tumefaciens. Nat Protoc. 2006;1:1105–1111. doi: 10.1038/nprot.2006.176. [DOI] [PubMed] [Google Scholar]
- McCormick AA, Reddy S, Reinl SJ, Cameron TI, Czerwinkski DK, Vojdani F, Hanley KM, Garger SJ, White EL, Novak J, Barrett J, Holtz RB, Tusé D, Levy R. Plant-produced idiotype vaccines for the treatment of non-Hodgkin’s lymphoma: safety and immunogenicity in a phase I clinical study. Proc Natl Acad Sci USA. 2008;105:10131–10136. doi: 10.1073/pnas.0803636105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick AA. Tobacco derived cancer vaccines for non-Hodgkin’s lymphoma: perspectives and progress. Hum Vaccin. 2011;7:305–312. doi: 10.4161/hv.7.3.14163. [DOI] [PubMed] [Google Scholar]
- Medina-Bolivar F, Wright R, Funk V, Sentz D, Barroso L, Wilkins TD, Petri W, Jr, Cramer CL. A non-toxic lectin for antigen delivery of plant-based mucosal vaccines. Vaccine. 2003;21:997–1005. doi: 10.1016/S0264-410X(02)00551-0. [DOI] [PubMed] [Google Scholar]
- Meyers B, Zaltsman A, Lacroix B, Kozlovsky SV, Krichevsky A. Nuclear and plastid genetic engineering of plants: comparison of opportunities and challenges. Biotechnol Adv. 2010;28:747–756. doi: 10.1016/j.biotechadv.2010.05.022. [DOI] [PubMed] [Google Scholar]
- Monsurrò V, Nagorsen D, Wang E, Provenzano M, Dudley ME, Rosenberg SA, Marincola FM. Functional heterogeneity of vaccine-induced CD8+ T cells. J Immunol. 2002;168:5933–5942. doi: 10.4049/jimmunol.168.11.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi A, Sugano N. Growth and division of carrot cells in suspension culture. Plant Cell Phsiol. 1970;11:757–765. [Google Scholar]
- Park SR, Lim CY, Kim DS, Ko K. Optimization of ammonium sulfate concentration for purification of colorectal cancer vaccine candidate recombinant protein GA733-FcK isolated from plants. Front Plant Sci. 2015;6:1040. doi: 10.3389/fpls.2015.01040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Maldonado LM, González-Ramírez JE. Genetically Engineered Plants as a Source of Vaccines against Wide Spread Diseases. Springer; New York: 2014. Bioreactors for plant biomass production and bioprocessing. pp. 129–140. [Google Scholar]
- Peruzzi PP, Chiocca EA. Cancer immunotherapy: A vaccine from plant virus proteins. Nat Nanotechnol. 2016;11:214–215. doi: 10.1038/nnano.2015.306. [DOI] [PubMed] [Google Scholar]
- Pineo CB, Hitzeroth II, Rybicki EP. Immunogenic assessment of plant-produced human papillomavirus type 16 L1/L2 chimaeras. Plant Biotechnol J. 2013;11:964–975. doi: 10.1111/pbi.12089. [DOI] [PubMed] [Google Scholar]
- Pinkhasov J, Alvarez ML, Rigano MM, Piensook K, Larios D, Pabst M, Grass J, Mukherjee P, Gendler SJ, Walmsley AM, Mason HS. Recombinant plant-expressed tumour-associated MUC1 peptide is immunogenic and capable of breaking tolerance in MUC1.Tg mice. Plant Biotechnol J. 2011;9:991–1001. doi: 10.1111/j.1467-7652.2011.00614.x. [DOI] [PubMed] [Google Scholar]
- Plchova H, Moravec T, Hoffmeisterova H, Folwarczna J, Cerovska N. Expression of human papillomavirus 16 E7ggg oncoprotein on N- and C-terminus of potato virus X coat protein in bacterial and plant cells. Protein Expr Purif. 2011;77:146–152. doi: 10.1016/j.pep.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Pleass RJ. Fc-receptors and immunity to malaria: from models to vaccines. Parasite Immunol. 2009;31:529–538. doi: 10.1111/j.1365-3024.2009.01101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao L, Lee KJ, Ko K. Characterization of the glycan structures of glycoprotein GA733-Fc expressed in a baculovirus-insect cell system. Bull Korean Chem Soc. 2015;36:139–149. doi: 10.1002/bkcs.10035. [DOI] [Google Scholar]
- Radford KJ, Higgins DE, Pasquini S, Cheadle EJ, Carta L, Jackson AM, Lemoine NR, Vassaux G. A recombinant E. coli vaccine to promote MHC class I-dependent antigen presentation: application to cancer immunotherapy. Gene Ther. 2002;9:1455–1463. doi: 10.1038/sj.gt.3301812. [DOI] [PubMed] [Google Scholar]
- Rao SR, Ravishankar GA. Plant cell cultures: Chemical factories of secondary metabolites. Biotechnol Adv. 2002;20:101–153. doi: 10.1016/S0734-9750(02)00007-1. [DOI] [PubMed] [Google Scholar]
- Rappuoli R. Bridging the knowledge gaps in vaccine design. Nat Biotechnol. 2007;25:1361–1366. doi: 10.1038/nbt1207-1361. [DOI] [PubMed] [Google Scholar]
- Raskin I, Ribnicky DM, Komarnytsky S, Ilic N, Poulev A, Borisjuk N, Brinker A, Moreno DA, Ripoll C, Yakoby N, O’Neal JM, Cornwell T, Pastor I, Fridlender B. Plants and human health in the twenty-first century. Trends Biotechnol. 2002;20:522–531. doi: 10.1016/S0167-7799(02)02080-2. [DOI] [PubMed] [Google Scholar]
- Rigano MM, Walmsley AM. Expression systems and developments in plant-made vaccines. Immunol Cell Biol. 2005;83:271–277. doi: 10.1111/j.1440-1711.2005.01336.x. [DOI] [PubMed] [Google Scholar]
- Ruf S, Hermann M, Berger IJ, Carrer H, Bock R. Stable genetic transformation of tomato plastids and expression of a foreign protein in fruit. Nat Biotechnol. 2001;19:870–875. doi: 10.1038/nbt0901-870. [DOI] [PubMed] [Google Scholar]
- Rymerson RT, Babiuk L, Menassa R, Vanderbeld B, Brandle JE. Immunogenicity of the capsid protein VP2 from porcine parvovirus expressed in low alkaloid transgenic tobacco. Mol Breed. 2003;11:267–276. doi: 10.1023/A:1023426906756. [DOI] [Google Scholar]
- Shaaltiel Y, Bartfeld D, Hashmueli S, Baum G, Brill-Almon E, Galili G, Dym O, Boldin-Adamsky SA, Silman I, Sussman JL, Futerman AH, Aviezer D. Production of glucocerebrosidase with terminal mannose glycans for enzyme replacement therapy of Gaucher’s disease using a plant cell system. Plant Biotechnol J. 2007;5:579–590. doi: 10.1111/j.1467-7652.2007.00263.x. [DOI] [PubMed] [Google Scholar]
- Sharp JM, Doran PM. Characterization of monoclonal antibody fragments produced by plant cells. Biotechnol Bioeng. 2001;73:338–346. doi: 10.1002/bit.1067. [DOI] [PubMed] [Google Scholar]
- Sheludko YV, Sindarovska YR, Gerasymenko IM, Bannikova MA, Kuchuk NV. Comparison of several Nicotiana species as hosts for high-scale Agrobacterium-mediated transient expression. Biotechnol Bioeng. 2007;96:608–614. doi: 10.1002/bit.21075. [DOI] [PubMed] [Google Scholar]
- Shivprasad S, Pogue GP, Lewandowski DJ, Hidalgo J, Donson J, Grill LK, Dawson WO. Heterologous sequences greatly affect foreign gene expression in tobacco mosaic virus-based vectors. Virology. 1999;255:312–323. doi: 10.1006/viro.1998.9579. [DOI] [PubMed] [Google Scholar]
- Singh D, Basu C, Meinhardt-Wollweber M, Roth B. LEDs for energy efficient greenhouse lighting. Renew Sustainable Energy Rev. 2015;49:139–147. doi: 10.1016/j.rser.2015.04.117. [DOI] [Google Scholar]
- Smith ML, Mason HS, Shuler ML. Hepatitis B surface antigen (HBsAg) expression in plant cell culture: Kinetics of antigen accumulation in batch culture and its intracellular form. Biotechnol Bioeng. 2002;80:812–822. doi: 10.1002/bit.10444. [DOI] [PubMed] [Google Scholar]
- Song I, Kim DS, Kim MK, Jamal A, Hwang KA, Ko K. Comparison of total soluble protein in various horticultural crops and evaluation of its quantification methods. Hortic Environ Biotechnol. 2015;56:123–129. doi: 10.1007/s13580-015-0097-y. [DOI] [Google Scholar]
- Spangenberg G, Wang ZY, Potrykus I. Biotechnology in forage and turf grass improvement. Vol. 23. Springer Science & Business Media; New York: 2012. [Google Scholar]
- Staib L, Birebent B, Somasundaram R, Purev E, Braumüller H, Leeser C, Küttner N, Li W, Zhu D, Diao J, Wunner W, Speicher D, Beger HG, Song H, Herlyn D. Immunogenicity of recombinant GA733-2E antigen (CO17-1A, EGP, KS1-4, KSA, Ep-CAM) in gastro-intestinal carcinoma patients. Int. J. Cancer. 2001;92:79–87. doi: 10.1002/1097-0215(200102)9999:9999<::AID-IJC1164>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Strasser R, Bondili JS, Vavra U, Schoberer J, Svoboda B, Glössl J, Léonard R, Stadlmann J, Altmann F, Steinkellner H, Mach L. A unique beta1,3-galactosyltransferase is indispensable for the biosynthesis of N-glycans containing Lewis a structures in Arabidopsis thaliana. Plant Cell. 2007;19:2278–2292. doi: 10.1105/tpc.107.052985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacket CO. Plant-based oral vaccines: results of human trials. Curr Top Microbiol Immunol. 2009;332:103–117. doi: 10.1007/978-3-540-70868-1_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaiwa F. A rice-based edible vaccine expressing multiple T-cell epitopes to induce oral tolerance and inhibit allergy. Immunol Allergy Clin North Am. 2007;27:129–139. doi: 10.1016/j.iac.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Tepfer M, Casse-Delbart F. Agrobacterium rhizogenes as a vector for transforming higher plants. Microbiol Sci. 1987;4:24–28. [PubMed] [Google Scholar]
- Vasilev N, Grömping U, Lipperts A, Raven N, Fischer R, Schillberg S. Optimization of BY-2 cell suspension culture medium for the production of a human antibody using a combination of fractional factorial designs and the response surface method. Plant Biotechnol J. 2013;11:867–874. doi: 10.1111/pbi.12079. [DOI] [PubMed] [Google Scholar]
- Verch T, Hooper DC, Kiyatkin A, Steplewski Z, Koprowski H. Immunization with a plant-produced colorectal cancer antigen. Cancer Immunol Immunother. 2004;53:92–99. doi: 10.1007/s00262-003-0428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZY, Ge Y. Recent advances in genetic transformation of forage and turf grasses. In vitro Cell. Dev. Biol., Plant. 2006;42:1–18. doi: 10.1079/IVP2005726. [DOI] [Google Scholar]
- Zhang S, Zhang H, Zhao J. The role of CD4 T cell help for CD8 CTL activation. Biochem Biophys Res Commun. 2009;384:405–408. doi: 10.1016/j.bbrc.2009.04.134. [DOI] [PubMed] [Google Scholar]
- Zhang X, Mason H. Bean Yellow Dwarf Virus replicons for high-level transgene expression in transgenic plants and cell cultures. Biotechnol Bioeng. 2006;93:271–279. doi: 10.1002/bit.20695. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li J, Pu H, Jin J, Zhang X, Chen M, Wang B, Han C, Yu J, Li D. Development of Tobacco necrosis virus A as a vector for efficient and stable expression of FMDV VP1 peptides. Plant Biotechnol J. 2010;8:506–523. doi: 10.1111/j.1467-7652.2010.00500.x. [DOI] [PubMed] [Google Scholar]