Abstract
Transcriptional co-activator with a PDZ-binding motif (TAZ) is an important factor in lysophosphatidic acid (LPA)-induced promotion of migration and proliferation of human mesenchymal stem cells (MSCs). The expression of TAZ significantly increased at 6 h after LPA treatment, and TAZ knockdown inhibited the LPA-induced migration and proliferation of MSCs. In addition, embryonic fibroblasts from TAZ knockout mice exhibited the reduction in LPA-induced migration and proliferation. The LPA1 receptor inhibitor Ki16425 blocked LPA responses in MSCs. Although TAZ knockdown or knockout did not reduce LPA-induced phosphorylation of ERK and AKT, the MEK inhibitor U0126 or the ROCK inhibitor Y27632 blocked LPA-induced TAZ expression along with the reduction in the proliferation and migration of MSCs. Our data suggest that TAZ is an important mediator of LPA signaling in MSCs in the downstream of MEK and ROCK signaling.
Keywords: LPA, TAZ, Mesenchymal stem cells, MEK, ROCK
INTRODUCTION
Human mesenchymal stem cells (MSCs) are multipotent stem cells that can be isolated from a variety of tissues, including adipose tissues, bone marrow, umbilical cord blood, and other fetal tissues (Murphy et al., 2013). MSCs possess the ability of self-renewal, long-term viability, and the potential to differentiate into diverse cell types (e.g., adipocytes, osteoblasts, chondrocytes, and myogenic lineages) (Lindroos et al., 2011). MSCs exhibit immunomodulatory responses to inflammatory molecules through complex feedback mechanisms involving various immune cells (Aggarwal and Pittenger, 2005; Iyer and Rojas, 2008). In addition, MSCs have the ability to rescue cells from apoptosis induced by hypoxia, mechanical damage, radiation, or chemicals (Cselenyak et al., 2010; Li et al., 2010a). Therefore, MSCs are valuable resources in regenerative medicine, and it is important to increase the survival time of MSCs following transplantation to maximize their therapeutic efficacy.
Lysophosphatidic acid (LPA) is a naturally present lipid implicated in oncogenesis, cell proliferation, migration, and survival (van Meeteren and Moolenaar, 2007). LPA-induced signals are primarily mediated through three closely related G protein-coupled receptors referred to as LPA1, LPA2, and LPA3 (also known as EDG2, EDG4, and EDG7) (Anliker and Chun, 2004). LPA-induced activation of LPA receptors led to stimulate diverse signaling pathways involving Ras, ERK, and phosphoinositide 3-kinase (PI3K) (Frankel and Mills, 1996; Hu et al., 2005). Accumulating evidence suggests that LPA promotes the proliferation and survival of MSCs (Chen et al., 2008; Liu et al., 2009; Li et al., 2010b; Binder et al., 2014); however, the signaling mechanism associated with LPA-induced survival has not been elucidated in human MSCs.
The Hippo signaling pathway is a well-conserved pathway that regulates cellular growth, and it is also involved in stem cell function, regeneration, and tumor suppression (Halder and Johnson, 2011; Ramos and Camargo, 2012). Moreover, the Hippo pathway negatively regulates the downstream mediator Yes-associated protein (YAP) as well as the transcriptional co-activator with a PDZ binding motif (TAZ), which are two homologous transcriptional co-activators (Hong and Guan, 2012). Active YAP and TAZ stimulate cellular proliferation and prevent cell death (Overholtzer et al., 2006; Chan et al., 2008). When the Hippo pathway is activated, YAP and TAZ are phosphorylated, exported from the nucleus, and subjected to degradation by the proteasome (Hao et al., 2008; Liu et al., 2010). Recently, LPA has been shown to regulate YAP and TAZ activity through G protein-coupled receptors and Rho GTPases to induce gene expression, cell migration, and proliferation (Yu et al., 2012). In addition, ERK1/2 and PI3K have been shown to be involved in promoting cell survival through the Hippo pathway (Chen et al., 2008). A careful analysis of Hippo signaling will provide an opportunity to increase the therapeutic efficacy of stem cells in the development of novel forms of regenerative medicine.
In the present study, we investigated the effects of LPA on human MSC migration and proliferation and clarified the role of TAZ in the LPA-induced cellular responses. In addition, we explored the signaling mechanisms associated with the LPA-induced activation of TAZ and cellular responses.
MATERIALS AND METHODS
Materials
α-Minimum essential medium (α-MEM), Hank’s balanced salt solution (HBSS), trypsin, fetal bovine serum, and Lipofectamine reagent were purchased from Invitrogen (Carlsbad, CA, USA). 1-Oleoyl-sn-glycero-3-phosphate (LPA), U0126, and Y27632 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Antibodies for TAZ, YAP, p-YAP, ERK, p-ERK, AKT, p-AKT, p38, p-p38, JNK, and p-JNK were obtained from Cell Signaling Technology (Beverly, MA, USA). Anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was purchased from EMD Millipore (Billerica, MA, USA).
Cell culture
Subcutaneous adipose tissue was obtained from elective surgeries with patient consent as approved by the Institution Review Board of Pusan National University Hospital (H-2008-116; Pusan, Korea). To isolate human MSCs, the adipose tissues were washed at least three times using sterile phosphate-buffered saline and treated with an equal volume of collagenase type I suspension (1 g/L of HBSS buffer with 1% bovine serum albumin) for 60 min at 37°C with intermittent shaking. The floating adipocytes were separated from the stromal-vascular fraction by centrifugation at 300 g for 5 min. The cell pellet was resuspended in α-MEM supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin, after which the cells were plated in tissue culture dishes at 3500 cells/cm2. The primary MSCs were cultured for 4–5 days until they reached confluence and were defined as passage “0.” The passage number of MSCs used in these experiments was between three and 10. Mouse embryonic fibroblasts (MEF) derived from WT and TAZ KO mice were maintained in DMEM supplemented with 10% heat-inactivated fetal bovine serum.
Western blotting
Serum-starved MSCs were treated with appropriate conditions, washed with ice-cold phosphate-buffered saline, and then lysed in lysis buffer (20 mM Tris-HCl, 1 mM EGTA, 1 mM EDTA, 10 mM NaCl, 0.1 mM phenylmethylsulfonyl fluoride, 1 mM Na3VO4, 30 mM sodium pyrophosphate, 25 mM β-glycerol phosphate, and 1% Triton X-100, pH 7.4). The cell lysates were centrifuged for 15 min at 4°C, and the supernatants were used for immunoblotting. The lysates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred onto a nitrocellulose membrane, and then stained with 0.1% Ponceau S solution (Sigma-Aldrich). After blocking with 5% nonfat milk, the membranes were immunoblotted with various antibodies overnight, and the bound antibodies were visualized with horseradish peroxidase-conjugated secondary antibodies using the enhanced chemiluminescence Western blotting system (ECL, Amersham Biosciences, Little Chalfont, UK).
Migration assay
MSCs migration assay was performed using disposable 96-well chemotaxis chamber (Neuro Probe, Inc., Gaithersburg, MD, USA). MSCs were harvested using 0.05% trypsin containing 0.02% EDTA and suspended in α-MEM at a concentration of 1×104 cells/mL. A membrane filter with 8 μm pores of the chemotaxis chamber was pre-coated with 20 μg/mL rattail collagen at room temperature. Aliquots (50 μL/well) of the MSC suspension were loaded into the upper chambers, and LPA was placed in the lower chamber. To investigate the signaling pathways involved in LPA-induced migration, the cells were pre-incubated with various pharmacological inhibitors for 15 min before loading. MSCs were transfected with empty vector (pCMV-flag vector) or TAZ overexpression vector (TAZ-flag overexpression construct). After incubation of the cells with LPA in the absence or presence of the inhibitors for 12 h at 37°C, the filters were disassembled and the upper surface of each filter was scraped free of cells by wiping it with a cotton swab. The numbers of cells that had migrated to the lower surfaces of each filter were determined by counting the cells in four random places under the microscope (Leica DE/DM IRB, Leica Microsystems AG, Wetzlar, Germany) after staining with Hoechst 33342 (Thermo Fisher Scientific, Waltham, MA, USA).
Cell proliferation assay
MSCs were seeded in a 24-well culture plate at a density of 1×104 cells/well and were cultured for 24 h in normal growth medium; MSCs were then washed twice with HBSS. After being serum-starved for 12 h and treated with LPA for the indicated number of days, the cell numbers were determined using a trypan blue inclusion assay.
Transfection of small interfering RNA (siRNA)
siRNA duplexes were synthesized and purified by Samchully Pharm. Co. Ltd. (Siheung, Korea) as follows: TAZ, 5′ AGGAACAAACGUUGACUUATT-3′ (sense) and 5′-UAAGUCAACGUUUGUUCCUTT-3′ (antisense). Nonspecific control siRNA (D-001206-13-05) was purchased from Dharmacon, Inc (Chicago, IL, USA). MSCs were seeded on 60-mm dishes at 70% confluence, and they were then transfected with siRNAs using the Lipofectamine Plus™ reagent (Thermo Fisher Scientific) according to the manufacturer’s instructions. The Lipofectamine Plus™ reagent was incubated with OPTI-MEM for 15 min, and the respective siRNAs were then added to the mixtures. After an incubation period of 15 min at room temperature, the mixtures were diluted with serum-free medium and added to each well. The final concentration of siRNAs in each well was 100 nM. After incubating MSCs in serum-free medium containing siRNAs for 4 h, the cells were cultured in growth medium for 24 h, and the expression levels of TAZ and GAPDH were assessed.
Reverse transcription-polymerase chain reaction (RT-PCR)
The total RNA was extracted from MSCs using TRIzol reagent (Sigma-Aldrich), and the subsequent synthesis of cyclic DNA (Thermo Reverse Transcriptase, NanoHelix, Daejeon, Korea) was carried out according to the manufacturers’ protocols. Real-time quantitative reverse-transcription polymerase chain reaction (RT-PCR) was performed using a Real-Time PCR system (ABI 7500) with SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) according to the manufacturers’ instructions. The following primer sequences were used: 5′-CTGCAATGTGGATGAGATGG-3′ (sense) and 5′-TCATTGAAGAGGGGGATCAG-3′ (antisense) for TAZ.
Luciferase reporter assay
MSCs were plated in 12-well culture plates at a density of 3×105 cells per well and transfected at 16 h after cell seeding using Lipofectamine/Lipofectamine Plus reagents (Thermo Fisher Scientific) according to the manufacturer’s instructions. The transfection mixtures contained 400 ng of YAP/TAZ-responsive reporrt construct (8xGTIIC-luciferase, #34615, Addgene, Cambridge, MA, USA) and 10 ng of internal control plasmid (pCMV-RL vector containing Renilla luciferase, Promega, Madison, WI, USA). At 24 h after transfection, cells were pretreated with 10 μM Ki16425 for 15 min, and then treated with 10 μM LPA for 6 h. After LPA treatment, cells were harvested and centrifuged at 2000×g for 3 min at 4°C, followed by determination of the luciferase activity using the Dual-Luciferase Reporter Assay System (Promega) and VICTOR3 (Perkin Elmer, Waltham, MA, USA).
Statistical analysis
Results of multiple observations were presented as mean ± SD. For multivariate data analysis, group differences were assessed with one-way or two-way ANOVA, followed by Scheffé’s post hoc test.
RESULTS
LPA treatment regulates hippo signaling and promotes the migration and proliferation of MSCs
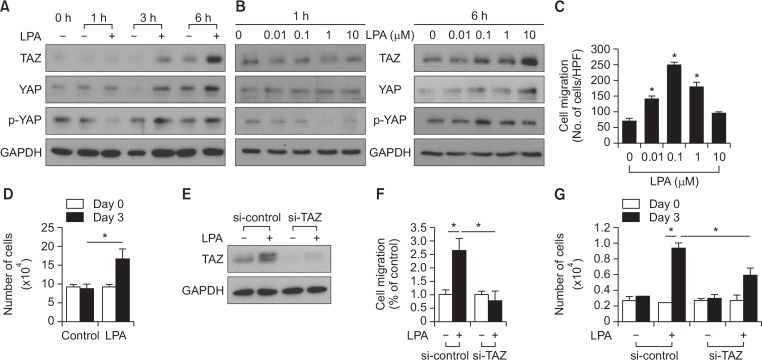
To explore whether LPA could modulate Hippo signaling in MSCs, human adipose tissue-derived MSCs were treated with LPA, and analyzed the expression and phosphorylation levels of TAZ and YAP. As shown in Fig. 1A, the expression levels of TAZ increased at 3 h following LPA treatment and exhibited a further increase at 6 h. The LPA-induced increase of YAP expression were less potent than that of TAZ expression; however, the phosphorylation of YAP decreased at 1 h after LPA treatment. To confirm these results, we next examined the dose-dependent effect of LPA on the expression levels of TAZ and YAP. At 1 h following LPA treatment, the expression level of neither TAZ nor YAP was not significantly affected, whereas the phosphorylation of YAP decreased with increasing doses of LPA. At 6 h after LPA treatment, the expression levels of TAZ and YAP increased in a dose-dependent manner in response to LPA. However, the phosphorylation levels of YAP was not significantly affected by LPA treatment at 6 h (Fig. 1B). These results suggest that LPA treatment regulates hippo signaling and thus stimulates the expression of the effector molecules of hippo signaling, TAZ and YAP.
Fig. 1.
Effects of LPA on hippo signaling and the requirement of TAZ for LPA-induced cellular responses in MSCs. (A) The time course of LPA-induced TAZ, YAP, and p-YAP expression is shown. MSCs were treated with serum-free medium containing 10 mM LPA or vehicles for the indicated amount of time. The expression levels of TAZ, YAP, p-YAP, and GAPDH were determined using Western blotting. (B) MSCs were treated with various doses of LPA for 1 h and 6 h. The expression levels of TAZ, YAP, p-YAP, and GAPDH were determined using Western blotting. (C) Migration of MSCs in response to various concentrations of LPA after 12 h incubation was measured using the chemotaxis chamber. Data indicate mean ± SD. *p<0.05 (n=8). (D) The effect of LPA on MSC proliferation is shown. The number of cells were counted and compared with those of the control cells. MSCs exhibited increased LPA-induced proliferation for 3 days. MSCs exhibited increased LPA-induced proliferation for 3 days. The cellular proliferation rate was determined by the cell count using a trypan blue inclusion assay. Data indicate mean ± SD. *p<0.05 (n=6). (E) siRNA-mediated silencing of LPA-induced TAZ expression is shown. MSCs were transfected with control siRNA and a pool of siRNA against TAZ. The expression levels of TAZ and GAPDH were determined using Western blotting. (F) Knockdown of TAZ abrogates LPA (1 uM)-stimulated migration in MSCs. Data indicate mean ± SD. *p<0.05 (n=8). (G) TAZ is required for the LPA-induced cellular proliferation of MSCs. Control and TAZ knockdown-MSCs were treated with LPA (10 μM) for 3 days, followed by cell counting using a trypan blue inclusion assay. Data indicate mean ± SD. *p<0.05 (n=6).
To determine the effect of LPA on the cellular responses, we examined the effects of LPA on the migration and proliferation of MSCs. As shown in Fig. 1C, LPA promoted the migration of MSCs with a maximal effect at 0.1 μM. In addition, LPA treatment significantly increased the proliferation of MSCs (Fig. 1D). To investigate the role of TAZ in LPA-induced migration and proliferation, we evaluated the effects of small interfering RNA (siRNA)-mediated knockdown of TAZ on migration and proliferation of MSCs. LPA-induced expression of TAZ was completely abrogated by transfection with TAZ-targeting siRNA (Fig. 1E). Furthermore, knockdown of TAZ markedly inhibited the LPA-induced migration and proliferation of MSCs (Fig. 1F, 1G). These results suggest the involvement of TAZ in LPA-induced migration and proliferation of MSCs.
TAZ knockout MEFs exhibit decreased cellular responses to LPA treatment
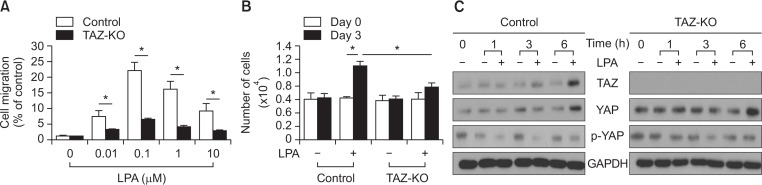
To verify whether TAZ is involved in LPA-induced cellular responses, we examined the effects of LPA on cell migration and proliferation in TAZ knockout and wild type MEFs. As shown in Fig. 2A, LPA treatment led to the increase of migration of wild type MEFs with a maximum stimulation at 0.1 μM. However, the LPA-induced migration was greatly attenuated in TAZ-knockout MEFs. When the proliferation of MEFs was analyzed, TAZ-knockout MEFs showed a significant decrease in LPA-stimulated proliferation (Fig. 2B).
Fig. 2.
Role of TAZ in LPA-induced migration and proliferation of MEFs. (A) Wild-type MEFs (control) and TAZ-knockout MEFs (TAZ-KO) were treated with the various concentrations of LPA for 12 h, followed by a chemotactic migration assay. Data indicate mean ± SD. *p<0.05 (n=8). (B) Inhibition of LPA-induced proliferation in the TAZ-knockout MEFs is shown. Wild-type MEFs (control) and TAZ-knockout MEFs (TAZ-KO) were treated with vehicles or LPA (10 μM) for 3 days. The number of cells were counted and compared with those of the control cells. TAZ-knockout MEFs exhibited decreased LPA-induced proliferation for 3 days. The cellular proliferation rate was determined via cell counting using trypan blue inclusion assay. Data indicate mean ± SD. *p<0.05 (n=6). (C) Time-course of LPA-induced TAZ expression is shown. Wild-type MEFs (control) and TAZ-knockout MEFs (TAZ-KO) were treated with vehicle or LPA (10 μM) for the indicated periods. The expression levels of TAZ and GAPDH were determined using Western blotting.
To investigate the effects of LPA on Hippo signaling in MEFs, the expression of TAZ, YAP and the phosphorylation of YAP were analyzed in TAZ-knockout MEFs in comparison with the control MEFs. As shown in Fig. 2C, the expression levels of TAZ and YAP increased 6 h after LPA treatment and the phosphorylation of YAP decreased 1 h following LPA treatment in the control MEFs. TAZ-knockout MEFs did not express TAZ in the absence or presence of LPA, whereas the expression of YAP increased at 6 h after LPA treatment. Taken together, these results suggest that TAZ plays a key role in LPA-induced migration and proliferation of MEFs.
LPAR1 is required for the LPA-induced regulation of hippo signaling and cellular responses in MSCs
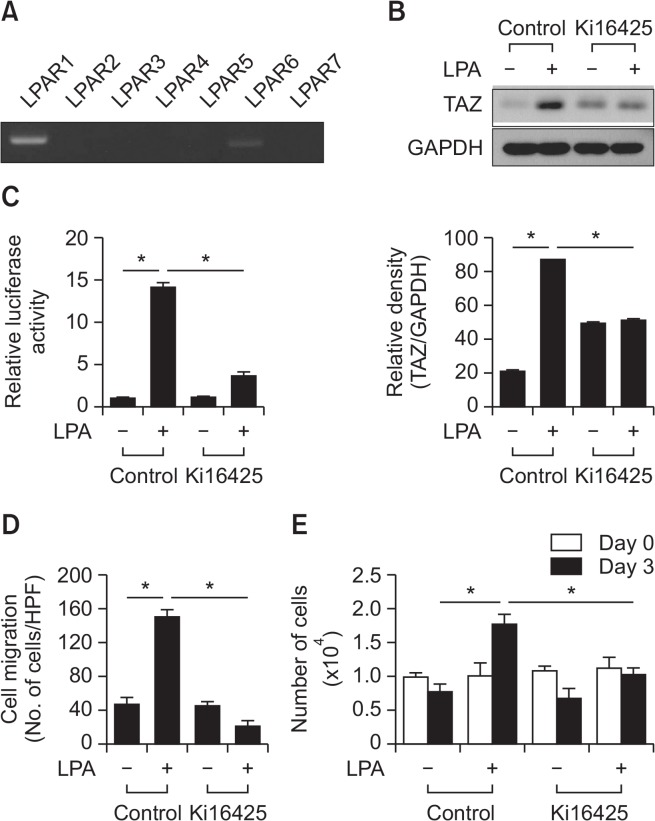
We next attempted to determine whether LPA receptors (LPARs) are necessary for the LPA-induced molecular and cellular responses of MSCs. The expression of seven different LPARs (LPAR1-7) in MSCs was tested using RT-PCR, and the results showed that LPAR1 was the most abundantly expressed in MSCs (Fig. 3A). To investigate the involvement of LPAR1 in mediating LPA-induced cellular response, the effects of Ki16425, an inhibitor of LPAR1 and LPAR3 (Ohta et al., 2003), on the LPA-induced TAZ expression and cellular responses. As shown in Fig. 3B, LPA treatment increased the expression of TAZ. However, pretreatment of MSCs with Ki16425 abrogated the LPA-induced TAZ expression. To investigate whether LPA could regulate hippo signaling, we examined the effects of LPA on the YAP/TAZ-responsive reporter activities in MSCs. Cells were transfected with a reporter construct of YAP/TAZ-responsive synthetic promoter driving luciferase expression (8×GTIIC-luciferase), followed by measurement of luciferase activities after LPA treatment. As shown in Fig. 3C, LPA treatment increased YAP/TAZ-dependent reporter activity, whereas blockade of LPA signaling by Ki16425 significantly attenuated the LPA-induced promoter activity. When cellular chemotactic migration was analyzed, blockade of LPA signaling by Ki16425 significantly inhibited LPA-induced migration of MSCs (Fig. 3D). We next tested the effect of Ki16425 on the LPA-induced proliferation of MSCs. The LPA-induced proliferation of MSCs was abrogated by pretreatment with Ki16425 (Fig. 3E). These results suggest that LPAR1 plays a role in the LPA-induced expression of TAZ and cellular responses in MSCs.
Fig. 3.
Involvement of LPAR1 in the LPA-induced migration and proliferation of MSCs. (A) The expression level of the LPA receptors was determined using RT-PCR. (B) MSCs were treated with vehicle control or Ki16425 (10 μM), LPAR1 and 3 inhibitor, followed by Western blotting analysis with indicated antibodies (left panel). Quantification of TAZ expression normalized by GAPDH expression is shown (right panel). Data indicate mean ± SD. *p<0.05 (n=4). (C) YAP/TAZ-responsive reporter (8×GTIIC-luciferase) activities in MSCs after treatment of LPA (10 μM) in the presence or absence of 10 μM Ki16425 are shown. Data indicate mean ± SD. *p<0.05 (n=4). (D) The inhibitory effect of Ki16425 (10 μM) on LPA-induced (1 μM) MSC migration is shown. Data indicate mean ± SD. *p<0.05 (n=8). (E) The inhibitory effect of Ki16425 (10 μM) on LPA-induced (10 μM) MSC proliferation is shown. The cell proliferation rate was determined by cell count using a trypan blue inclusion assay. Data indicate mean ± SD. *p<0.05 (n=6).
MEK and ROCK signaling are involved the LPA-regulated hippo signaling and cellular responses
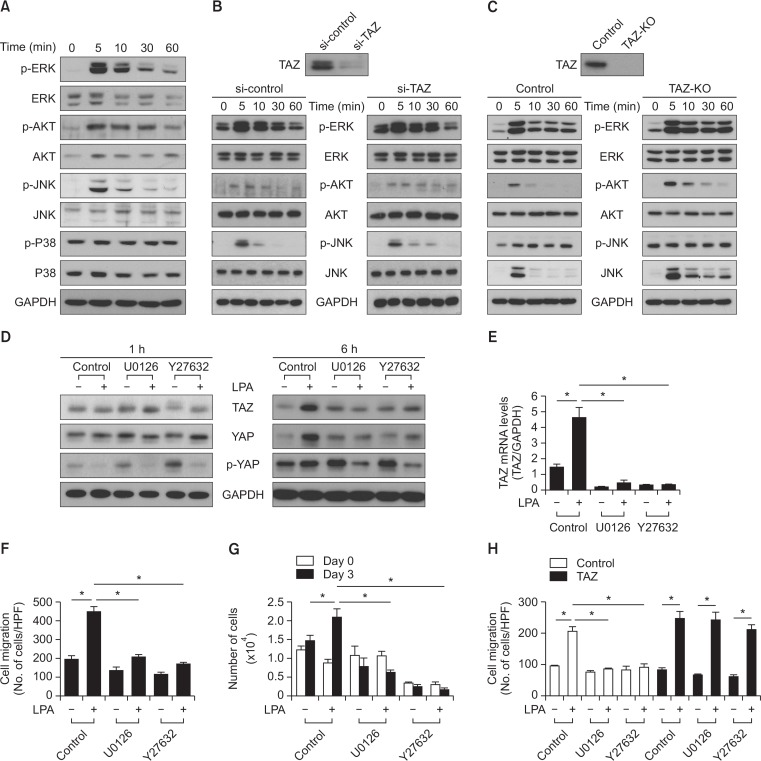
ERK and AKT are major signal transduction molecules involved in various cellular responses, including cell death, cell survival, proliferation, and migration (Chen et al., 2008; Li et al., 2010b). When MSCs were treated with LPA, the phosphorylation of ERK, JNK, and AKT were detected at 5 min after LPA treatment (Fig. 4A). Unlike ERK, JNK, or AKT, the phosphorylation of p38 was not affected by LPA treatment. Therefore, we next examined whether TAZ had an effect on LPA-induced activation of ERK, AKT, and JNK. siRNA-mediated silencing of TAZ expression had no significant effects on the LPA-induced phosphorylation of ERK, AKT, and JNK (Fig. 4B). To confirm these results, we next examined the effects of LPA on the phosphorylation levels of the protein kinases in TAZ-knockout MEFs. Knockout of TAZ had no significant effects on the LPA-induced phosphorylation of ERK, JNK, and AKT at the early time points (5 min) with the sustained phosphorylation of JNK and AKT at the later time points (Fig. 4C). These results suggest that TAZ is not required for the activation of LPA-induced activation of ERK, AKT, and JNK.
Fig. 4.
Effects of the MEK inhibitor and the ROCK inhibitor on LPA-induced regulation of hippo signaling in MSCs. (A) The LPA stimulated phosphorylation of MAP kinases in MSCs is shown. MSCs were treated with LPA (10 μM) for the indicated times and subjected to Western blotting analysis with indicated antibodies. (B) siRNA-mediated silencing of TAZ expression is shown. LPA-stimulated phosphorylation of MAP kinases in both the control and TAZ knockdown-MSCs. (C) The wild-type MEFs (control) and TAZ-knockout MEFs (TAZ-KO) were treated with LPA (10 μM) for the indicated times and subjected to Western blotting analysis. The expression level of the indicated antibodies and GAPDH were determined using Western blotting. (D) The effect of U0126 and Y-27632 on LPA-induced TAZ, YAP, and p-YAP expressions are shown. MSCs were treated with vehicle control, U0126 (10 μM) or Y-27632 (10 μM), followed by stimulation with LPA (10 μM) for the indicated times. The expression levels of TAZ, YAP, p-YAP, and GAPDH were determined by Western blotting analysis. (E) Real-time PCR analysis revealed the mRNA expression levels of TAZ in MSCs. MSCs were pretreated with U0126 (10 μM) and Y-27632 (10 μM), followed by treatment with LPA (10 μM) for 6 h. (F) The effect of U0126 (10 μM) and Y-27632 (10 μM) on LPA-induced (1 μM) migration of MSCs. Data indicate mean ± SD. *p<0.05 (n=8). (G) The effect of U0126 (10 μM) and Y-27632 (10 μM) on the LPA-induced (10 μM) cellular proliferation of MSCs. The cellular proliferation rate was determined by cell counting using a trypan blue inclusion assay. Data indicate mean ± SD. *p<0.05 (n=6). (H) TAZ overexpression reversed the inhibitory effect of U0126 (10 μM) or Y27632 (10 μM) on LPA-induced (1 μM) MSCs migration. Data indicate mean ± SD. *p<0.05 (n=8).
We investigated the signaling pathways implicated in the LPA-induced modulation of hippo signaling and cellular responses. When AKT signaling was blocked by the PI3K inhibitor, LY294002, there was no significant effect on the increase of TAZ expression 6 h after LPA treatment (data not shown). Because Rho GTPase has been reported to be involved in LPA-regulated Hippo signaling (Yu et al., 2012), we tested the effects of not only the MEK inhibitor U0126 but also the ROCK inhibitor Y27632 on LPA-regulated hippo signaling and cellular responses. When MSCs were treated with the U0126 MEK inhibitor or Y27632 in combination with LPA, both U0126 and Y27632 had no effect on the LPA-induced de-phosphorylation of YAP after 1 h treatment. The LPA-stimulated protein levels of TAZ were not affected by treatment with U0126 or Y27632 treatment at 1 h; however, the TAZ expression was attenuated by the treatment of U0126 or Y27632 after 6 h treatment (Fig. 4D). Consistently, LPA-induced mRNA levels of TAZ were also inhibited by treatment with U0126 or Y27632 (Fig. 4E). Interestingly, the LPA-induced decrease of YAP phosphorylation was not restored at 6 h in the presence of U0126 or Y27632 (Fig. 4D), suggesting a key role of ERK and ROCK in the phosphorylation of YAP. Taken together, these results propose that both ERK and ROCK are involved in the LPA-induced expression of TAZ through activation of gene transcription.
We next analyzed the effect of the inhibition of MEK or ROCK signaling on LPA-induced cell migration and proliferation. Inhibition of MEK or ROCK abrogated the LPA-induced migration of MSCs (Fig. 4F). Moreover, inhibition of MEK or ROCK completely blocked the LPA-induced proliferation of MSCs (Fig 4G). These results suggest that MEK and ROCK signaling play an important role in mediating LPA-induced migration and proliferation of MSCs.
To further clarify whether TAZ plays a role in LPA-induced cell migration, TAZ was transiently overexpressed in MSCs, followed by treatment with LPA in the absence or presence of U0216 and Y27632. As shown in Fig. 4H, inhibition of MEK or ROCK reduced LPA-induced migration of MSCs. However, overexpression of TAZ abrogated the inhibition of LPA-induced migration of MSCs by treatment with U0126 or Y27632. These results suggest that LPA treatment stimulates migration of MSCs through mechanisms involving ERK- and ROCK-dependent TAZ expression.
DISCUSSION
In the present study, we demonstrated LPA stimulated migration and proliferation of human MSCs by activating TAZ expression. The effects of LPA on cellular responses on MSCs significantly reduced by LPAR1 blockade. Furthermore, MEK or ROCK inhibition in MSCs decreased LPA-induced TAZ expression, migration, and proliferation. Recently, MSCs have gained considerable interest because the transplantation of MSCs has shown significant therapeutic potential in regenerating and repopulating injured tissues (Orlic et al., 2001; Mangi et al., 2003). Although MSCs are the ideal resource in regenerative medicine because they can be readily obtained for autologous transplantation, the largest hurdle is the loss of MSCs following transplantation due to cell death presumably induced by the ischemic environment into which MSCs are introduced (Geng, 2003). LPA is a natural phospholipid, which has been found to accumulate following various pathologic conditions including acute myocardial infarction, fibrosis, and cancer (Chen et al., 2003; Willier et al., 2013; Tang et al., 2014). In addition, LPA plays a substantial modulatory role in the cellular responses such as proliferation, survival, differentiation, and maintenance of stem cells and progenitor cells (Pebay et al., 2007). LPA has recently been reported to reduce the apoptosis of MSCs under hypoxic conditions (Chen et al., 2008; Liu et al., 2009; Li et al., 2010b; Binder et al., 2014). Moreover, LPA enhanced the migration of resident MSCs through the β-catenin signaling pathway (Badri and Lama, 2012). These reports support the conclusion of the current study, in which LPA promotes MSCs migration and proliferation. Our results may provide a novel application of LPA in enhancing the therapeutic potential of MSCs for the development of regenerative medicine.
LPA is a glycerophospholipid known to regulate the Hippo-YAP pathway, which is involved in controlling organ size, regeneration, tumorigenesis, and stem cell function (Yu et al., 2012; Johnson and Halder, 2014). Moreover, both YAP and TAZ are transcriptional co-activators and regarded as functionally redundant because of high homology and a shared regulation mechanism (Varelas, 2014); however, accumulating evidence also indicates that there are divergent functions of YAP and TAZ (Cordenonsi et al., 2011). Multiple post-translational modifications, mainly phosphorylation, control the localization and activity of YAP and TAZ. The functions of YAP and TAZ are often context-dependent, and the events that distinctly regulate YAP or TAZ have been poorly explained to date. We observed a strong induction of TAZ expression at 6 h after LPA treatment in MSCs. Although YAP expression also increased following LPA stimulation, de-phosphorylation, which is required for the activation of YAP, was not observed at 6 h. Moreover, treatment with the MEK or ROCK inhibitor decreased YAP phosphorylation in response to LPA stimulation, which inhibited LPA-induced cellular responses as the net result. Because the knockdown or knockout of TAZ alone was sufficient to block LPA-induced cellular proliferation and migration, YAP did not exhibit redundancy in the absence of TAZ. These results suggest that in LPA-induced responses of MSCs, TAZ and YAP may have an additive effect to rapidly induce cellular responses or have distinct roles.
The extensive death of MSCs following transplantation remains a major obstacle to the success of MCS-based treatment for tissue repair. In previous reports, LPA has been shown to inhibit hypoxia and serum deprivation-induced apoptosis of MSCs, which was found to be dependent on both ERK1/2 and PI3K/AKT signaling (Chen et al., 2008). In addition, p38 activation was inhibited by LPA for antagonizing hypoxia and serum deprivation-induced apoptosis and was independent of PI3K/AKT pathway (Li et al., 2010b). We treated MSCs with LPA under normoxic conditions without changing the media and observed that LPA stimulation did not decrease the phosphorylation of p38. Moreover, inhibition of PI3K by LY294002 did not stably block the LPA-induced increase of TAZ expression (data not shown). However, the effect of LPA was blocked by the inhibition of MEK with U0126 or ROCK with Y27632. These results suggest that LPA may use different pathways when promoting the proliferation and the migration of MSCs under normoxic conditions, which is the common culture condition for expanding and maintaining MSCs prior to transplantation.
In summary, we observed that LPA stimulation increases the expression of TAZ in MSCs, as well as the proliferation and migration of MSCs. A knockdown or knockout of TAZ demonstrated that TAZ is necessary for LPA-induced cellular responses. Furthermore, MEK and ROCK are also involved in LPA-induced molecular and cellular responses. Our findings may provide a novel opportunity for intervention through the preparation of MSCs for successful treatment in the field of regenerative medicine.
Acknowledgments
This work was supported by two year grant of Pusan National University.
Footnotes
CONFLICT OF INTEREST
Authors declare no conflict of interest.
REFERENCES
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- Anliker B, Chun J. Cell surface receptors in lysophospholipid signaling. Semin Cell Dev Biol. 2004;15:457–465. doi: 10.1016/j.semcdb.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Badri L, Lama VN. Lysophosphatidic acid induces migration of human lung-resident mesenchymal stem cells through the β-catenin pathway. Stem Cells. 2012;30:2010–2019. doi: 10.1002/stem.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder BY, Genetos DC, Leach JK. Lysophosphatidic acid protects human mesenchymal stromal cells from differentiation-dependent vulnerability to apoptosis. Tissue Eng. Part A. 2014;20:1156–1164. doi: 10.1089/ten.tea.2013.0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SW, Lim CJ, Guo K, Ng CP, Lee I, Hunziker W, Zeng Q, Hong W. A role for TAZ in migration, invasion, and tumorigenesis of breast cancer cells. Cancer Res. 2008;68:2592–2598. doi: 10.1158/0008-5472.CAN-07-2696. [DOI] [PubMed] [Google Scholar]
- Chen J, Baydoun AR, Xu R, Deng L, Liu X, Zhu W, Shi L, Cong X, Hu S, Chen X. Lysophosphatidic acid protects mesenchymal stem cells against hypoxia and serum deprivation-induced apoptosis. Stem Cells. 2008;26:135–145. doi: 10.1634/stemcells.2007-0098. [DOI] [PubMed] [Google Scholar]
- Chen X, Yang XY, Wang ND, Ding C, Yang YJ, You ZJ, Su Q, Chen JH. Serum lysophosphatidic acid concentrations measured by dot immunogold filtration assay in patients with acute myocardial infarction. Scand J Clin Lab Invest. 2003;63:497–503. doi: 10.1080/00365510310003265. [DOI] [PubMed] [Google Scholar]
- Cordenonsi M, Zanconato F, Azzolin L, Forcato M, Rosato A, Frasson C, Inui M, Montagner M, Parenti AR, Poletti A, Daidone MG, Dupont S, Basso G, Bicciato S, Piccolo S. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147:759–772. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- Cselenyak A, Pankotai E, Horvath EM, Kiss L, Lacza Z. Mesenchymal stem cells rescue cardiomyoblasts from cell death in an in vitro ischemia model via direct cell-to-cell connections. BMC Cell Biol. 2010;11:29. doi: 10.1186/1471-2121-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel A, Mills GB. Peptide and lipid growth factors decrease cis-diamminedichloroplatinum-induced cell death in human ovarian cancer cells. Clin Cancer Res. 1996;2:1307–1313. [PubMed] [Google Scholar]
- Geng YJ. Molecular mechanisms for cardiovascular stem cell apoptosis and growth in the hearts with atherosclerotic coronary disease and ischemic heart failure. Ann N Y Acad Sci. 2003;1010:687–697. doi: 10.1196/annals.1299.126. [DOI] [PubMed] [Google Scholar]
- Halder G, Johnson RL. Hippo signaling: growth control and beyond. Development. 2011;138:9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Chun A, Cheung K, Rashidi B, Yang X. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J Biol Chem. 2008;283:5496–5509. doi: 10.1074/jbc.M709037200. [DOI] [PubMed] [Google Scholar]
- Hong W, Guan KL. The YAP and TAZ transcription coactivators: key downstream effectors of the mammalian Hippo pathway. Semin Cell Dev Biol. 2012;23:785–793. doi: 10.1016/j.semcdb.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Haney N, Kropp D, Kabore AF, Johnston JB, Gibson SB. Lysophosphatidic acid (LPA) protects primary chronic lymphocytic leukemia cells from apoptosis through LPA receptor activation of the anti-apoptotic protein AKT/PKB. J Biol Chem. 2005;280:9498–9508. doi: 10.1074/jbc.M410455200. [DOI] [PubMed] [Google Scholar]
- Iyer SS, Rojas M. Anti-inflammatory effects of mesenchymal stem cells: novel concept for future therapies. Expert Opin Biol Ther. 2008;8:569–581. doi: 10.1517/14712598.8.5.569. [DOI] [PubMed] [Google Scholar]
- Johnson R, Halder G. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat Rev Drug Discov. 2014;13:63–79. doi: 10.1038/nrd4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Sarojini H, An J, Wang E. Prosaposin in the secretome of marrow stroma-derived neural progenitor cells protects neural cells from apoptotic death. J Neurochem. 2010a;112:1527–1538. doi: 10.1111/j.1471-4159.2009.06565.x. [DOI] [PubMed] [Google Scholar]
- Li Z, Wei H, Liu X, Hu S, Cong X, Chen X. LPA rescues ER stress-associated apoptosis in hypoxia and serum deprivation-stimulated mesenchymal stem cells. J Cell Biochem. 2010b;111:811–820. doi: 10.1002/jcb.22731. [DOI] [PubMed] [Google Scholar]
- Lindroos B, Suuronen R, Miettinen S. The potential of adipose stem cells in regenerative medicine. Stem Cell Rev. 2011;7:269–291. doi: 10.1007/s12015-010-9193-7. [DOI] [PubMed] [Google Scholar]
- Liu CY, Zha ZY, Zhou X, Zhang H, Huang W, Zhao D, Li T, Chan SW, Lim CJ, Hong W, Zhao S, Xiong Y, Lei QY, Guan KL. The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCF{beta}-TrCP E3 ligase. J Biol Chem. 2010;285:37159–37169. doi: 10.1074/jbc.M110.152942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hou J, Shi L, Chen J, Sang J, Hu S, Cong X, Chen X. Lysophosphatidic acid protects mesenchymal stem cells against ischemia-induced apoptosis in vivo. Stem Cells Dev. 2009;18:947–954. doi: 10.1089/scd.2008.0352. [DOI] [PubMed] [Google Scholar]
- Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:e54. doi: 10.1038/emm.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta H, Sato K, Murata N, Damirin A, Malchinkhuu E, Kon J, Kimura T, Tobo M, Yamazaki Y, Watanabe T, Yagi M, Sato M, Suzuki R, Murooka H, Sakai T, Nishitoba T, Im DS, Nochi H, Tamoto K, Tomura H, Okajima F. Ki16425, a subtype-selective antagonist for EDG-Family lysophosphatidic acid receptors. Mol Pharmacol. 2003;64:994–1005. doi: 10.1124/mol.64.4.994. [DOI] [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci USA. 2001;98:10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overholtzer M, Zhang J, Smolen GA, Muir B, Li W, Sgroi DC, Deng CX, Brugge JS, Haber DA. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci USA. 2006;103:12405–12410. doi: 10.1073/pnas.0605579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pebay A, Bonder CS, Pitson SM. Stem cell regulation by lysophospholipids. Prostaglandins Other Lipid Mediat. 2007;84:83–97. doi: 10.1016/j.prostaglandins.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Ramos A, Camargo FD. The Hippo signaling pathway and stem cell biology. Trends Cell Biol. 2012;22:339–346. doi: 10.1016/j.tcb.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N, Zhao Y, Feng R, Liu Y, Wang S, Wei W, Ding Q, An MS, Wen J, Li L. Lysophosphatidic acid accelerates lung fibrosis by inducing differentiation of mesenchymal stem cells into myofibroblasts. J Cell Mol Med. 2014;18:156–169. doi: 10.1111/jcmm.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meeteren LA, Moolenaar WH. Regulation and biological activities of the autotaxin-LPA axis. Prog Lipid Res. 2007;46:145–160. doi: 10.1016/j.plipres.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Varelas X. The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development. 2014;141:1614–1626. doi: 10.1242/dev.102376. [DOI] [PubMed] [Google Scholar]
- Willier S, Butt E, Grunewald TG. Lysophosphatidic acid (LPA) signalling in cell migration and cancer invasion: a focussed review and analysis of LPA receptor gene expression on the basis of more than 1700 cancer microarrays. Biol. Cell. 2013;105:317–333. doi: 10.1111/boc.201300011. [DOI] [PubMed] [Google Scholar]
- Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, Fu XD, Mills GB, Guan KL. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]