Abstract
Autism spectrum disorder (ASD) remains unexplained and untreated despite the high attention of research in recent years. Aside from its various characteristics is the baffling male preponderance over the female population. Using a validated animal model of ASD which is the telomerase reverse transcriptase overexpressing mice (TERT-tg), we conducted ASD-related behavioral assessments and protein expression experiments to mark the difference between male and females of this animal model. After statistically analyzing the results, we found significant effects of TERT overexpression in sociability, social novelty preference, anxiety, nest building, and electroseizure threshold in the males but not their female littermates. Along these differences are the male-specific increased expressions of postsynaptic proteins which are the NMDA and AMPA receptors in the prefrontal cortex. The vGluT1 presynaptic proteins, but not GAD, were upregulated in both sexes of TERT-tg mice, although it is more significantly pronounced in the male group. Here, we confirmed that the behavioral effect of TERT overexpression in mice was male-specific, suggesting that the aberration of this gene and its downstream pathways preferentially affect the functional development of the male brain, consistent with the male preponderance in ASD.
Keywords: Sex difference, TERT transgenic mice, Autism spectrum disorder, Synapse, Excitatory/Inhibitory imbalance
INTRODUCTION
Neurodevelopmental disorders have increasingly been in the spotlight of neuroscience research due to their increasing prevalence, lifetime effect, and barely understood pathologic mechanisms. Autism spectrum disorder (ASD) is on the top spot among them for its complexity, heterogeneity, and lack of therapeutic treatments. ASD is diagnosed based on the two core symptoms of social communication deficits and repetitive/stereotypic behaviors (American Psychiatric Association, 2013). ASD also has various comorbidities such as anxiety (Storch et al., 2012), ADHD, seizure, depression (Bakken et al., 2010), and learning disorders (Larson et al., 2010). Aside from the lack of a connecting mechanism for ASD, one of the biggest questions is the yet unexplained high prevalence in the male population over females (Kim et al., 2011). Despite this discrepancy, only a few researchers have delved into investigating the reasons behind the sex-differential phenomenon.
Some researchers attempted to explain that females have some “Female Protective Effect” (FPE) which shields them from a load of genetic interruption or other etiologic factors enough to cause ASD in males (Skuse, 2000; Robinson et al., 2013; Jacquemont et al., 2014). Thus, most females who reached the diagnosis of autism have higher mutational load and severity than their male counterparts (Jacquemont et al., 2014). In our previous studies using the valproic acid (VPA) induced rat model of ASD, we found autistic-like phenotypes in a male-dependent manner (Kim et al., 2013, 2016a). VPA-exposed male rats have downregulated methyl CpG binding protein 2 (MeCP2) expression in the brain cortex during early development which could be responsible for the overexpression of glutamate receptors (Kim et al., 2016a). Thus, by the FPE theory, female mice could have the natural physiological capacity to resist abnormal changes in brain development caused by environmental insults. In the same manner, the identification of sex differences in the behavior of a genetic animal model is of great interest and can present straightforward links in the underlying mechanisms of ASD.
Telomerase is a ribonucleoprotein which consists of an RNA (non-coding RNA; TER) and protein (telomerase reverse transcriptase, TERT) components (Giardini et al., 2014). TER is a non-coding RNA that is essential for telomere synthesis while TERT is a protein component that acts as a reverse transcriptase. When telomeres shorten during repeated cell cycles, telomerase adds the TTAGGG repeat to elongate the telomeres and continue cell proliferation. Telomerase is considered to be closely related to the proliferation of stem cells such as tumor cells and neuronal stem cells (Kim et al., 1994). TERT is expressed in proliferating cells and its expression level normally goes down at cellular differentiation stages (Harley et al., 1990). When TERT expression was maintained in human fibroblasts, the cells underwent robust replications with reduced senescence (Bodnar et al., 1998). In the brain, TERT plays a role in the prevention of neuronal apoptosis from ischemic brain injury (Kang et al., 2004). In contrast, TERT knockout in mice resulted in a marked decline of neuronal stem cell proliferation along with specific impairments in neuronal differentiation and survival (Ferron et al., 2009). Previously, we have reported that the TERT overexpressing transgenic mice (TERT-tg) have ASD-like behavioral phenotypes and have increased glutamatergic neuronal differentiation, increased postsynaptic protein expressions and increased excitability in the prefrontal cortex (Kim et al., 2016b). All of these studies confirm the involvement of TERT in neuronal development and could be implicated in the excitatory/inhibitory imbalance theory of ASD (Rubenstein and Merzenich, 2003). Moreover, it is paramount to investigate whether TERT overexpression in mice has sex-differential effects, related to the male preponderance bias in ASD. Hence, this study is directed to this point of inquiry, and the results could add knowledge to the mechanisms surrounding male preponderance and the female protective effect in ASD. We asked whether the autistic-like phenotypes in TERT-tg mice is male-specific. We also assessed the postsynaptic excitatory neuronal markers of the TERT-tg mice to explore the possible mechanisms involved.
MATERIALS AND METHODS
Animals
We bred a total of 20 pairs of TERT-tg male and female adult mice to produce TERT-tg offspring used in different sets of experiments. TERT overexpressing mice have an FVB background, so we purchased pregnant FVB wild-type mice at embryonic day 10 (DaeHan BioLink, Daejeon, Korea) and their offspring served as the controls. We arranged the time of pregnancy during purchase so that FVB mice will give birth nearest to the time that the TERT-tg mice will give birth. The generation and identification of TERT-tg mice (FVB/N genetic background) along with their behavioral features was described previously (Kang et al., 2004; Kim et al., 2016b). Animals were kept in a room with automatic 12-h light cycle (lights on at 06:00), at a certain range of temperature (22 ± 2°C) and humidity (55 ± 5%). Animal care and treatment were approved by the Animal Care and Use Committee of Konkuk University, Seoul, Korea (KU12016) and were carried out in accordance with the Principle of Laboratory Animal Care (NIH publication No. 85-23, revised 1985). The number of animals used in this study was kept to a minimum. Behavioral experiments were done between 10:00 and 16:00 o’clock in a dedicated test room and all animals were acclimated for 1 h before the actual tests.
Experimental design
All offspring of TERT-tg and FVB wild-type mice were weaned and grouped at postnatal day 21 (P21). In each litter, male and female mice were randomly selected to make experimental groups according to sex and genotype for a total of four groups. The number of animals in each group depends on the type of test performed as indicated in the tests below. All behavior tests and neurochemical analysis were performed during the mice juvenile age of 4 weeks. Different sets of mice were used for each behavior test, and another set was used for western blot analysis of the brain cortex.
Social interaction test
The social interaction test was adapted from Silverman et al. (2010) and was performed in 4 weeks old mice. The test mice were chosen randomly from each litter during the weaning period. A three-chamber apparatus was used for the trial where each side chamber can be accessed through an opening connected to the center area. Three phases completed one trial. First was the 5-min habituation period. Second was the 10-min sociability test which allowed the subject to explore all chambers with one side housing a stranger mouse inside a wire cage and the other end with an empty wire cage. Third was the social novelty preference test which introduced a novel stranger mouse in the previously empty cage for the subject mouse to explore for another 10 min. The stranger mice used were wild type FVB mice of the same age and sex with the test mouse. In each test, the total duration in each compartment was measured using the Ethovision software (Noldus, VA, USA). In separate trials using the same test conditions, the sniffing length of the subject mouse to each wire cage (nose and cage distance within 2 cm) was measured by an experimenter “blind” to the experimental conditions of the mice.
Open field locomotor activity
An open field test was conducted in 4 weeks old mice naïve from any other test. The apparatus consisted of five open fields made of plastic boxes (42×42 cm) with 42 cm-high side walls. Mice were placed into the test boxes for a 5-min habituation before the actual recording. The total distance moved, and the duration of movement was tracked for 10 min (Noldus et al., 2001; Park et al., 2007) using a charge-coupled device (CCD) camera-assisted motion tracking apparatus and software (EthoVision 3.1, Noldus information Technology, the Netherlands). The time spent and movement duration in the center area, defined as the central 20×20 cm square in the field, were also recorded as measures of anxiety-like behaviors.
Elevated plus maze
Four weeks old mice were tested for anxiety behavior in the elevated plus maze (EPM). The maze consists of four perpendicular arms that comprise two open arms (30×6 cm) and two close arms (30×6 cm) enclosed by 20 cm high walls. Each arm meets in a central delimited area of 6×6 cm. The EPM was elevated to 50 cm above the floor. The subject mice were placed in the center of the maze facing one of the open arms to explore the maze for 5 min. An arm entry is defined as the entry of all 4 paws to the lined boundary of each arm. The observed parameters included the time spent and the number of entries into the open arms (Pellow and File, 1986). The percentage of open arms exploration against the exploration of all arms was calculated.
Nest building
The assessment of mice’s ability to build a nest was based on a method previously reported (Deacon, 2012). The test was done when mice were at the age of 4 weeks. The floor of each cage was covered with 0.5 cm deep corncob bedding and a ‘Nestlet’ on the surface. The Nestlet is a 5 cm square of pressed cotton batting provided by Ancare. Each subject mouse was placed into individual nesting cages about one hour before the dark phase and carefully removed the next morning to assess the formed nest. The nests were scored on a 5-point scale, and the amount of untorn Nestlet was weighed.
Electroshock seizure threshold test
The electroshock seizure threshold was evaluated in the four weeks old mice, as reported previously (Park et al., 2007). The seizure was induced by a constant current stimulator, and the subsequent seizure was considered full when there is an overt hind limb extension. A convulsive current 50 (CC50) eliciting convulsion to 50% of mice, was elicited through a ‘staircase’ procedure to determine the electroshock seizure threshold (Browning et al., 1990). The results were calculated by the Litchfield-Wilcoxon II method (Litchfield and Wilcoxon, 1949). Mice were individually given electroshocks through ear clips for 1 s to determine the current-convulsion relationship.
Mouse brain dissection
At four weeks of postnatal age, one mouse from 4 litters of each group was sacrificed for protein analysis of the prefrontal cortex (PFC), a region implicated in executive and social behaviors functions. Mice were anesthetized by inhalation of ethyl ether followed by decapitation. The skull was carefully opened to gain access to the whole brain. The whole brain was taken out using curved forceps starting from the olfactory bulb area and making sure that no mechanical damage is caused. The entire prefrontal cortex section, anatomically defined as the frontal part of the frontal lobe in the cerebral cortex, was isolated following a previously established method (Spijker, 2011). Each isolated PFC was put in a 1.7 ml Eppendorf tube and snap-frozen with liquid nitrogen then stored in the −80°C freezer until used for analysis.
Western blot analysis
PFC tissues of mice at four weeks of age were homogenized with 500 μl homogenization buffer (50 mM Tris-HCl [pH.7.4], 150 mM NaCl, and protein inhibitor cocktail). BCA assay was performed to measure the protein concentration for each sample. Aliquots containing 50 μg of total proteins were separated by 10% SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blocked with 1% polyvinyl alcohol in PBS including 0.2% tween-20 for 1 h. We then incubated the membranes overnight with primary antibody at 4°C and then with peroxidase-conjugated secondary antibody (Santa Cruz, CA, USA) for 2 h at room temperature. Specific bands were detected using the ECL system (Amersham, Buckinghamshire, UK) and exposed to the Bio-rad electrophoresis image analyzer (Bio-rad, Hemel Hampstead, UK).
Antibodies
Antibodies were purchased from the following companies: anti-β-actin from Sigma (St. Louis, MO, USA), vGluT1, GluR1, GluR2 antibodies from Abcam (Cambridgeshire, England), GAD, NR1, NR2A, NR2B antibodies from Millipore (Billerica, MA, USA), and Tuj-1 antibody from Covance (Princeton, NJ, USA).
Statistical analysis
The results of each test were reflected in the graphs as the mean ± standard error of mean (SEM). We analyzed the statistical significance of each data using one-way analysis of variance (ANOVA) followed by Newman-Keuls test as a posthoc test. Two-way ANOVA was used to identify genotype or sex effects followed by Bonferroni’s post tests as posthoc comparisons. Statistical differences were considered significant when the p-value was less than 0.05 (p<0.05). All statistical analyzes were conducted using PASW Statistics (18.0; SPSS Inc., Chicago, IL, USA).
RESULTS
TERT-tg mice displayed male-specific impairment in sociability
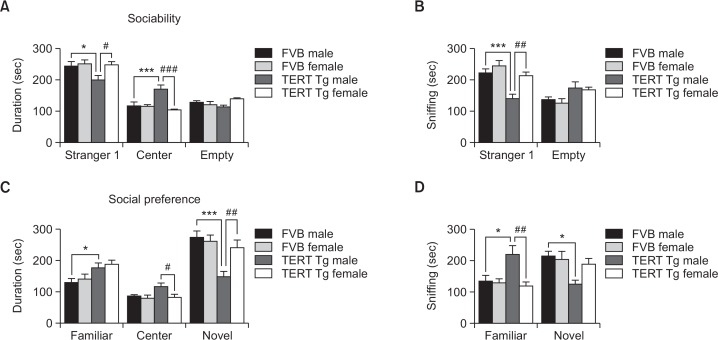
In the sociability test (Fig. 1A), two-way ANOVA revealed that the stay duration in the stranger mouse side differed according to sex [F(1,36)=4.287, p=0.046]. Post-hoc comparisons showed that TERT-tg males stayed less in the stranger mouse side than the control FVB males (p<0.01) and the TERT-tg females (p<0.05). In the empty compartment, posthoc comparisons revealed that TERT-tg males stayed more in the central compartment than the control FVB males (p<0.001) or the TERT-tg females (p<0.001). In separate trials, we measured the sniffing duration of subject mice to the stranger mouse or empty wire cage stimulus as a more sensitive parameter of sociability (Fig. 1B). Sniffing to the stranger mouse was significantly different according to genotype [F(1,36)=17.48, p=0.0002] and sex [F(1,36)=12.75, p=0.001]. The post hoc results made it clearer that TERT-tg males have decreased sniffing interaction to the stranger mouse compared to the FVB males (p<0.001) and TERT-tg females (p<0.01). Sniffing to the empty cage did not significantly differ between each group. These results demonstrated that the TERT-tg males, but not female, mice display less social interest than their FVB control counterparts.
Fig. 1.
Male-specific social impairments in TERT transgenic mice. (A, B) Sociability test. The total duration in each chamber (A) and duration of sniffing to wire cages (B) in the presence of a stranger mouse in one wire cage were compared between male and female TERT-tg mice versus the control groups. (C, D) Social novelty preference test. The total duration in each chamber (C) and the length of sniffing to wire cages (D) in the presence of a familiar or novel mouse were also evaluated. All data are expressed as the mean ± SEM. One male, and one female mouse was chosen from each of 10 litters for a total 10 mice per group. *p<0.05, ***p<0.001 vs. FVB male group; #p<0.05, ##p<0.01, ###p<0.001 vs. TERT-tg female group, as revealed by Bonferroni’s posthoc comparisons following two-way ANOVA.
TERT-tg mice displayed male-specific impairment in social novelty preference
In the social novelty preference test (Fig. 1C), the stay duration in the familiar side was affected by genotype [F(1,36)=10.97, p=0.002], but not according to sex [F(1,36)=0.661, p=0.422]. Post-hoc comparisons showed that the TERT-tg males stayed more in the familiar side than the FVB males (p<0.05). In the central compartment, the duration was not changed by genotype [F(1,36)=3.255, p=0.080] but differed according to sex [F(1,36)=4.859, p=0.0340]. Post-hoc comparisons revealed that TERT-tg males stayed more in the central compartment than TERT-tg females (p<0.05). There was no significant interaction between genotype and sex [F(1,36)=2.143, p=0.152]. The duration in the novel mouse side was affected by genotype [F(1,36)=13.41, p=0.0008] and marginally according to sex [F(1,36)=3.968, p=0.054]. Post-hoc comparisons revealed that TERT-tg males stayed less in the novel side than FVB males (p<0.001) and TERT-tg females (p<0.01). In separate trials, we also measured the sniffing duration of subject mice to either the familiar or novel mouse in the social novelty preference test (Fig. 1D). Sniffing to the novel mouse was significantly different according to genotype [F(1,36)=7.443, p=0.0098] and sex [F(1,36)=2.053, p=0.1605]. Post hoc test showed that TERT-tg males have decreased sniffing interaction to the novel mouse than FVB males (p<0.05) but not TERT-tg females (p>0.05). In addition, sniffing to the familiar cage differed only according to sex [F(1,36)=7.448, p=0.0098] but marginally according to genotype [F(1,36)=3.667, p=0.0635]. Post hoc test revealed the increased sniffing of TERT-tg mice to the familiar mouse than the FVB males (p<0.05) and TERT-tg females (p<0.01). These results suggest that TERT-tg male mice displayed more approaches to familiar mice and fewer approaches to the socially novel mice compared to their FVB male counterparts and the TERT-tg female mice.
TERT-tg mice display normal locomotor activity but have male-specific decreased anxiety-like behaviors
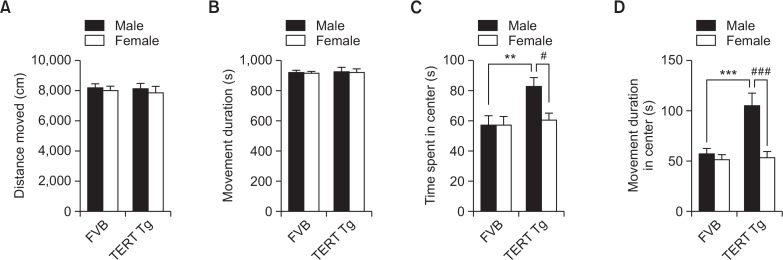
In the open-field test, locomotor activities did not show significant differences between FVB mice and TERT-tg mice (Fig. 2A), as we also reported previously (Kim et al., 2016b). The distance moved was not affected by genotype [F(1,60)=0.085, p=0.772] nor according to sex [F(1,60)=0.4165, p=0.521]. The movement duration (Fig. 2B) was not also different according to genotype [F(1,60)=0.053, p=0.818] or sex [F(1,60)=0.057, p=0.812]. However, movement in the marked center area of the open-field, which represent reduced anxiety of animals, were increased in the TERT-tg male mice as shown by the time spent in the center area of the open field (Fig. 2C). TERT-tg male mice spent more time in the center than the FVB males (p<0.01) and TERT-tg females (p<0.05). Also, TERT-tg male mice had higher movement duration in the central area than the FVB male mice (p<0.001) and the TERT-tg female mice (p<0.001) (Fig. 2D).
Fig. 2.
Locomotor activity of TERT-tg mice. Locomotor activity test was performed with FVB mice and TERT-tg mice when they reach 4 weeks of age. The distance moved (A), and movement duration (B) parameters were presented. The time spent (C) and movement duration (D) in the center area [20×20 cm] were also calculated as preliminary measures of anxiety-like behaviors. All data are expressed as the mean ± SEM. Two male, and two female mice were chosen from each of 8 litters for a total of 16 mice per group. **p<0.01, ***p<0.001 vs. FVB male group; #p<0.05, ###p<0.001 vs. TERT-tg female group, as revealed by Bonferroni’s posthoc comparisons following two-way ANOVA.
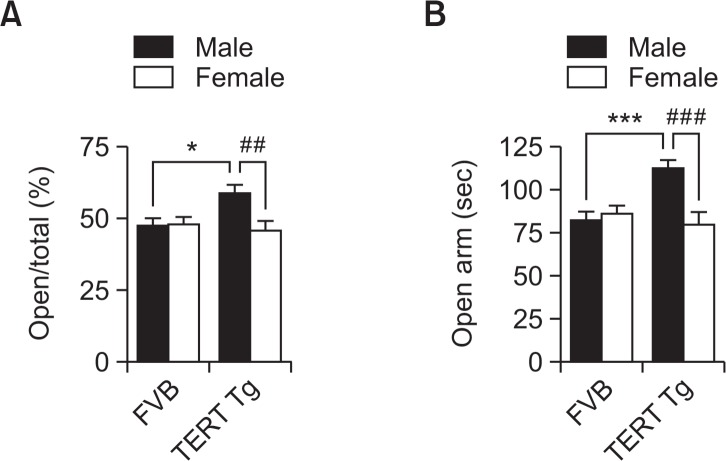
To confirm the lower anxiety level represented by TERT-tg male mice, we performed the elevated plus maze test (Fig. 3). The percentage of the frequency of entry was not affected by genotype [F(1,56)=2.552, p=0.1158], but changed according to sex [F(1,56)=4.926, p=0.0305]. Post-hoc comparisons showed that TERT-tg male mice entered the open arms more frequently than the FVB male mice (p<0.05) and the TERT-tg female mice (p<0.01). Furthermore, the duration in the open arms was affected according to genotype [F(1,56)=4.521, p=0.0379], and sex [F(1,56)=6.796, p=0.0117]. Post-hoc comparisons showed that TERT-tg male mice stayed more in open arms than the FVB male mice (p<0.001) and TERT-tg female mice (p<0.001). These pieces of evidence demonstrate the lower anxiety-like behavior of TERT-tg male mice.
Fig. 3.
Male-specific lower anxiety-like behavior in TERT-tg mice. Elevated plus maze test was performed with FVB mice and TERT-tg mice when they reach 4 weeks of age. The frequency of entry in the open arms (A) was calculated using the following formula: (total open arms entries/total open+close arms entries×100). Meanwhile, the total time spent in the open arms (B) were also compared per group. All data are expressed as the mean ± SEM. Fifteen of each male and female mice were chosen from 8 litters for a total of 15 mice per group. *p<0.05, ***p<0.001 vs. FVB male group; ##p<0.01, ###p<0.001 vs. TERT-tg female group, as revealed by Bonferroni’s posthoc comparisons following two-way ANOVA.
TERT-tg mice have male-specific impairments in nest-building ability
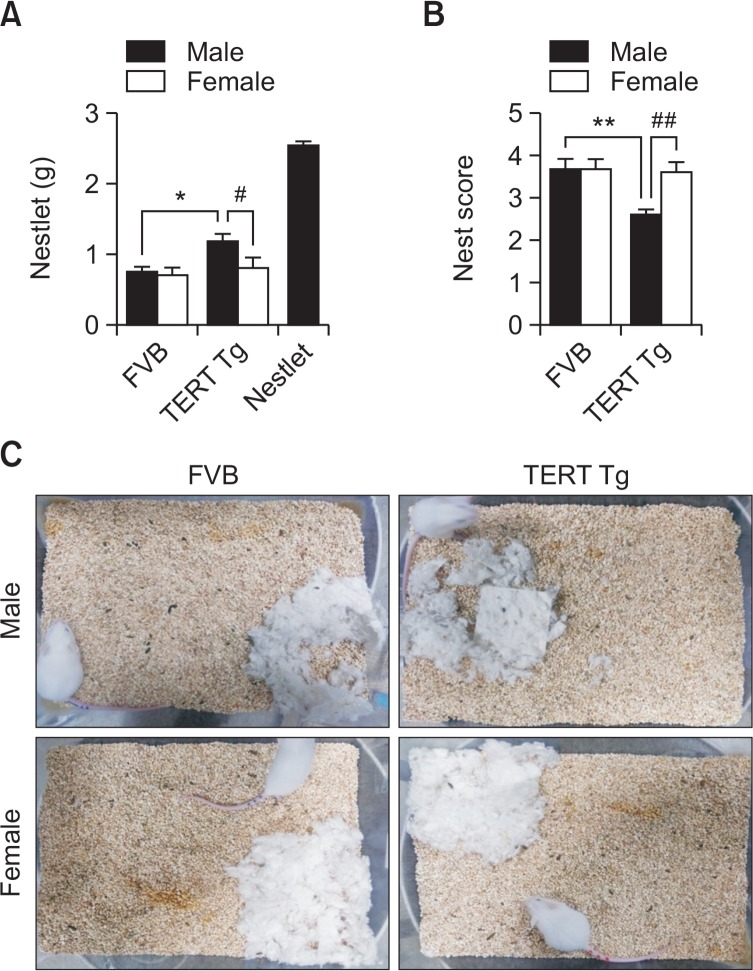
In the nest building test, the remaining weight of intact nest- let was affected by genotype [F(1,56)=6.464, p=0.0138], but not according to sex [F(1,56)=3.855, p=0.0546] (Fig. 4A). Post-hoc comparisons showed that TERT-tg male mice left more intact nestlet than the FVB male mice (p<0.05) and the TERT-tg female mice (p<0.05). The nest building scores, based on Deacon’s method (Deacon, 2012), were affected by genotype [F(1,56)=6.766, p=0.0119], and according to sex [F(1,56)=5.268, p=0.0255] (Fig. 4B, 4C). Post-hoc comparisons showed that TERT-tg male mice had lower nest scores than the FVB male mice (p<0.01) and the TERT-tg female mice (p<0.01). Both these parameters support the decreased ability of TERT-tg male mice to build a nest.
Fig. 4.
Nest building ability of TERT-tg mice. Nest building test was performed with FVB mice and TERT-tg mice when they reach 4 weeks of age. The remaining nestlet were weighed (A) in each mouse and averaged per group. The nest scores (B) were measured according to a previously established protocol (Deacon, 2012). Representative images of the actual nests built by each mouse were shown (C). All data are expressed as the mean ± SEM. Fifteen of each male and female mice were chosen from 8 litters for a total of 15 mice per group. *p<0.05, **p<0.01 vs. FVB male group; #p<0.05, ##p<0.01 vs. TERT-tg female group, as revealed by Bonferroni’s posthoc comparisons following two-way ANOVA.
TERT-tg mice have male-specific lower electroshock seizure threshold
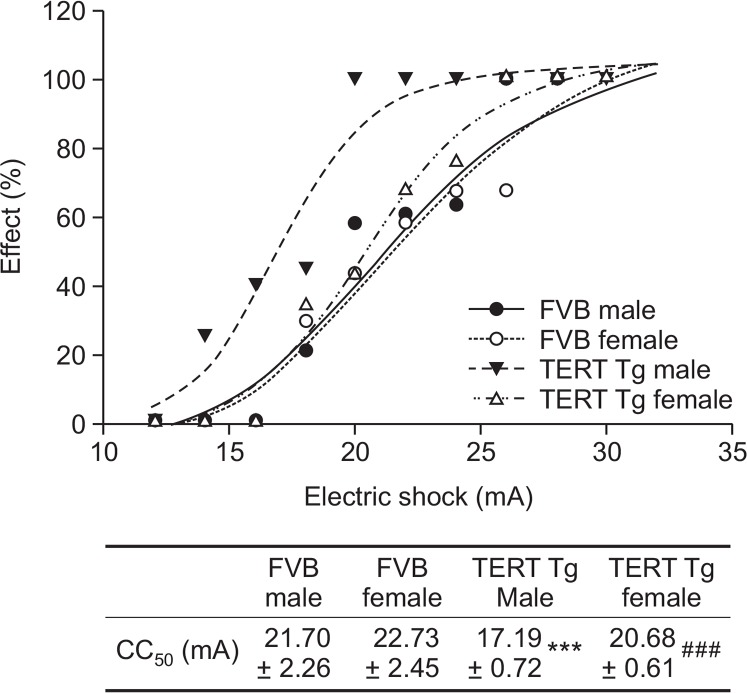
Increased excitatory tone may affect the excitability connections of the brain, which is often increased in ASD. To measure the electroshock seizure threshold of TERT-tg mice, we conducted the seizure threshold test using a staircase method. Two-way ANOVA revealed that electroshock seizure thresholds were affected by genotype [F(1,28)=28.69, p<0.0001] and according to sex [F(1,28)=13.62, p=0.001] (Fig. 5). Post-hoc comparisons showed that TERT-tg males had a lower threshold than the FVB males (p<0.001) and the TERT-tg females (p<0.001). This result suggests that TERT overexpression in mice have a male preponderance in response to seizure-provoking stimuli.
Fig. 5.
Electroshock seizure threshold of TERT-tg mice. Measurement of electroshock seizure threshold was performed as described in materials and methods. The CC50 of each animal group was presented. The table showed the actual value of CC50 with upper and lower confidence limits. All data are expressed as the mean ± SEM. One male and one female mouse was chosen from each of 8 litters for a total of 8 mice per group. ***p<0.001 vs. FVB male group; ###p<0.001 vs. TERT transgenic female group, as revealed by Bonferroni’s posthoc comparisons following two-way ANOVA.
TERT-tg mice have male-specific up-regulation of pre- and post-synaptic neuronal markers
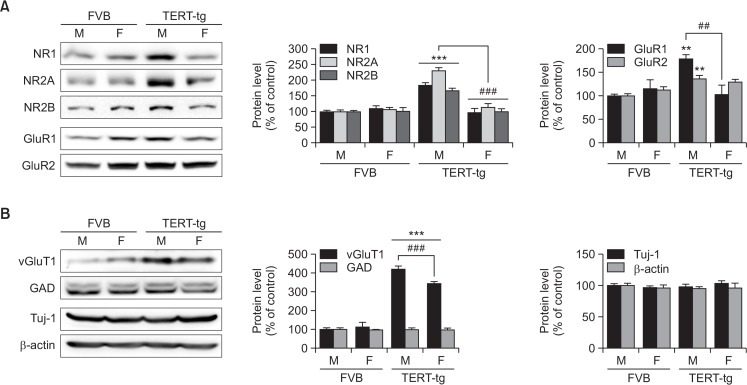
Postsynaptic receptors and markers are implicated in ASD phenotypes. Thus, we investigated the synaptic maturation markers in the TERT-tg mice and inquired whether these markers have sex-dependent variabilities. The prefrontal cortical regions (PFC) were analyzed by Western blot. The expressions of NMDA receptor proteins were significantly increased in the PFC of TERT-tg male mice (Fig. 6A). The manifestations of NR1, NR2A and NR2B proteins in the TERT-tg males were higher than the FVB males (p<0.001) and TERT-tg females (p<0.001). The expressions of AMPA receptor proteins in the PFC of TERT-tg male mice were also upregulated (Fig. 6A). The GluR1 expression in the TERT-tg males was higher than in the FVB males (p<0.01) and the TERT-tg females (p<0.01). Meanwhile, the GluR2 expression in the TERT-tg males was higher than the FVB males (p<0.01), but not the TERT-tg females (p>0.05).
Fig. 6.
Western blot analysis of synaptic proteins in the prefrontal cortex of TERT-tg mice (A) Postsynaptic protein levels including NR1, NR2A, NR2B, GluR1 and GluR2 in the PFC of 4 weeks old FVB mice and TERT transgenic mice were analyzed by Western blot. (B) Also, the pre-synaptic proteins including vGluT1 and GAD, and somatic proteins including Tuj-1 and β-actin in the PFC of FVB mice and TERT transgenic mice were analyzed. All data are expressed as the mean ± SEM. One male, and one female mouse was chosen from each of 4 litters for a total of 4 mice per group. **p<0.01, ***p<0.001 vs. FVB male group; ##p<0.01, ###p<0.001 vs. TERT transgenic female group, as revealed by Bonferroni’s posthoc comparisons following two-way ANOVA
The proteins predominantly localized in the somatic or presynaptic regions were also investigated (Fig. 6B). The vGluT1 expression in the TERT-tg males was higher than the FVB males (p<0.001) and the TERT-tg females (p<0.001). Interestingly, vGluT1 expression in the TERT-tg females was greater than the FVB females (p<0.001). On the other hand, GAD and Tuj-1 protein expressions were similar in all groups. β-actin was used as the loading control. Overall, these results suggest that TERT overexpression induced excitatory neuronal differentiation both in male and female mice, but post-synaptic changes (especially NMDA and AMPA receptor subunits) in the PFC are mostly confined to males.
DISCUSSION
In mice, telomerase activity is regulated during brain development. High levels of telomerase induce stem cell proliferation while low levels of telomerase induce stem cell death (Klapper et al., 2001). The abnormally expressed TERT proteins influence the proliferation of neuronal stem cells (Liu et al., 2012). Thus, TERT overexpression could delay the cell apoptosis leading to the increased cell proliferation and differentiation. This study demonstrated the sex-dependent autistic-like effects of TERT overexpression in TERT-tg male mice and not in the TERT-tg female mice. The autistic-like behaviors of TERT-tg male mice include decreased sociability and social novelty preference, decreased nest building ability and decreased electro-seizure threshold. A reduced anxiety behavior is also observed in these animals. The prefrontal cortex of TERT-tg male mice exhibited the increased expressions of postsynaptic NMDA and AMPA receptor subunit proteins. Also, the presynaptic proteins vGluT1, but not GAD, were upregulated in both sexes of TERT-tg mice.
These results offer important implications in the explanation and elucidation of the “Female Protective Effect” theory (Robinson et al., 2013). Digging deeper into the molecular pathways that help the female brain compensate the abnormal transcription of TERT or other genes will give us clearer answers to this theory. Based on the results, we can infer that females possess molecular substrates in their brain architecture that protected them from the autistic-like effects of TERT overexpression in brain development and neuronal architecture. These factors could be involved in the structural differentiation in males and females during brain development (Giedd et al., 1996; Luders et al., 2005), differences in their psychological response [the E-S theory of psychological sex differences] (Baron-Cohen et al., 2005), and the effects of hormonal differences in brain development and behavior (De Vries and Simerley, 2002; Baron-Cohen et al., 2005). Interestingly, females could carry more burdens of mutation in their genes than males, but they could remain carriers of the mutation but do not show symptoms, supporting a “female protective model” (Jacquemont et al., 2014). A similar phenomenon could have occurred in the sex-differential abnormal phenotypes of our current study where female behaviors remain intact, and only the vGluT1 protein marker was changed in the synaptic area. Indeed, further studies should be focused on isolating or identifying these protective factors present in the female brains during development, as the current study gives us valuable insights.
The autistic-like behavioral phenotypes found in the TERT-tg mice demonstrate a face validation of this model as what has been previously reported (Kim et al., 2016b). More importantly, this study determined that only the male TERT-tg offspring but not their female littermates were affected. The fact that males are more prone to bouts of ASD than females is reflective of male-related factors to diseases in contrast or in addition to the “Female Protective Theory.” This fact presents an idea of innate tendencies between male and female in being susceptible or resistant to certain disorders. A popular theory where this idea was rooted is the Empathizing-Systemizing (E-S) theory of psychological sex differences developed by Baron-Cohen (Baron-Cohen et al., 2005; Baron-Cohen, 2009). Indeed, the observed differences in the psychological symptoms between sexes can be a possible way of explaining the male preponderance in ASD. In support, a recent study showed that the innate strong empathizing capacity of females might mitigate the underlying autistic alterations in the brain as most of these diagnosed or undiagnosed female patients can socialize and communicate without difficulty and have higher IQ scores than male patients (Halladay et al., 2015). This theory could, at least, partially answer the outstanding question of higher male diagnosis than females in ASD. While still in the early stage of development, designing behavioral paradigms to assess specific empathetic behaviors of mice will be useful tools for the study of sex differences (Panksepp and Lahvis, 2011). It is also important to establish and identify the brain regions and specific neural networks governing empathizing and systemizing to quantify the changes in male and female brains with ASD to confirm its validity based on evidence.
Of note are the increases in the presynaptic marker vGluT1 in both sexes of the TERT-tg mice, although more pronounced in males than females. On the other hand, the postsynaptic markers of NMDA and AMPA receptor subtypes were upregulated only in the male brains. NMDA receptors are involved in glutamate-mediated excitatory signaling which accelerates synaptic transmission and synaptic plasticity (Zorumski and Izumi, 2012). By this, we could infer that the autistic-like behaviors in the males of TERT-tg mice could be related to the abnormal postsynaptic development and abnormal excitatory neurotransmission as implicated in previous studies (Rinaldi et al., 2007). These are further supported by the increased seizure susceptibility of TERT-tg male mice. Along with this path, the results of the present study provide a positive contribution to the excitatory/inhibitory (E/I) imbalance theory of ASD (Rubenstein and Merzenich, 2003; Bourgeron, 2009) and support the hypothesis that male brains are more prone to E/I imbalance than female brains. The comparison of ASD-like phenotypes between sexes in other validated transgenic animal models of ASD is needed to bridge the missing links for the biased prevalence in ASD. For now, we take note that presynaptic terminal proteins including vGluT1 are upregulated at a comparable level in both sexes while postsynaptic proteins are upregulated only in males. These results may strongly support the existence of a molecular mechanism in the female brain to counteract abnormally-high presynaptic inputs leading to the corresponding increase in postsynaptic maturation.
The male-specific manifestations of ASD in TERT-tg mice may have important implications for the epigenetic regulations during brain development though further investigations will be required to satisfy this presumption. It was established that male and female brains have different epigenetic regulations and have differential histone modification kinetics (McCarthy et al., 2009; Tsai et al., 2009). Given these differences, the sex-dependent distinctive expressions of various genes surrounding TERT overexpression could play a role in brain development or the protection of the brain from abnormalities in mice. For example, the expression of MECP2, a gene implicated in DNA methylation, is lower in certain brain areas of male rats at postnatal day 1 when compared to their female counterparts, and vice versa on day 10 (Kurian et al., 2007). Also, the MeCP2 expression could be male-specifically downregulated when exposed to VPA at E12, preceding the increased excitatory postsynaptic development in the male offspring and the autistic-like behavioral phenotypes (Kim et al., 2013, 2016a). It is then interesting to see whether VPA prenatal injection could enhance the expression of TERT in the male VPA rat models and cultured NPCs. This path suggests an important role of TERT in brain development and in the manifestation of ASD, which has a possible link to differential sex prevalence.
Another finding worth mentioning in the male TERT-tg mice is the decreased anxiety-like behavior, an accompanying symptom of ASD. Previously, we observed reduced anxiety levels in the VPA-exposed mice model of ASD (Kim et al., 2014a), which could be interpreted as a manifestation of impulsive behavior and could be a comorbid symptom related to ADHD (Ueno et al., 2002). TSC2 mutant mice models of ASD also displayed reduced anxiety levels in the elevated plus maze (Yuan et al., 2012). In contrast, other animal models of ASD have increased anxiety-like behaviors such as the rat VPA models (Markram et al., 2008; Schneider et al., 2008). In such cases, the differences in species and age during tests (they tested 90 days old rats while we tested four weeks old mice) could explain this discrepancy. Thus, the comorbid symptoms of ASD are also expected to be varied and unique in each animal model.
In summary, this study demonstrates that the high male prevalence in ASD is also found in the TERT-tg animal model through the manifestations of abnormal social behaviors and increased excitatory postsynaptic expression in the prefrontal cortex of the brain. The possible role of TERT expression in the imbalanced sex prevalence of ASD should be delved deeper in the next step of this study. It is also important to compare whether the male-specific autism-like phenotypes in the TERT-tg mice model can be replicated in other transgenic animal models to converge the pathologic mechanism of ASD, which is relevant to the high male prevalence and the protective mechanisms in the female genetic/epigenetic makeup.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (NRF-2014R1A2A2A01003079) and by a grant from the Korean Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (No. HI12C0021).
Footnotes
CONFLICT OF INTEREST
There is no conflict of interest to declare.
REFERENCES
- American Psychiatric Association . The Diagnostic and Statistical Manual of Mental Disorders: DSM 5. bookpoint; USA: 2013. [Google Scholar]
- Bakken TL, Helverschou SB, Eilertsen DE, Heggelund T, Myrbakk E, Martinsen H. Psychiatric disorders in adolescents and adults with autism and intellectual disability: a representative study in one county in Norway. Res Dev Disabil. 2010;31:1669–1677. doi: 10.1016/j.ridd.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Knickmeyer RC, Belmonte MK. Sex differences in the brain: implications for explaining autism. Science. 2005;310:819–823. doi: 10.1126/science.1115455. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. Autism: the empathizing-systemizing (E-S) theory. Ann N Y Acad Sci. 2009;1156:68–80. doi: 10.1111/j.1749-6632.2009.04467.x. [DOI] [PubMed] [Google Scholar]
- Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- Bourgeron T. A synaptic trek to autism. Curr Opin Neurobiol. 2009;19:231–234. doi: 10.1016/j.conb.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Browning RA, Wang C, Lanker ML, Jobe PC. Electroshock- and pentylenetetrazol-induced seizures in genetically epilepsy-prone rats (GEPRs): differences in threshold and pattern. Epilepsy Res. 1990;6:1–11. doi: 10.1016/0920-1211(90)90002-D. [DOI] [PubMed] [Google Scholar]
- De Vries G, Simerley R. Development of Hormone-Dependent Neuronal Systems. In: Pfaff DW, Joels M, editors. Hormones, Brain and Behaviour. Elsevier; 2002. p. 137. [Google Scholar]
- Deacon R. Assessing burrowing, nest construction, and hoarding in mice. J. Vis. Exp. 2012;(59):e2607. doi: 10.3791/2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferron SR, Marques-Torrejon MA, Mira H, Flores I, Taylor K, Blasco MA, Farinas I. Telomere shortening in neural stem cells disrupts neuronal differentiation and neuritogenesis. J Neurosci. 2009;29:14394–14407. doi: 10.1523/JNEUROSCI.3836-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardini MA, Segatto M, da Silva MS, Nunes VS, Cano MI. Telomere and telomerase biology. Prog Mol Biol Transl Sci. 2014;125:1–40. doi: 10.1016/B978-0-12-397898-1.00001-3. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Vauss YC, Rapoport JL. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. J Comp Neurol. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Halladay AK, Bishop S, Constantino JN, Daniels AM, Koenig K, Palmer K, Messinger D, Pelphrey K, Sanders SJ, Singer AT, Taylor JL, Szatmari P. Sex and gender differences in autism spectrum disorder: summarizing evidence gaps and identifying emerging areas of priority. Mol Autism. 2015;6:36. doi: 10.1186/s13229-015-0019-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- Jacquemont S, Coe BP, Hersch M, Duyzend MH, Krumm N, Bergmann S, Beckmann JS, Rosenfeld JA, Eichler EE. A higher mutational burden in females supports a “female protective model” in neurodevelopmental disorders. Am J Hum Genet. 2014;94:415–425. doi: 10.1016/j.ajhg.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HJ, Choi YS, Hong SB, Kim KW, Woo RS, Won SJ, Kim EJ, Jeon HK, Jo SY, Kim TK, Bachoo R, Reynolds IJ, Gwag BJ, Lee HW. Ectopic expression of the catalytic subunit of telomerase protects against brain injury resulting from ischemia and NMDA-induced neurotoxicity. J Neurosci. 2004;24:1280–1287. doi: 10.1523/JNEUROSCI.4082-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Seung H, Kwon KJ, Ko MJ, Lee EJ, Oh HA, Choi CS, Kim KC, Gonzales EL, You JS, Choi DH, Lee J, Han SH, Yang SM, Cheong JH, Shin CY, Bahn GH. Subchronic treatment of donepezil rescues impaired social, hyperactive, and stereotypic behavior in valproic acid-induced animal model of autism. PLoS ONE. 2014a;9:e104927. doi: 10.1371/journal.pone.0104927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KC, Choi CS, Kim JW, Han SH, Cheong JH, Ryu JH, Shin CY. MeCP2 modulates sex differences in the postsynaptic development of the valproate animal model of autism. Mol Neurobiol. 2016a;53:40–56. doi: 10.1007/s12035-014-8987-z. [DOI] [PubMed] [Google Scholar]
- Kim KC, Kim P, Go HS, Choi CS, Park JH, Kim HJ, Jeon SJ, Dela Pena IC, Han SH, Cheong JH, Ryu JH, Shin CY. Male-specific alteration in excitatory postsynaptic development and social interaction in pre-natal valproic acid exposure model of autism spectrum disorder. J Neurochem. 2013;124:832–843. doi: 10.1111/jnc.12147. [DOI] [PubMed] [Google Scholar]
- Kim KC, Rhee J, Park JE, Lee DK, Choi CS, Kim JW, Lee HW, Song MR, Yoo HJ, Shin CY. Overexpression of telomerase reverse transcriptase induces autism-like excitatory phenotypes in mice. Mol Neurobiol. 2016b;53:7312–7328. doi: 10.1007/s12035-015-9630-3. [DOI] [PubMed] [Google Scholar]
- Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- Kim YS, Leventhal BL, Koh Y-J, Fombonne E, Laska E, Lim E-C, Cheon K-A, Kim S-J, Kim Y-K, Lee H, Song DH, Grinker RR. Prevalence of autism spectrum disorders in a total population sample. Am. J. Psychiatry. 2011;168:904–912. doi: 10.1176/appi.ajp.2011.10101532. [DOI] [PubMed] [Google Scholar]
- Klapper W, Shin T, Mattson MP. Differential regulation of telomerase activity and TERT expression during brain development in mice. J Neurosci Res. 2001;64:252–260. doi: 10.1002/jnr.1073. [DOI] [PubMed] [Google Scholar]
- Kurian JR, Forbes-Lorman RM, Auger AP. Sex difference in mecp2 expression during a critical period of rat brain development. Epigenetics. 2007;2:173–178. doi: 10.4161/epi.2.3.4841. [DOI] [PubMed] [Google Scholar]
- Larson T, Anckarsater H, Gillberg C, Stahlberg O, Carlstrom E, Kadesjo B, Rastam M, Lichtenstein P, Gillberg C. The autism - tics, AD/HD and other comorbidities inventory (ATAC): further validation of a telephone interview for epidemiological research. BMC Psychiatry. 2010;10:1. doi: 10.1186/1471-244X-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litchfield JT, Jr, Wilcoxon F. A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther. 1949;96:99–113. [PubMed] [Google Scholar]
- Liu M, Hu Y, Zhu L, Chen C, Zhang Y, Sun W, Zhou Q. Overexpression of the mTERT gene by adenoviral vectors promotes the proliferation of neuronal stem cells in vitro and stimulates neurogenesis in the hippocampus of mice. J Biomed Res. 2012;26:381–388. doi: 10.7555/JBR.26.20110078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Narr K, Thompson P, Woods R, Rex D, Jancke L, Steinmetz H, Toga A. Mapping cortical gray matter in the young adult brain: effects of gender. Neuroimage. 2005;26:493–501. doi: 10.1016/j.neuroimage.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Markram K, Rinaldi T, La Mendola D, Sandi C, Markram H. Abnormal fear conditioning and amygdala processing in an animal model of autism. Neuropsychopharmacology. 2008;33:901–912. doi: 10.1038/sj.npp.1301453. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Auger AP, Bale TL, De Vries GJ, Dunn GA, Forger NG, Murray EK, Nugent BM, Schwarz JM, Wilson ME. The epigenetics of sex differences in the brain. J Neurosci. 2009;29:12815–12823. doi: 10.1523/JNEUROSCI.3331-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noldus LP, Spink AJ, Tegelenbosch RA. EthoVision: a versatile video tracking system for automation of behavioral experiments. Behav Res Methods Instrum Comput. 2001;33:398–414. doi: 10.3758/BF03195394. [DOI] [PubMed] [Google Scholar]
- Panksepp JB, Lahvis GP. Rodent empathy and affective neuroscience. Neurosci Biobehav Rev. 2011;35:1864–1875. doi: 10.1016/j.neubiorev.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HG, Yoon SY, Choi JY, Lee GS, Choi JH, Shin CY, Son KH, Lee YS, Kim WK, Ryu JH, Ko KH, Cheong JH. Anticonvulsant effect of wogonin isolated from Scutellaria baicalensis. Eur J Pharmacol. 2007;574:112–119. doi: 10.1016/j.ejphar.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Pellow S, File SE. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol Biochem Behav. 1986;24:525–529. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- Rinaldi T, Kulangara K, Antoniello K, Markram H. Elevated NMDA receptor levels and enhanced postsynaptic long-term potentiation induced by prenatal exposure to valproic acid. Proc Natl Acad Sci USA. 2007;104:13501–13506. doi: 10.1073/pnas.0704391104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson EB, Lichtenstein P, Anckarsäter H, Happé F, Ronald A. Examining and interpreting the female protective effect against autistic behavior. Proc Natl Acad Sci USA. 2013;110:5258–5262. doi: 10.1073/pnas.1211070110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein J, Merzenich M. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183X.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider T, Roman A, Basta-Kaim A, Kubera M, Budziszewska B, Schneider K, Przewłocki R. Gender-specific behavioral and immunological alterations in an animal model of autism induced by prenatal exposure to valproic acid. Psychoneuroendocrinology. 2008;33:728–740. doi: 10.1016/j.psyneuen.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuse DH. Imprinting, the X-chromosome, and the male brain: explaining sex differences in the liability to autism. Pediatr Res. 2000;47:9–16. doi: 10.1203/00006450-200001000-00006. [DOI] [PubMed] [Google Scholar]
- Spijker S. Dissection of rodent brain regions. Neuroproteomics. 2011;57:13–26. doi: 10.1007/978-1-61779-111-6_2. [DOI] [Google Scholar]
- Storch EA, Wood JJ, Ehrenreich-May J, Jones AM, Park JM, Lewin AB, Murphy TK. Convergent and discriminant validity and reliability of the pediatric anxiety rating scale in youth with autism spectrum disorders. J Autism Dev Disord. 2012;42:2374–2382. doi: 10.1007/s10803-012-1489-9. [DOI] [PubMed] [Google Scholar]
- Tsai H-W, Grant PA, Rissman EF. Sex differences in histone modifications in the neonatal mouse brain. Epigenetics. 2009;4:47–53. doi: 10.4161/epi.4.1.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno KI, Togashi H, Mori K, Matsumoto M, Ohashi S, Hoshino A, Fujita T, Saito H, Minami M, Yoshioka M. Behavioural and pharmacological relevance of stroke-prone spontaneously hypertensive rats as an animal model of a developmental disorder. Behav Pharmacol. 2002;13:1–13. doi: 10.1097/00008877-200202000-00001. [DOI] [PubMed] [Google Scholar]
- Yuan E, Tsai PT, Greene-Colozzi E, Sahin M, Kwiatkowski DJ, Malinowska IA. Graded loss of tuberin in an allelic series of brain models of TSC correlates with survival, and biochemical, histological and behavioral features. Hum. Mol. Genet. 2012. dds262. [DOI] [PMC free article] [PubMed]
- Zorumski CF, Izumi Y. NMDA receptors and metaplasticity: mechanisms and possible roles in neuropsychiatric disorders. Neurosci Biobehav Rev. 2012;36:989–1000. doi: 10.1016/j.neubiorev.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]