Abstract
4-O-methylhonokiol, a neolignan compound from Magnolia Officinalis, has been reported to have various biological activities including hair growth promoting effect. However, although transforming growth factor-β (TGF-β) signal pathway has an essential role in the regression induction of hair growth, the effect of 4-O-methylhonokiol on the TGF-β signal pathway has not yet been elucidated. We thus examined the effect of 4-O-methylhonokiol on TGF-β-induced canonical and noncanonical pathways in HaCaT human keratinocytes. When HaCaT cells were pretreated with 4-O-methylhonokiol, TGF-β1-induced G1/G0 phase arrest and TGF-β1-induced p21 expression were decreased. Moreover, 4-O-methylhonokiol inhibited nuclear translocation of Smad2/3, Smad4 and Sp1 in TGF-β1-induced canonical pathway. We observed that ERK phosphorylation by TGF-β1 was significantly attenuated by treatment with 4-O-methylhonokiol. 4-O-methylhonokiol inhibited TGF-β1-induced reactive oxygen species (ROS) production and reduced the increase of NADPH oxidase 4 (NOX4) mRNA level in TGF-β1-induced noncanonical pathway. These results indicate that 4-O-methylhonokiol could inhibit TGF-β1-induced cell cycle arrest through inhibition of canonical and noncanonical pathways in human keratinocyte HaCaT cell and that 4-O-methylhonokiol might have protective action on TGF-β1-induced cell cycle arrest.
Keywords: 4-O-methylhonokiol, Transforming growth factor-β1, Cell cycle arrest, Smads, Reactive oxygen species, NADPH oxidase 4
INTRODUCTION
The growth and development of hair follicles are regulated by a variety of growth factors and cytokines. Insulin-like growth factor 1 (IGF-1), fibroblast growth factor 7 (FGF-7), and hepatocyte growth factor (HGF) are known to positively regulate hair growth, whereas IL-1, FGF-5, and transforming growth factor-β (TGF-β) are negatively regulate hair growth.
It was reported that TGF-β plays a critical role in the growth of hair follicles as well as their morphogenesis (Foitzik et al., 1999; Paus et al., 1999). The localization of the TGF-β iso-forms in hair follicles has also been observed (Soma et al., 2002). Particularly, TGF-β1 was strongly detected in the hair cuticle, hair cortex and connective tissue sheath cells of the anagen hair follicle (Soma et al., 2002). TGF-β1 has been considered to be a potent triggering factor of catagen induction in the murine hair cycle (Foitzik et al., 2000). In addition, TGF-β1 is involved in the regulation of hair follicle regression, and is capable of inducing premature catagen in vivo via the induction of apoptosis and inhibition of keratinocyte proliferation (Seiberg et al., 1995; Welker et al., 1997). Furthermore, androgen-inducible TGF-β1 derived from dermal papilla cells (DPCs) has been shown to mediate the suppression of hair growth and epithelial cell growth in androgenetic alopecia (AGA) (Inui et al., 2002). Collectively, TGF-β1 operates as a catagen inducer and indirectly suppresses hair growth.
TGF-β1 is a well-known growth inhibitory modulator in a variety of cell types including epithelial, endothelial, and myeloid cells (Silberstein and Daniel, 1987; Bascom et al., 1989). Most of TGF-β effects arise from its ability to regulate transcription of specific sets of genes. Among the genes, induction of p21 gives rise tothe effect of TGF-β on the growth inhibition (Li et al., 1995). Increase of the p21 expression in response to TGF-β results from Smad-mediated transcriptional activation. TGF-β-activated Smad2 or Smad3 forms a complex with Smad4 and the complex induces transcription by interacting with Sp1 at the p21 promoter (Pardali et al., 2000). On the other hand, TGF-β has been reported to increase intracellularreactive oxygen species (ROS) in several cell types, such as vascular endothelial cells (Hong et al., 1997) and human lung fibroblast cells (Thannickal et al., 1998). ROS is an important regulator of cell cycle and apoptosis in many cell types (Guyton et al., 1996; Reagan-Shaw et al., 2006). Previous study showed that elevated ROS could induce apoptosis by activation of extracellular signal-regulated protein kinase (ERK) (Guyton et al., 1996). In addition, several studies have documented significant generation of ROS in a variety of cells which is usually the consequence of mitochondrial respiration and NADPH oxidase (NOX) activity (Cheng et al., 2001). NOX is expressed in many cell types and exists in at least five isoforms termed NOX1, NOX2, NOX3, NOX4 and NOX5 (Cheng et al., 2001). Little is known about the role of NOX, except that it was designed for ROS generation. However, previous studies revealed that the level of NOX4 increase in response to TGF-β, which result in the apoptotic cell death (Carmona-Cuenca et al., 2008; Yan et al., 2014).
4-O-Methylhonokiol has been reported to have anti-inflammatory, anti-cancer, and memory improving effects (Oh et al., 2009; Lee et al., 2009b, 2011, 2013; Hyun et al., 2015). We also found that 4-O-Methylhonokiol purified from Magnolia Officinalis can promote the proliferation of DPCs and induce anagen phase in vivo via down-regulation of TGF-β1 and TGF-β2 in the outer root sheath (ORS) and epithelial strand (Kim et al., 2011). In this report, we address if 4-O-Methylhonokiol can potentially protect HaCaT human keratinocytes from TGF-β1-induced cell cycle arrest and how 4-O-Methylhonokiol regulates TGF-β1 signaling pathway in HaCaT human keratinocytes.
MATERIALS AND METHODS
Compound preparation
4-O-Methylhonokiol was supplied by Bioland Ltd (Chungnam, Korea). In brief, 4-O-Methylhonokiol was isolated from the dried bark of Magnolia officinalis Rehd. et Wils. as described (Lee et al., 2009b). A voucher specimen was deposited at the Herbarium of Chungbuk National University, Chungbuk, Korea (voucher specimen # CNBU2009006) (Zhang et al., 2014). The dried bark of Magnolia officinalis Rehd. et Wils. was extracted twice with 95% (v/v) ethanol and concentrated under reduced pressure. The ethanol extract was suspended in H2O and then extracted with n-hexane, ethyl acetate, and n-BuOH, respectively. The n-hexane faction was subjected silica gel chromatography with n-hexane:ethyl acetate (9:1) gradient. The purified compound (4-O-Methylhonokiol; purity>99.5%) was then identified by 1H-NMR and 13C-NMR as described elsewhere (Lee et al., 2009b). Stock solution of 4-O-Methylhonokiol was diluted in 0.05% dimethyl sulfoxide (DMSO) (final concentration, 50 mM).
Reagents
Antibodies against ERK1/2, phospho-pERK1/2, p38, phospho-p38, SAPK/JNK and phospho-SAPK/JNK were obtained from Cell signaling Technology (Beverly, MA, USA). Recombinant human TGF-β1 was purchased from R&D systems (Minneapolis, MN, USA). Antibodies against TGF-β1, PCNA, Smad2/3, Smad4 and monoclonal β-actin were obtained from Sigma (St. Louis, MO, USA) and p21 was obtained from BD Biosciences (San Diego, CA, USA). Antibody against Sp1 was purchased from Millipore (Billerica, MA, USA). HRP-conjugated goat anti-rabbit and horse anti-mouse IgGs were obtained from Vector (Vector Laboratories, Burlingame, CA, USA). 2′,7′-Dichlorofluorescin diacetate (DCFH-DA) and dihydroethidium (DHE) were obtained from Sigma.
Cell culture
HaCaT cells, immortalized human keratinocytes were obtained from Amore Pacific Company (Yongin, Korea). HaCaT cells were cultured in DMEM (HycloneInc, UT, USA) supplemented with 10% fetal bovine serum (Gibco Inc., Grand Island, NY, USA) and penicillin/streptomycin (100 unit/mL and 100 μg/mL, respectively) at 37°C in a humidified atmosphere under 5% CO2.
RNA preparation and RT-PCR
To extract total RNA, we used the Tri-Reagent (Molecular Research Center, Cincinnati, OH, USA) protocol following the manufacturer’s instructions. RNA isolation was carried out in an RNase-free environment. The 1 μg aliquots of RNA were reverse transcribed using MuLV reverse transcriptase (Promega, Madison, WI, USA), oligo (dT)15 primer, deoxyribonucleotide triphosphate (dNTP) (0.5 μM) and 1 URNase inhibitor. The primer sequences of NOX1, NOX2, and NOX4 were as follows; NOX1-sense: gatcaaattvagtgtgavgaccac; antisense: cagactgcaatatcggtgacagca (420 bp); NOX2-sense: ggagtttcaagatgcgtggaaacta; antisense: cagactgcaatatcggtgacagca (550 bp); NOX4-sense: ctcagcggaatcaatcagctgtg; antisense: agaggaacacgacaatcagccttag (251 bp). The polymerase chain reaction (PCR) was performed with a C1000TM Thermal cycler (Bio-Rad, HC, USA), and the amplification was followed by 35 cycles of 94°C for 30 sec (denaturing), 60°C for 30 sec for (annealing) and 72°C for 30 sec (extension). The PCR products were electrophoresed on a 1.2% agarose gel and visualized by ethidium bromide staining.
Cell cycle analysis
HaCaT cells (1.0×105 cells/mL) were pre-incubated for 24 h, and then washed 3 times with PBS, cultured 48 h in serum-free DMEM. Serum starvation-arrested cells were stimulated by 10% FBS without or with TGF-β1 (10 ng/mL) and in the absence or presence of 4-O-Methylhonokiol. For the flow cytometric analysis to determine cell cycle phase distribution, the treated cell were harvested, washed twice with PBS, and fixed in 70% ethanol for 30 min at 4°C. The fixed cells were washed twice with cold PBS, incubated with 50 μg/mL RNase A at 37°C for 30 min, and stained with 50 μg/mL propidium iodide (PI) in the dark for 30 min at 37°C.The stained cells were analyzed using fluorescence activated cell sorter (FACS) caliber flow cytometry (Becton Dickinson, San Jose, CA, USA).Histograms were analyzed with the software program Cell Quest (Becton Dickinson) (Krishan, 1975).
Western blot analysis
To obtain whole cell protein, the HaCaTcells were lysed with a lysis buffer (50 mMTris-HCl (pH 7.5), 150 mM NaCl, 2 mM EDTA, 1 mM EGTA, 1 mM NaVO3, 10 mM NaF, 1 mM dithiothreitol, 1 mM Phenylmethylsulfonylfluoride, 25 μg/mL aprotinin, 25 μg/mL leupeptin, 1% Nonidet P-40) for 30 min at 4°C. The cell lysates were centrifuged at 15,000 rpm at 4°C for 15 min. The supernatant was stored at −20°C until analysis. In some experiments, nuclear fraction was prepared using NE-PER nuclear extraction reagents (Pierce Biotechnology, Rockford, IL, USA) by following the manufacturer’s instructions. Protein concentration was determined by Bradford method (Bradford, 1976). Equal amount of protein was loaded onto a SDS-PAGE gel. After electrophoretic separation, proteins were transferred onto a polyvinylidene fluoride membrane (Bio-Rad) with a glycine transfer buffer (192 mM glycine, 25 mMTris-HCl (pH 8.8), 20% MeOH (v/v)) at 120 V for 1.5 h. After blocking with 1% bovine serum albumin in TBS-T (50 mM Tris, pH 7.6, 150 mM NaCl, 0.1% Tween-20), the membrane was incubated with specific primary antibodies at 4°C overnight. Primary antibody (1:500–1:1000) incubation was followed by washing with TBS-T and then incubating with a secondary HRP antibody (1:5000; Vector Laboratories) at room temperature for 1 h. A chemiluminescence reaction (ECL, Intron Biotechnology, Sungnam, Korea) was used to visualize protein bands on X-ray films (AGFA, Mortsel, Belgium). All blots were probed with β-actin to confirm that equal amounts of protein were loaded.
Measurement of intracellular ROS
Intracellular ROS was analyzed by flow cytometry using DCFH-DA and DHE. HaCaT cells (1.0×105 cells/mL) were seeded on 35-mm dishes and incubated for 24 h, then washed 3 times with PBS, cultured for 24 h in serum-free DMEM. After 24 h, HaCaT cells were preincubated with 4-O-Methylhonokiol (3, 30 and 300 nM) for 1 h and treated with TGF β1 (10 ng/mL) for different times. The cells were washed with PBS and incubated in the medium containing DCFH-DA (10 μM) or DHE (10 μM) for 30 min at 37°C with 5% CO2 in the dark. The HaCaT cells were washed twice with PBS and intracellular accumulation of fluorescence DCF-DA or DHE were measured (10,000 cells each) using a FACScan Flow Cytometer (Becton Dickinson). For DCF-DA assay, excitation was at 488 nm with an emission at 540 nm. For DHE assay, excitation was at 535 nm with an emission at 610 nm. Histograms were analyzed with the software program Cell Quest (Becton Dickinson).
Superoxide dismutase (SOD) and catalase (CAT) activity
The HaCaT cells were seeded at 1×105 cells/mL. After 16 h incubation, the cells were treated with various concentrations of 4-O-Methylhonokiol for 1 h and harvested. The cells were suspended in 10 mM potassium phosphate buffer (pH 7.5) and then lysed on ice by sonication twice for 15 sec. Triton X-100 (0.1%) was then added to the lysates and the lysates were incubated for 10 min on ice. The lysates were centrifuged at 5000×g for 30 min at 4°C to remove the cellular debris. The protein amount of the supernatant was determined by Bradford method (Bradford, 1976).
The SOD activity has been used to detect the inhibition level of epinephrine auto-oxidation (Misra and Fridovich, 1972). 5 μg of the protein was added to 50 mM potassium phosphate buffer (pH 10.2) containing 0.1 mM EDTA and 0.4 mM epinephrine. Epinephrine rapidly undergoes auto-oxidation at pH 10 to produce adrenochrome, a pink colored product, which can be measured at 480 nm using a UV/VIS spectrophotometer in kinetic mode. SOD inhibits the auto-oxidation of epinephrine. The rate of inhibition was monitored at 480 nm. The SOD activity was expressed as units/mg protein and one unit of enzyme activity was defined as the amount of enzyme required for 50% inhibition of auto-oxidation of epinephrine.
For measurement of the CAT activity, 5 μg of protein was added to 50 mM potassium phosphate buffer (pH 7) containing 100 mM (v/v) H2O2. The reaction mixture was incubated for 2 min at 37°C and the absorbance was monitored at 240 nm for 5 min. The change in absorbance with time was proportional to the breakdown of H2O2 (Carrillo et al., 1991). The CAT activity was expressed as units/mg protein and one unit of enzyme activity was defined as the amount of enzyme required to breakdown of 1 mM H2O2.
RESULTS
4-O-Methylhonokiol inhibited TGF-β1-induced G1 arrest in HaCaT cell
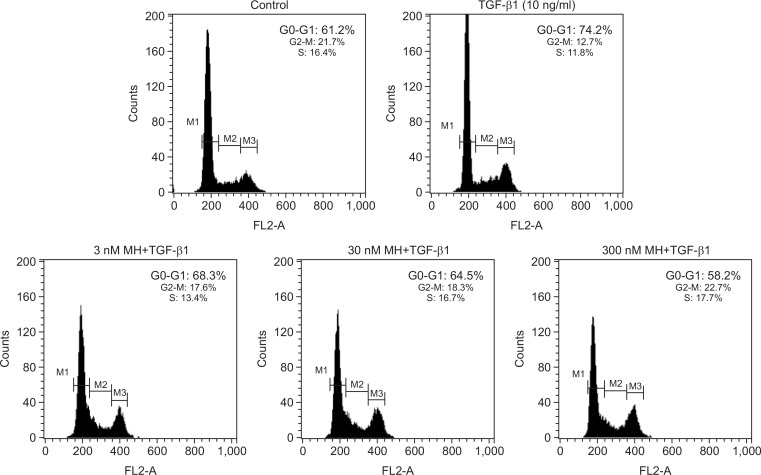
To assess whether 4-O-Methylhonokiol can inhibit TGF-β1-induced G1 arrest in HaCaT cells, we performed propidium iodide staining followed by flowcytometric analysis. As shown in Fig. 1, TGF-β1 (10 ng/mL) arrested cells in the G0-G1 phase (74.2%) of the cell cycle. Pretreatment with 4-O-Methylhonokiol at 3, 30 and 300 nM before TGF-β1 (10 ng/mL) treatment decreased the percentage of G1 phase cells to 68.3%, 64.5% and 58.2%, respectively, in a dose-dependent manner.
Fig. 1.
The effect of 4-O-Methylhonokiol (MH) on TGF-β1-induced G1 arrest in HaCaT cell. After serum starvation for 48 h, HaCaT were pretreated with MH at final concentrations of 3, 30 and 300 nM for 1 h, and then treated with TGF-β1 (10 ng/mL) for 24 h. Cellular DNA was stained with PI staining solution and analyzed by flowcytometry.
4-O-Methylhonokiol inhibited the increase of TGF-β1-induced p21 expression in HaCaT cell
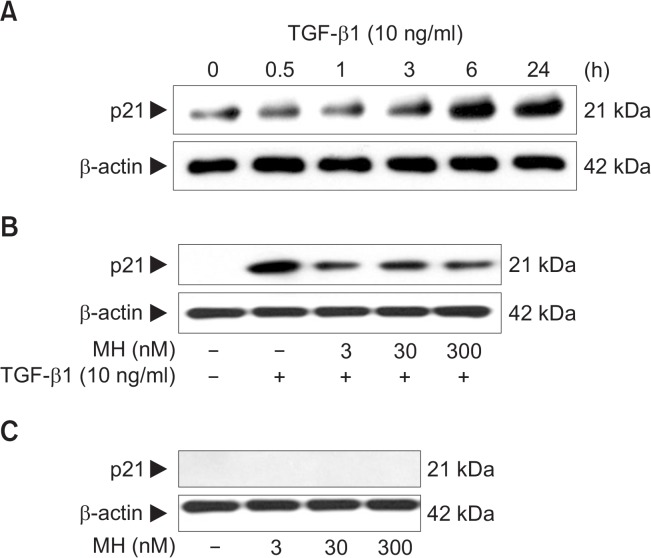
Previous studies have shown that p21 is an important cell cycle regulator and is induced by TGF-β1. The induction of p21 by TGF-β1 plays a causative role in TGF-β1-mediatied inhibition of cell growth (Moustakas et al., 2002). Firstly, we confirmed that TGF-β1 treatment led to an increase in the expression level of p21 in human keratinocytes HaCaT cells in a time-dependent manner (Fig. 2A).We examined 4-O-Methylhonokiol effect on TGF-β1-induced p21 expression. HaCaT cells were pretreated with 4-O-Methylhonokiol at final concentrations of 3, 30 and 300 nM for 60 min, and then treated with 10 ng/mL of TGF-β1 for 24 h. As shown in Fig. 2B, 4-O-Methylhonokiol treatment decreased TGF-β1-induced p21 expression. When treated with 4-O-Methylhonokiol alone (3, 30 and 300 nM) for 24 h, the expression of p21 was not observed (Fig. 2C).
Fig. 2.
The effect of 4-O-Methylhonokiol (MH) on TGF-β1-induced p21 expression in HaCaT cell. (A) HaCaT cells were treated with TGF-β1 (10 ng/mL) for the indicated times. Whole lysates (30 μg proteins) were examined by 10% SDS-PAGE and analyzed with Western blotting using for p21. (B) After pretreatment with MH (3, 30 and 300 nM) for 1 h, HaCaT cells were treated with TGF-β1 (10 ng/mL) for another 24 h. The expression level of p21 was analyzed by Western blotting. (C) HaCaT cells were incubated with MH (3, 30 and 300 nM) for 24 h, and the expression level of p21 was analyzed. Blots were then probed with the corresponding antibodies after which they were stripped and reprobed with an antibody to β-actin to ensure equivalent loading and transfer.
4-O-Methylhonokiol inhibited TGF-β1-mediated nuclear translocation of Smads
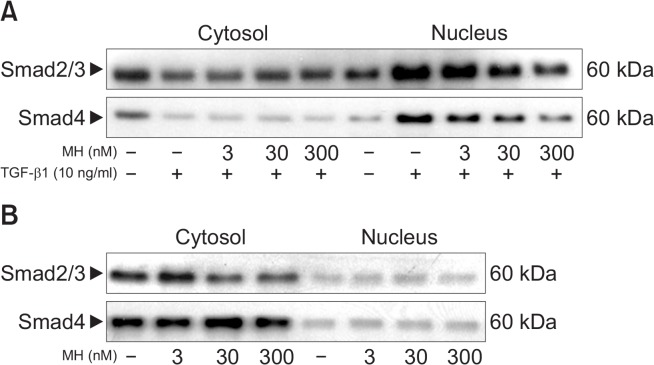
It has been reported that Smads are translocated into the nucleus compartment in response to TGF-β1 stimulation, which was essential for induction of TGF-β1-target gene (Massague and Wotton, 2000). To determine the effect of 4-O-Methylhonokiol on TGF-β1-mediated nuclear translocation of Smad proteins, we analyzed levels of Smads in the cytoplasmic and nuclear fractions after treating HaCaT cells with TGF-β1 and/or 4-O-Methylhonokiol. As shown in Fig. 3, TGF-β1 induced nuclear translocation of Smad2/3, and Smad4 within 60 min, which was blocked by 4-O-Methylhonokiol. 4-O-Methylhonokiol alone did not show any effect on the localization of Smad proteins. These results demonstrated that 4-O-Methylhonokiol could block TGF-β1-mediated nuclear translocation of Smad2/3, and Smad4.
Fig. 3.
The effect of 4-O-Methylhonokiol (MH) on TGF-β1-induced Smads translocation in HaCaT cell. Nuclear translocation of Smad proteins was analyzed by separating cytoplasmic and nuclear fractions after treating HaCaT cells with TGF-β1 and/or MH for 1 h. Smad proteins were detected by Western blotting using antibodies against Smad2/3 and Smad4, respectively.
4-O-Methylhonokiol inhibited TGF-β1-induced nuclear translocation of Sp1 in HaCaT cell
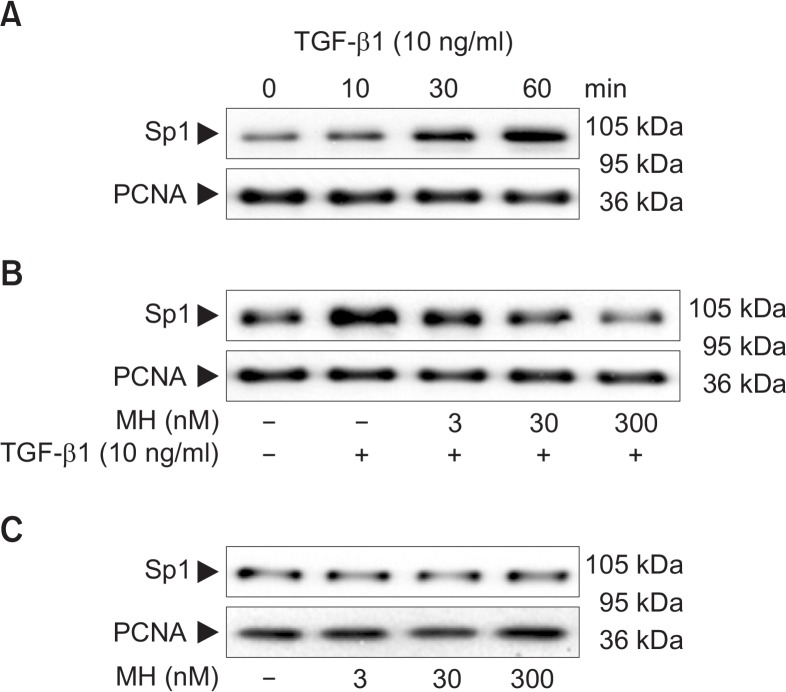
It has been demonstrated that p21 induction by TGF-β1 is mediated through binding of Sp1 transcription factor to Sp1 binding site in the promoter region (Pardali et al., 2000), and interaction between Sp1 transcription factor and Smad proteins is critical for the induction of TGF-β1 target genes (Pardali et al., 2000). To determine whether 4-O-Methylhonokiol could affect the TGF-β1-induced nuclear translocation of Sp1, HaCaT cells were pretreated with 4-O-Methylhonokiolat final concentrations of 3, 30 and 300 nM for 1 h, and then treated with TGF-β1 (10 ng/mL) for 1 h. Treatment with TGF-β1 (10 ng/mL) increased the level of Sp1 in the nucleus of HaCaT cells (Fig. 4A), while pretreatment with 4-O-Methylhonokiol-inhibited the TGF-β1-mediated increase of Sp1 nuclear level in a dose-dependent manner (Fig. 4B). However, 4-O-Methylhonokiol alone did not affect level of Sp1 (Fig. 4C).
Fig. 4.
The effect of 4-O-Methylhonokiol (MH) on TGF-β1-induced nuclear tranlocation of Sp1 inHaCaT cell. (A) HaCaT cells were treated with TGF-β1 (10 ng/mL) for the indicated times. Nuclear fractions were examined by 8% SDS-PAGE and analyzed with Western blotting using antibody against Sp1. (B) After pretreatment with MH (3, 30 and 300 nM) for 1 h, HaCaT cells were treated with TGF-β1 (10 ng/mL) for another 1 h. The nuclear tranlocation of Sp1 was analyzed by Western blotting. (C) HaCaT cells were incubated with MH (3, 30 and 300 nM) for 1 h, and the expression of Sp1 was analyzed. Blots were then probed with the corresponding antibodies after which they were stripped and reprobed with an antibody to PCNA to ensure equivalent loading and transfer.
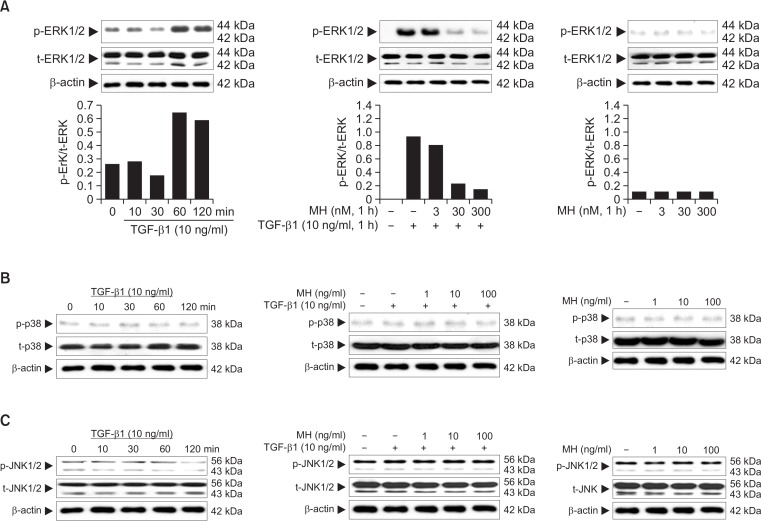
Effect of 4-O-Methylhonokiol on TGF-β1-induced MAPKs activation in HaCaT cells
It has been known that the mitogen-activated protein kinase kinase (MEK) pathway is required for the TGF-β1-induced stimulation of p21 (Hu et al., 1999). We examined the effect of U0126, a pharmacological inhibitor of MEK, on the TGF-β1-induced increase of p21. As shown in Fig. 5, pretreatment of HaCaT cells with U0126 dramatically inhibited the TGF-β1-induced increase of p21. To assess whether the other ERK family members such as p38 and JNK, are involved in induction of p21by TGF-β1, we examined the effect of SB203580 and SP600125, specific inhibitors of p38 and JNK, respectively, on the TGF-β1-induced increase of p21. Pretreatment of HaCaT cells with these inhibitors did not affect the level of TGF-β1-induced p21 (Fig. 5). These data indicate that induction of p21 by TGF-β1 requires the activation of ERK signaling pathway, but not of p38 or JNK pathway.
Fig. 5.
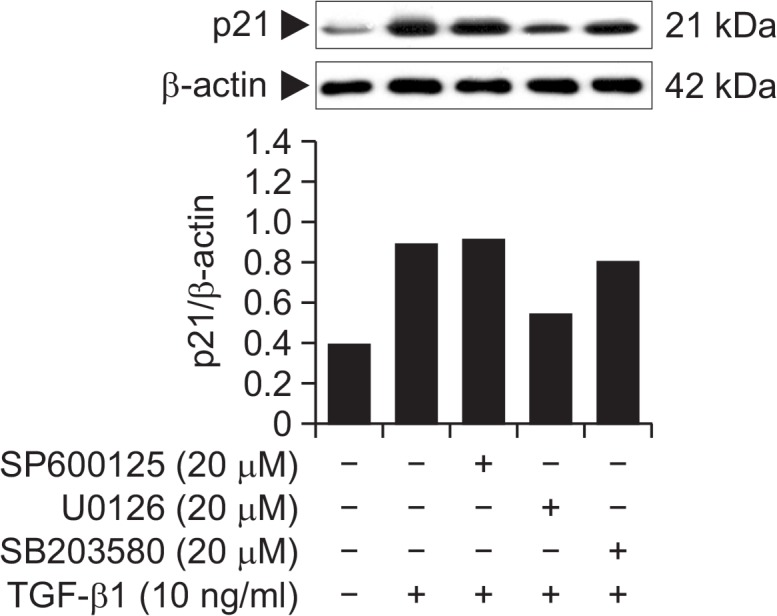
Effect of MAPK inhibitors on TGF-β1-induced p21 expression in HaCaT cells. HaCaT cells were pretreated with U0126 (20 μM), SB203580 (20 μM), and SP600125 (20 μM) for 1 h, and further incubated in the absence or presence of TGF-β1 (10 ng/mL) for 24 h. Whole lysates (30 μg proteins) of the HaCaT cells were examined by 10% SDS-PAGE and analyzed with Western blotting using antibodies for p21.
We addressed if 4-O-Methylhonokiol could affect the TGF-β1-induced activation of ERK pathway. The increase of ERK phosphorylation following TGF-β1 treatment was apparent with maximum activity at 60 min, which was sustained longer than 120 min thereafter (Fig. 6A). 4-O-Methylhonokiol decreased the TGF-β1-induced activation of ERK in a dose-dependent manner (Fig. 6A), whereas TGF-β1 did not affect the activation of p38 and JNK (Fig. 6B, 6C).
Fig. 6.
Effect of 4-O-Methylhonokiol (MH) on TGF-β1-induced MAPKs activation in HaCaT cells. After serum starvation for 24 h, HaCaT cells were stimulated with TGF-β1 (10 ng/mL) for the indicated times. Whole lysates (30 μg proteins) were examined by 10% SDS-PAGE and analyzed with Western blotting using antibodies for phospho-ERK1/2 or ERK1/2 (A), phospho-p38 or p38 (B), phospho-JNK or JNK (C). After pretreatment with MH (3, 30 and 300 nM) for 1 h, HaCaT cells were stimulated with TGF-β1 (10 ng/mL) for another 1 h. Phosphorylations of ERK1/2, p38, and JNK were analyzed by Western blotting.
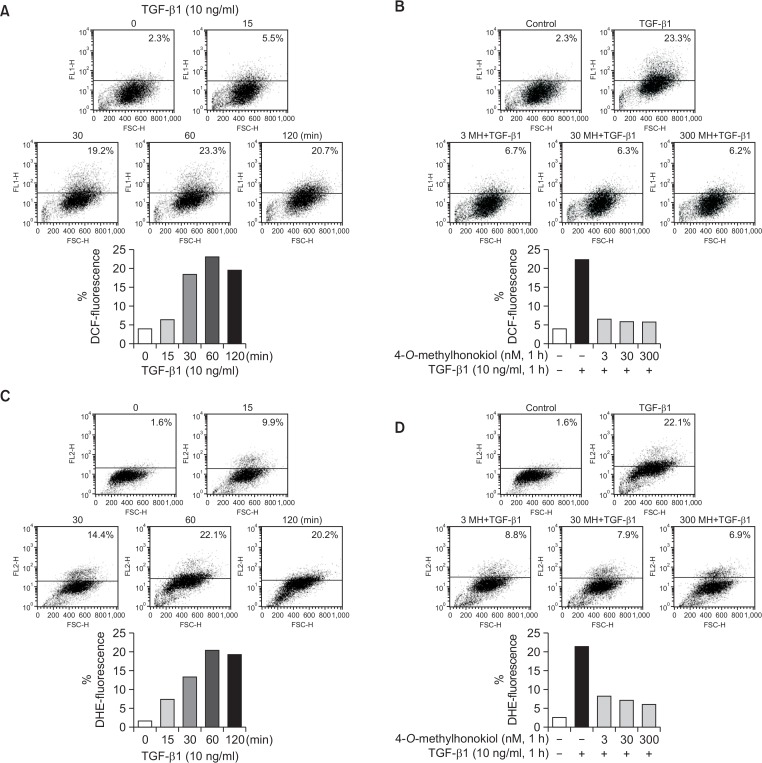
Effect of 4-O-Methylhonokiol on the TGF-β1-induced ROS production in HaCaT cells
Recent accumulating evidences suggest that ROS is an important intracellular messenger to mediate the activation of various signaling molecules in various cells (Yoon et al., 2005). We examined the effect of 4-O-Methylhonokiol on the TGF-β1-induced ROS production. When HaCaT cells were incubated with TGF-β1 (10 ng/mL) for 15, 30, 60 and 120 min, the increase of DCF fluorescence appeared from 30 min, with maximum production at 60 min, which was slightly decreased at 120 min (Fig. 7A). However, when HaCaT cells were pretreated with 4-O-Methylhonokiol followed by the treatment with TGF-β1 (10 ng/mL) for 60 min, pretreatment of 4-O-Methylhonokiol significantly reduced the TGF-β1-induced ROS generation (Fig. 7B). In addition, we also evaluated whether 4-O-Methylhonokiol could block production of superoxide by TGF-β1. Fig. 7C shows that the DHE fluorescence increased in TGF-β1-treated HaCaT cells, whereas the pretreatment of 4-O-Methylhonokiol reduced the TGF-β1-induced ROS generation (included superoxide form) (Fig. 7D).
Fig. 7.
Effect of 4-O-Methylhonokiol (MH) on the TGF-β1-induced ROS production in HaCaT cells. (A) After incubation of serum-starved HaCaT cells in the presence of TGF-β1 (10 ng/mL) for the indicated times, DCF fluorescence was measured with flow cytometry. (B) HaCaT cells were pretreated with MH (3, 30 and 300 nM) for 1 h, and further incubated following treatment with TGF-β1 (10 ng/mL) for 1 h. DCF fluorescence was measured with flow cytometry. (C) After incubation of serum-starved HaCaT cells in the presence of TGF-β1 (10 ng/mL) for the indicated times, DHE fluorescence was measured with flow cytometry. (D) HaCaT cells were pretreated with MH (3, 30 and 300 nM) for 1 h, and further incubated following treatment with TGF-β1 (10 ng/mL) for 1 h. DHE fluorescence was measured with flow cytometry.
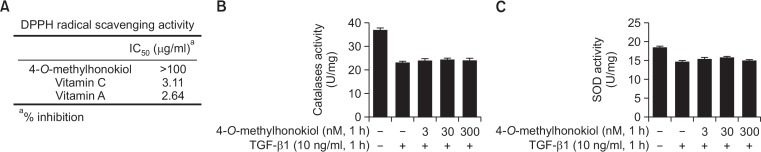
To assess how 4-O-Methylhonokiol could inhibit TGF-β1-induced ROS production, we examined the effect of 4-O-Methylhonokiol on the DPPH free radical scavenging activity, the SOD and CAT activities. As shown in Fig. 8, 4-O-Methylhonokiol did not affect the DPPH free radical scavenging activity, the SOD and CAT activities.
Fig. 8.
Effect of 4-O-Methylhonokiol (MH) on enzymatic and non-enzymatic antioxidant systems. (A) DPPH radical scavenging activity. Data are expressed as the concentration necessary to scavenge 50% of DPPH radical. (B) Catalase and (C) SOD activity in HaCaT cells. The enzyme activities were expressed as average enzyme unit per milligram protein.
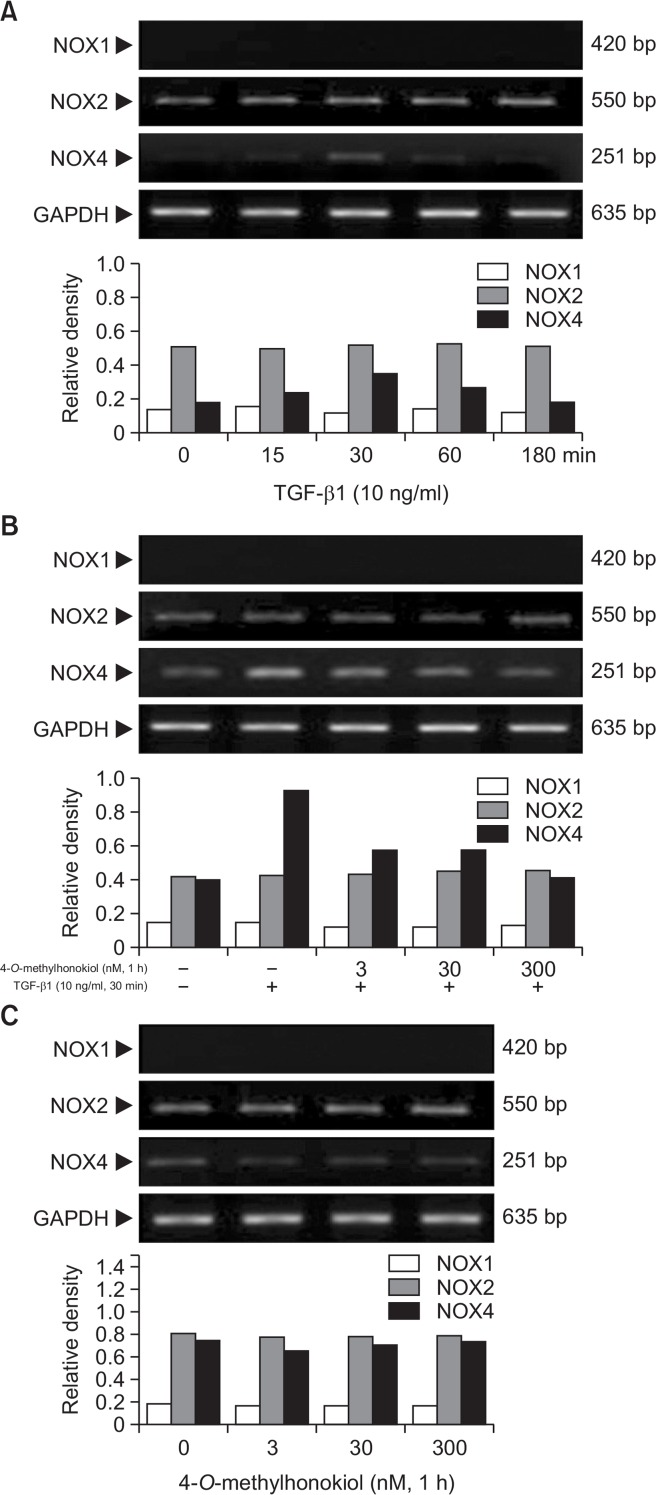
Effect of 4-O-Methylhonokiol on the TGF-β1-induced NADPH oxidase NOX4 in HaCaTcells
It has been previously reported that NOX4 mediates TGF-β1-induced ROS production (Carmona-Cuenca et al., 2008). We examined the effect of 4-O-Methylhonokiol on TGF-β1-induced NOXs expressions. As shown in Fig. 9A, NOX4 mRNA expression increased at 30 min after TGF-β1 treatment. TGF-β1 did not affect the expression of NOX1 and NOX2. However, pretreatment of 4-O-Methylhonokiol reduced increase of NOX4 mRNA level upon TGF-β1 (Fig. 9B).
Fig. 9.
Effect of 4-O-Methylhonokiol (MH) on the TGF-β1-induced NADPH oxidase NOX4 in HaCaT cells. (A) After serum starvation for 24 h, HaCaT cells were treated with TGF-β1 (10 ng/mL) for the indicated times. RNA extraction was carried out in RNase-free environment and the mRNA expression of NOX1, NOX2 and NOX4 were determined by RT-PCR. (B) After pretreated with MH (3, 30, 300 nM) for 1 h, HaCaT cells were incubated with TGF-β1 (10 ng/mL) for 30 nim. The mRNA expression of NOX1, NOX2 and NOX4 were determined by RT-PCR as described above. (C) HaCaT cells were treated with MH (3, 30, 300 nM) for 1 h, and the mRNA expression of NOX1, NOX2 and NOX4 were determined by RT-PCR as described above.
DISCUSSION
In this study, we addressed the effect and mechanism of 4-O-Methylhonokiol, a neolignan compound from M. Officinalis, on TGF-β1-induced cell cycle arrest in human keratinocyte HaCaT cells. To the best of our knowledge, this is the first study to demonstrate that 4-O-Methylhonokiol could inhibit TGF-β1-induced cell cycle arrest through down regulation of canonical and noncanonical pathways.
TGF-β1 is a multifunctional cytokine that has the ability to regulate cell differentiation, survival, and death. In many cell types, TGF-β1 inhibits cellular proliferation by causing growth arrest in the G1 phase of the cell cycle (Senturket al., 2010). The TGF-β1 induced G1 arrest has been attributed to the regulatory effects of TGF-β1 on the levels and activities of cyclins-cyclin-dependent kinase (CDK) complexes and cyclin dependent kinase inhibitors (p21WAF1/Cip1, p27kip1, p18, p16, and p15) (Senturket al., 2010). We found that 4-O-Methylhonokiol could prevent TGF-β1 induced keratinocyte growth arrest in G1 phase via the decrease of p21, one of downstream effectors of TGF-β1 pathway (Fig. 1, 2). On the other hand, 4-O-methylhonokiol increased p21 and apoptotic proteins, which are followed by the inhibition of prostate tumor growth (Lee et al., 2013). Smad proteins may play a role in regulation of CDK inhibitor p21 gene expression by TGF-β1, because TGF-β1-induced p21 gene expression is further enhanced by overexpression of Smad3 and Smad4. In contrast, dominant negative mutants of Smad3 and Smad4 inhibit TGF-β1-induced p21 gene expression (Pardali et al., 2000). In the present study, Smad2/3, Samd4 and Sp1 were significantly translocated into the nucleus following treatment with TGF-β1, whereas this conventional TGF-β1 cascade was blocked by 4-O-Methylhonokiol (Fig. 3, 4). It has been demonstrated that the ability of Smads to induce specific transcription programs in response to TGF-β1 results from a functional cooperation with other transcription factors in multiprotein complexes in nucleus (Datta et al., 2000; Massague and Wotton, 2000). In addition, the requirement of functional and physical interaction between Smad proteins and Sp1 transcription factors for the induction of p21 in response to TGF-β1 has been reported (Ellenrieder, 2008). The interaction of SMAD3/4 with Sp1 and FoxO1 transcription factors by TGFβ signaling could modulate oligodendrocyte progenitor cell cycle withdrawal and differentiation through the transcriptional modulation of c-myc and p21 gene expression (Palazuelos et al., 2014). Collectively, 4-O-Methylhonokiol might inhibit TGF-β1-induced cell cycle arrest through inhibition of canonical pathway in human keratinocyte HaCaT cell.
In addition to the Smad pathway, the canonical pathway of TGF-β signaling, there are several examples of interactions between TGF-β signaling and noncanonical pathways such as mitogen-activated protein kinases (MAPKs), protein kinase C (PKC), β-catenin and phosphatidylinositol 3-kinase (PI3K) (Li et al., 2001). Importantly, several studies have demonstrated that MAPKsare involved in TGF-β-induced p21 expression on a variety of cell types (Hu et al., 1999; Senturk et al., 2010). In addition, both Smad- and Ras/MAPK mediated pathways target a proximal sequence of the p21 promoter that can recruit transcription factors of the Sp1 family together with Smads (Moustakas et al., 2002). The pretreatment of HaCaT keratinocytes with U0126, MEK inhibitor, dramatically inhibited induction of p21 by TGF-β1, whereas pretreatment of cells with SB203580 and SP600125, specific inhibitors of p38 and JNK, did not affect the induction of p21 by TGF-β1 (Fig. 5). These data indicate that induction of p21 by TGF-β1 may require the activation of ERK signaling pathway, but neither of p38 nor JNK signaling pathway. TGF-β1-induced ERK activation was completely inhibited by pretreatment with 4-O-Methylhonokiol (Fig. 6A). 4-O-Methylhonokiol induces neurotropic factors through ERK activation in rat embryonic neuronal cells (Lee et al., 2009a), whereas memory improving effect of 4-O-Methylhonokiol may be associated with the suppression of ERK activation in presenilin 2 mutant mice (Lee et al., 2011). On the other hand, the growth arrest in response to TGF-β1 was accompanied by ROS production (Yoon et al., 2005). Various mechanisms are involved in ROS production by TGF-β1. ROS generation in response to TGF-β1 stimulation is rapid and engages non-SMAD (e.g., EGFR, Src kinase, MAPKs, and p53) and SMAD2/3 pathways (Samarakoon et al., 2013). In addition, it has been previously reported that NOX4 mediates TGF-β1-induced ROS production (Carmona-Cuenca et al., 2008; Peshavariya et al., 2014). Pretreatment of HaCaT cells with 4-O-Methylhonokiol reduced the TGF-β1-induced ROS generation (Fig. 7). NOX4 mRNA expression increased at 30 min after TGF-β1 treatment, whereas the expression of NOX4 was reduced by pretreatment of 4-O-Methylhonokiol (Fig. 9). The results suggest that 4-O-Methylhonokiol could protect keratinocytes against TGF-β1-induced cell cycle arrest by blocking ROS production through NADPH oxidase system.
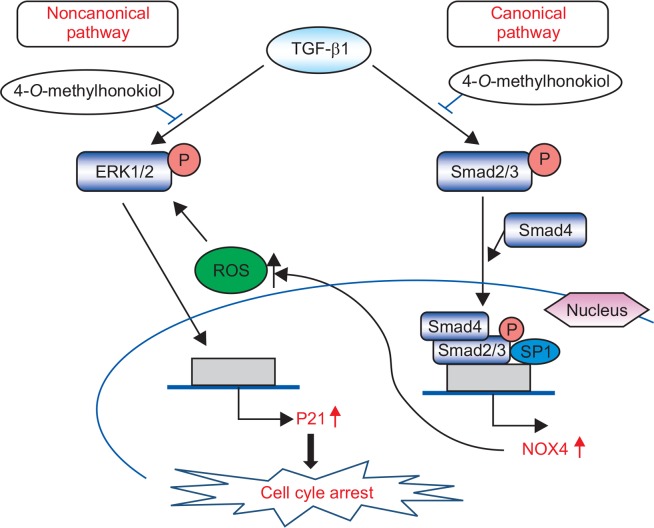
Taken together, the results of this study demonstrate that 4-O-Methylhonokiol could inhibit TGF-β1-induced cell growth arrest through inhibition of canonical and noncanonical pathways in human keratinocyte HaCaT cell and that the hair-growing activity of 4-O-Methylhonokiol might be at least related to its protective action on TGF-β-induced catagen induction in hair cycle (Fig. 10).
Fig. 10.
The scheme for target of 4-O-Methylhonokiol on the TGF-β pathway.
Acknowledgments
This work was supported by the research grant of Jeju National University in 2014.
Footnotes
CONFLICT OF INTEREST
No competing financial interests exist for any of the authors of this study.
REFERENCES
- Bascom CC, Sipes NJ, Coffey RJ, Moses HL. Regulation of epithelial cell proliferation by transforming growth factors. J Cell Biochem. 1989;39:25–32. doi: 10.1002/jcb.240390104. [DOI] [PubMed] [Google Scholar]
- Bradford LW. Problems of ethics and behavior in the forensic sciences. J Forensic Sci. 1976;21:763–768. doi: 10.1520/JFS10557J. [DOI] [PubMed] [Google Scholar]
- Carmona-Cuenca I, Roncero C, Sancho P, Caja L, Fausto N, Fernandez M, Fabregat I. Upregulation of the NADPH oxidase NOX4 by TGF-β in hepatocytes is required for its proapoptotic activity. J Hepatol. 2008;49:965–976. doi: 10.1016/j.jhep.2008.07.021. [DOI] [PubMed] [Google Scholar]
- Carrillo MC, Kanai S, Nokubo M, Kitani K. (−) deprenyl induces activities of both superoxide dismutase and catalase but not of glutathione peroxidase in the striatum of young male rats. Life Sci. 1991;48:517–521. doi: 10.1016/0024-3205(91)90466-O. [DOI] [PubMed] [Google Scholar]
- Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–140. doi: 10.1016/S0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- Datta PK, Blake MC, Moses HL. Regulation of plasminogen activator inhibitor-1 expression by transforming growth factor-β-induced physical and functional interactions between smads and Sp1. J Biol Chem. 2000;275:40014–40019. doi: 10.1074/jbc.C000508200. [DOI] [PubMed] [Google Scholar]
- Ellenrieder V. TGFβ regulated gene expression by Smads and Sp1/KLF-like transcription factors in cancer. Anticancer Res. 2008;28:1531–1539. [PubMed] [Google Scholar]
- Foitzik K, Lindner G, Mueller-Roever S, Maurer M, Botchkareva N, Botchkarev V, Handjiski B, Metz M, Hibino T, Soma T, Dotto GP, Paus R. Control of murine hair follicle regression (catagen) by TGF-β1 in vivo. FASEB J. 2000;14:752–760. doi: 10.1096/fasebj.14.5.752. [DOI] [PubMed] [Google Scholar]
- Foitzik K, Paus R, Doetschman T, Dotto GP. The TGF-β2 isoform is both a required and sufficient inducer of murine hair follicle morphogenesis. Dev Biol. 1999;212:278–289. doi: 10.1006/dbio.1999.9325. [DOI] [PubMed] [Google Scholar]
- Guyton KZ, Liu Y, Gorospe M, Xu Q, Holbrook NJ. Activation of mitogen-activated protein kinase by H2O2. Role in cell survival following oxidant injury. J Biol Chem. 1996;271:4138–4142. doi: 10.1074/jbc.271.8.4138. [DOI] [PubMed] [Google Scholar]
- Hong YH, Peng HB, La Fata V, Liao JK. Hydrogen peroxide-mediated transcriptional induction of macrophage colony-stimulating factor by TGF-β1. J Immunol. 1997;159:2418–2423. [PubMed] [Google Scholar]
- Hu PP, Shen X, Huang D, Liu Y, Counter C, Wang XF. The MEK pathway is required for stimulation of p21 (WAF1/CIP1) by transforming growth factor-β. J Biol Chem. 1999;274:35381–35387. doi: 10.1074/jbc.274.50.35381. [DOI] [PubMed] [Google Scholar]
- Hyun S, Kim MS, Song YS, Bak Y, Ham SY, Lee DH, Hong J, Yoon DY. Peroxisome proliferator-activated receptor-gamma agonist 4-O-Methylhonokiol induces apoptosis by triggering the intrinsic apoptosis pathway and inhibiting the PI3K/Akt survival pathway in SiHa human cervical cancer cells. J Microbiol Biotechnol. 2015;25:334–342. doi: 10.4014/jmb.1411.11073. [DOI] [PubMed] [Google Scholar]
- Inui S, Fukuzato Y, Nakajima T, Yoshikawa K, Itami S. Androgen-inducible TGF-β1 from balding dermal papilla cells inhibits epithelial cell growth: a clue to understand paradoxical effects of androgen on human hair growth. FASEB J. 2002;16:1967–1969. doi: 10.1096/fj.02-0043fje. [DOI] [PubMed] [Google Scholar]
- Kim SC, Kang JI, Kim MK, Boo HJ, Park DB, Lee YK, Kang JH, Yoo ES, Kim YH, Kang HK. The hair growth promoting effect of 4-O-Methylhonokiol. Eur J Dermatol. 2011;21:1012–1014. doi: 10.1684/ejd.2011.1533. [DOI] [PubMed] [Google Scholar]
- Krishan A. Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining. J Cell Biol. 1975;66:188–193. doi: 10.1083/jcb.66.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NJ, Oh JH, Ban JO, Shim JH, Lee HP, Jung JK, Ahn BW, Yoon DY, Han SB, Ham YW, Hong JT. 4-O-Methylhonokiol, a PPARγ agonist, inhibits prostate tumour growth: p21-mediated suppression of NF-κB activity. Br J Pharmacol. 2013;168:1133–1145. doi: 10.1111/j.1476-5381.2012.02235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Choi IS, Park MH, Lee YM, Song JK, Kim YH, Kim KH, Hwang DY, Jeong JH, Yun YP, Oh KW, Jung JK, Han SB, Hong JT. 4-O-Methylhonokiol attenuates memory impairment in presenilin 2 mutant mice through reduction of oxidative damage and inactivation of astrocytes and the ERK pathway. Free Radic Biol Med. 2011;50:66–77. doi: 10.1016/j.freeradbiomed.2010.10.698. [DOI] [PubMed] [Google Scholar]
- Lee YK, Choi IS, Kim YH, Kim KH, Nam SY, Yun YW, Lee MS, Oh KW, Hong JT. Neuriteoutgrowth effect of 4-O-Methylhonokiol by induction of neurotrophic factors through ERK activation. Neurochem Res. 2009a;34:2251–2260. doi: 10.1007/s11064-009-0024-7. [DOI] [PubMed] [Google Scholar]
- Lee YK, Yuk DY, Kim TI, Kim YH, Kim KT, Kim KH, Lee BJ, Nam SY, Hong JT. Protective effect of the ethanol extract of Magnolia officinalis and 4-O-Methylhonokiol on scopolamine-induced memory impairment and the inhibition of acetylcholinesterase activity. J Nat Med. 2009b;63:274–282. doi: 10.1007/s11418-009-0330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Garland JM, Kumar S. Re: Role of transforming growth factor-β signaling in cancer. J Natl Cancer Inst. 2001;93:555–556. doi: 10.1093/jnci/93.7.555. [DOI] [PubMed] [Google Scholar]
- Li CY, Suardet L, Little JB. Potential role of WAF1/Cip1/p21 as a mediator of TGF-β cytoinhibitory effect. J Biol Chem. 1995;270:4971–4974. doi: 10.1074/jbc.270.10.4971. [DOI] [PubMed] [Google Scholar]
- Massague J, Wotton D. Transcriptional control by the TGF-β/Smad signaling system. EMBO J. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- Moustakas A, Pardali K, Gaal A, Heldin CH. Mechanisms of TGF-β signaling in regulation of cell growth and differentiation. Immunol Lett. 2002;82:85–91. doi: 10.1016/S0165-2478(02)00023-8. [DOI] [PubMed] [Google Scholar]
- Oh JH, Kang LL, Ban JO, Kim YH, Kim KH, Han SB, Hong JT. Anti-inflammatory effect of 4-O-Methylhonokiol, compound isolated from Magnolia officinalis through inhibition of NF-κB. Chem Biol Interact. 2009;180:506–514. doi: 10.1016/j.cbi.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Palazuelos J, Klingener M, Aguirre A. TGFβ signaling regulates the timing of CNS myelination by modulating oligodendrocyte progenitor cell cycle exit through SMAD3/4/FoxO1/Sp1. J Neurosci. 2014;34:7917–7930. doi: 10.1523/JNEUROSCI.0363-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardali K, Kurisaki A, Moren A, ten Dijke P, Kardassis D, Moustakas A. Role of Smad proteins and transcription factor Sp1 in p21 (Waf1/Cip1) regulation by transforming growth factor-β. J Biol Chem. 2000;275:29244–29256. doi: 10.1074/jbc.M909467199. [DOI] [PubMed] [Google Scholar]
- Paus R, Muller-Rover S, Van Der Veen C, Maurer M, Eichmuller S, Ling G, Hofmann U, Foitzik K, Mecklenburg L, Handjiski B. A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J Invest Dermatol. 1999;113:523–532. doi: 10.1046/j.1523-1747.1999.00740.x. [DOI] [PubMed] [Google Scholar]
- Peshavariya HM, Chan EC, Liu GS, Jiang F, Dusting GJ. Transforming growth factor-β1 requires NADPH oxidase 4 forangiogenesis in vitro and in vivo. J Cell Mol Med. 2014;18:1172–1183. doi: 10.1111/jcmm.12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan-Shaw S, Breur J, Ahmad N. Enhancement of UVB radiation-mediated apoptosis by sanguinarine in HaCaT human immortalized keratinocytes. Mol Cancer Ther. 2006;5:418–429. doi: 10.1158/1535-7163.MCT-05-0250. [DOI] [PubMed] [Google Scholar]
- Samarakoon R, Overstreet JM, Higgins PJ. TGF-β signaling in tissue fibrosis: Redox controls, target genes and therapeutic opportunities. Cell Signal. 2013;25:264–268. doi: 10.1016/j.cellsig.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiberg M, Marthinuss J, Stenn KS. Changes in expression of apoptosis-associated genes in skin mark early catagen. J Invest Dermatol. 1995;104:78–82. doi: 10.1111/1523-1747.ep12613555. [DOI] [PubMed] [Google Scholar]
- Senturk S, Mumcuoglu M, Gursoy-Yuzugullu O, Cingoz B, Akcali KC, Ozturk M. Transforming growth factor-β induces senescence in hepatocellular carcinoma cells and inhibits tumor growth. Hepatology. 2010;52:966–974. doi: 10.1002/hep.23769. [DOI] [PubMed] [Google Scholar]
- Silberstein GB, Daniel CW. Reversible inhibition of mammary gland growth by transforming growth factor-β. Science. 1987;237:291–293. doi: 10.1126/science.3474783. [DOI] [PubMed] [Google Scholar]
- Soma T, Tsuji Y, Hibino T. Involvement of transforming growth factor-β2 in catagen induction during the human hair cycle. J Invest Dermatol. 2002;118:993–997. doi: 10.1046/j.1523-1747.2002.01746.x. [DOI] [PubMed] [Google Scholar]
- Thannickal VJ, Aldweib KD, Fanburg BL. Tyrosine phosphorylation regulates H2O2 production in lung fibroblasts stimulated by transforming growth factor β1. J Biol Chem. 1998;273:23611–23615. doi: 10.1074/jbc.273.36.23611. [DOI] [PubMed] [Google Scholar]
- Welker P, Foitzik K, Bulfone-Paus S, Henz BM, Paus R. Hair cycle-dependent changes in the gene expression and protein content of transforming factor β 1 and β 3 in murine skin. Arch Dermatol Res. 1997;289:554–557. doi: 10.1007/s004030050239. [DOI] [PubMed] [Google Scholar]
- Yan F, Wang Y, Wu X, Peshavariya H, Dusting G, Zhang M, Jiang F. Nox4 and redox signaling mediate TGF-β-induced endothelial cell apoptosis and phenotypic switch. Cell Death Dis. 2014;5:e1010. doi: 10.1038/cddis.2013.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon YS, Lee JH, Hwang SC, Choi KS, Yoon G. TGF β1 induces prolonged mitochondrial ROS generation through decreased complex IV activity with senescent arrest in Mv1Lu cells. Oncogene. 2005;24:1895–1903. doi: 10.1038/sj.onc.1208262. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Chen J, Jiang X, Wang J, Yan X, Zheng Y, Conklin DJ, Kim KS, Kim KH, Tan Y, Kim YH, Cai L. The magnolia bioactive constituent 4-O-Methylhonokiol protects against high-fat diet-induced obesity and systemic insulin resistance in mice. Oxid Med Cell Longev. 2014;2014:965954. doi: 10.1155/2014/965954. [DOI] [PMC free article] [PubMed] [Google Scholar]