Abstract
CD13/APN (aminopeptidase N) was first identified as a selective angiogenic marker expressed in tumor vasculature and is considered a target for anti-cancer therapy. CD13 was also reported to express in non-diabetic, hypoxia-induced retinal neovascularization. Whether diabetes induces upregulation of CD13 expression in the retina is unknown. We hypothesize that at an early stage of non-proliferative diabetic retinopathy (NPDR) characterized by disruption of blood-retinal barrier (BRB) permeability is related to upregulated expression of CD13 because of its known role in extracellular matrix (ECM) degradation. The purpose of this study is to evaluate the role of CD13/APN and the therapeutic efficacy of a CD13/APN inhibitor in a mouse model of streptozotocin-induced NPDR. Hyperglycemic C57Bl/6 mice 26 weeks after streptozotocin (STZ) injection were intravitreally injected with a sustained release formulation of CD13/APN inhibitor bestatin. At 15th day of post-bestatin treatment, mouse retinas were evaluated for vascular permeability by Evans blue dye extravasation assay, fluorescent angiography of retinal vascular permeability and leukostasis. Retinal protein extracts were analyzed by Western blot to determine the effects of bestatin treatment on the expression of CD13/APN related inflammatory mediators of ECM degradation and angiogenesis. Intravitreal bestatin treatment significantly inhibited retinal vascular permeability and leukostasis. This treatment also significantly inhibited retinal expression of CD13, ECM degrading proteases (heparanase and MMP9 and angiogenic molecules (HIF-1α and VEGF). Intravitreal CD13 inhibition may relate to furthering our knowledge on the protective effect of bestatin against diabetic retinal vasculature abnormalities through inhibition of retinal permeability, leukostasis, inflammatory molecules of ECM degradation and angiogenesis.
Keywords: CD13, Bestatin, Diabetic retinopathy, Leukotriene A4 hydrolase, Retina permeability, ECM
1. Introduction
Vision loss due to diabetic retinopathy (DR) is primarily due to vascular complications (Dorrell et al., 2007). The current therapeutic options for this blinding disease are inadequate because of the complex etiology and chronic progression of this disease. According to the January 2011 National Diabetes Fact Sheet, 8.3% (25.8 million) of Americans have diabetes and ~12% of diabetics will develop DR (Ribeiro et al., 2015). Based on clinical evidence of vascular changes, DR can be divided into early nonproliferative diabetic retinopathy (NPDR) and more advanced, neovascular or proliferative diabetic retinopathy (PDR) (Ribeiro et al., 2015). NPDR is also characterized by increased blood-retinal barrier permeability resulting in leaky capillaries, release of angiogenic growth factors, and development of microaneurysm. Each of these subclinical lesions can progress to the development of clinically evident late stage of PDR. The current treatment options for PDR are conventional laser photocoagulation, photodynamic therapy, intravitreal corticosteroid and anti-vascular endothelial growth factor (anti-VEGF) treatment – all of which have limitations. Laser photocoagulation causes irreversible tissue damage resulting in vision loss (Neubauer and Ulbig, 2007), photodynamic therapy results in reversal of blood vessel proliferation after stopping the treatment (Saito et al., 2014), anti-VEGF treatment causes apoptosis of photoreceptor cells (Truong et al., 2011), and corticosteroid as an anti-inflammatory has local and systemic complications (Comyn et al., 2013). Early identification and therapeutic intervention is expected to block progression toward more advanced stages of human PDR. CD13/APN (aminopeptidase N) is a selective epitope first identified by phage display peptide assays in the tumor vasculature and in hypoxia-induced retinal neovessels (Pasqualini et al., 2000). CD13/APN is also reported to have selective expression in angiogenic blood vessels and little or no expression in the blood vessels of normal tissues (Pasqualini et al., 2000). In a mouse model of hypoxia-induced (not diabetes-induced) retinal neo-vascularization that mimics human PDR, systemic intravenous injection of CD13/APN inhibitor (bestatin) blocked retinal neovascularization (Bhagwat et al., 2001). Most animal models including streptozotocin-induced rodent develop NPDR and there is no best animal model that mimics human PDR (Robinson et al., 2012). It remains unknown whether diabetes upregulates CD13 in the retina of diabetic rodents. The enzymatic activity of CD13/APN includes ECM degradation and release of angiogenic growth factor VEGF (Fujii et al., 1995). Inflammatory leukostasis precedes the release of VEGF. Leukostasis leads to local ischemia or capillary non-perfusion, which subsequently induces HIF-1α and VEGF upregulation (Lin et al., 2011). Upregulation of VEGF also increases vascular permeability or leak and PDR (Ishida et al., 2003). Aminopeptidase N (CD13) is the partial activity of a bifunctional enzyme leukotriene A4 hydrolase (LTA4H) (Orning et al., 1994). LTA4H is bifunctional either as hydrolase or aminopeptidase. The hydrolase activity of LTA4H catalyzes the conversion of leukotriene A4 to the inflammatory leukocyte attractant leukotriene B4 (LTB4) (Haeggstrom et al., 2002). The aminopeptidase activity of LTA4H is reported to protect cell survival in ischemia-injured retina (Husain et al., 2009). Bestatin is a small molecule inhibitor of LTA4H. Bestatin being the inhibitor of both aminopeptidase and hydrolase function of LTA4H is expected to block inflammatory leukostasis and BRB permeability through downregulation inflammatory mediators of ECM degradation and angiogenesis in the diabetic retina.
2. Materials and methods
2.1. Streptozotocin (STZ)-induced diabetes
Male C57Bl/6 mice (Taconic Labs) were injected intraperitoneally (i/p) with 55 mg/kg of STZ (Sigma Aldrich, St. Louis, MO) diluted in sterile citrate buffer (0.05 M, pH 4.5) were administered daily for four consecutive days. Control mice received i/p injection of citrate buffer (0.05 M, pH 4.5) alone. At 26 weeks after injection of STZ or citrate buffer all animals were tested for blood glucose level through tail vein puncture and using AlphaTRAK2 (Abbott Diabetes Care Inc., Alameda, CA). Animals with blood glucose levels more than 350 mg/dL were considered diabetic. All experimental protocols were approved by the Animal Care and Use Committee of Xavier University of Louisiana and were conducted in accordance with the ARVO statement for the use of Animals in Ophthalmic and Vision Research.
2.2. Treatment regimen
Mice were divided in three groups. Each group comprised of five mice. Group 1 (Normal standard control/non-diabetic), group 2 (diabetic treated with controlled release formulation only as vehicle) and group 3 (diabetic treated with bestatin in controlled release formulation). Treatment started at 26 weeks after STZ induction of diabetes. Intravitreal injection of 20 μM bestatin in 5 μL of controlled release formulation as vehicle was used. Animals were sacrificed at 15th day of post-bestatin treatment.
2.3. Preparation of alginate in-situ gel for ophthalmic controlled release formulation
The alginate in-situ gel formulation was prepared by first dissolving 0.4 g of sodium alginate (Sigma) in 20 mL of deionized water. Next, 45 mg of sodium chloride (Sigma) was added to a 10 mL volumetric flask along with approximately 2 mL of deionized water. After dissolution of the sodium chloride, 2 μL of a 100 mM bestatin and 5 mL of the 2% alginate solution were added. The sample was then brought to the dilution of 4 μM/μL with deionized water. For intravitreal injection, each eye received 20μM/5 μL of this formulation (Mandal et al., 2012; Liu et al., 2010; Gupta et al., 2015).
2.4. Evans blue dye assay
Each mouse was anesthetized and received an i/v injection (tail vein) 45 mg/kg Evans blue dye. Two hours later, a 0.1–0.2 mL of blood sample was obtained from re-anesthetized mice and the animals were perfused via the left ventricle with PBS followed by 1% paraformaldehyde. Eyes were enucleated and retina was dissected out. Retinas were treated with dimethylformamide (Sigma Aldrich, St. Louis, MO) overnight at 78 °C and centrifuged at 12,000 g for 15 min. Supernatant retinal extracts were tested spectrophotometrically at 620 nm (blue) and 740 nm (background). Blood samples were not treated with formamide and centrifuged at 3,500 g at 25 °C for 15 min and the supernatant was diluted 1:1000 before testing. BRB breakdown was calculated using the concentration of Evans blue in the blood and retina as follows
2.5. BSA-FITC fluorescent angiography
Flat-mount retinas were prepared from BSA-FITC injected mice to have a qualitative angiographic evidence of vascular exudation. Diffuse and patchy hyperfluorescence areas in the perivascular region were the indicators of vascular leakage. Retinal vascular permeability was measured in vivo by injecting BSA-FITC (Sigma Aldrich, St. Louis, MO), 100 μg/g into the tail vein of anesthetized mice. Thirty minutes later, mice were euthanized and enucleated eyes were collected and fixed in 4% paraformaldehyde for 1 h. After fixation, flat-mount retinas were isolated and viewed under Olympus IX71 inverted fluorescence-microscope with a 4× objective using a Coolsnap CCD camera.
2.6. Retinal leukostasis assay
Leukocytes are stained with concanavalin. At 15th day of post-bestatin treatment, mice retinas from each group were used for leukostasis assay. Following an anesthetic overdose, each mouse received an intracardiac perfusion of 250 mL/kg of PBS for 2 min to remove RBCs and non-adherent leukocytes. Immediately after perfusion, each animal was perfused with a 40 μg/mL of FITC-conjugated concanavalin lectin A (5 mg/kg) to stain adherent leukocytes. Perfusion with PBS is repeated to remove excess fluorescent dyes. Following euthanasia, enucleated eyeballs were used to collect flat-mount retinas. FITC-labeled leukocytes were visualized using an Olympus IX71 inverted fluorescence-microscope with a 20× objective using a Coolsnap CCD camera. Numbers of observed leukocytes were compared among eyes/groups of mice.
2.7. Western blot analysis
At 15th day of post-bestatin treatment, enucleated eyeballs from three different groups of mice were processed for collection of retinal extract. The protein concentration in the retinal extract was determined using a BCA protein assay kit (Pierce, Rockford, IL). An equal amount (50 μg/lane) of protein was loaded for electrophoresis on a 5–20% sodium dodecyl sulfate-polyacrylamide gel. The electrophoresed gel was transferred electrophoretically onto PVDF membranes (Amersham, little Chalfont, UK). Blocking of membrane is done for 1 h in 5% nonfat milk. Following blocking, the membranes were incubated at 4 °C overnight with anti-CD13 (CD13/APN (E1Y7U) rabbit mAb #13721, Cell Signaling Technology, Danvers, MA), anti-heparanse (Sc-25825, Santa Cruz Biotechnology, CA), anti-MMP9 (Sc-6841, Santa Cruz Biotechnology, CA), anti-VEGF (Sc-1836, Santa Cruz Biotechnology, CA), anti-HIF-1α (Sc-10700, Santa Cruz Biotechnology) and anti-β-actin (Sc-81178, Santa Cruz Biotechnology, CA). After overnight incubation with primary antibody, the membrane was washed with PBS-Tween20, and incubated with the secondary antibody for 1 h at room temperature in PBS-Tween20 and 1% nonfat milk. The same blots were then stripped and re-probed with an antibody against β-actin to ensure the equal loading of protein in each lane. Band intensities were analyzed using ImageJ (NIH).
2.8. Gelatin zymography
Retinal tissue homogenates were mixed with 4× non-reducing sample buffer (Thermo Fischer Scientific, MA) and electrophoresed using novex 10% zymogram gel containing 0.1% gelatin (Invitrogen, CA). Following electrophoresis, zymogram gel was treated with novex zymogram renaturing buffer (Invitrogen, CA) for 30 min under gentle shaking. The gel was then incubated overnight at 37 °C using novex zymogram developing buffer (Invitrogen, CA). On the next day, zymogram gel was stained using gel-code-blue stain (Thermo Fischer Scientific, MA) for 6 h and subsequent washing overnight to visualize the white gelatinolytic bands of respective molecular weights. Relative intensity of clear bands was measured using ImageJ (NIH).
2.9. Statistical analysis
Statistical analysis of quantitative data was analyzed by students t-test. Statistical analysis was set at p ≤ 0.05.
3. Results
3.1. Intravitreal bestatin treatment significantly reversed BRB permeability determined by fluorescent angiography of flat-mount retina
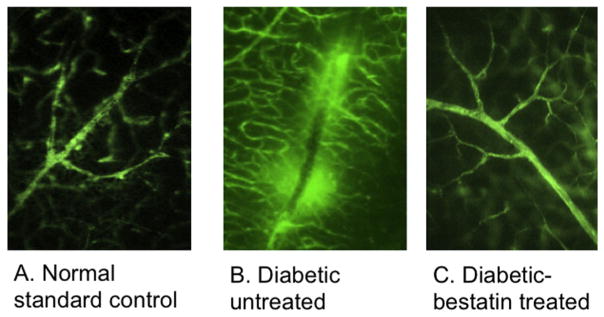
Thirty minutes after tail vein injection of BSA-FITC, enucleated eyeballs from euthanized mice were processed to isolate flat mount retinas. Exudation of albumin conjugated fluorescent dye (BSA-FITC) was examined to determine the extent of BRB leakage. (Fig. 1): perivascular hyperfluorescent area was not seen normal non-diabetic mouse retina (A), it was seen only in diabetic mice retinas (Fig. 1B) but not in bestatin treated mice (Fig. 1C).
Fig. 1.
Following intravenous injection of BSA-FITC, flat mount retinas were visualized using fluorescence microscopy to determine the extent of FITC diffusion around retinal blood vessels. (A) No spot of hyperfluorescence was seen in normal non-diabetic mice retinas. (B) Diabetic mice retina had multiple patchy areas of hyperfluorescence (white arrows) (C) Intravitreal bestatin treated mice eyes did not reveal any spot of hyperfluorescence.
3.2. Intravitreal bestatin treatment prevents retinal permeability determined by Evans blue tracer
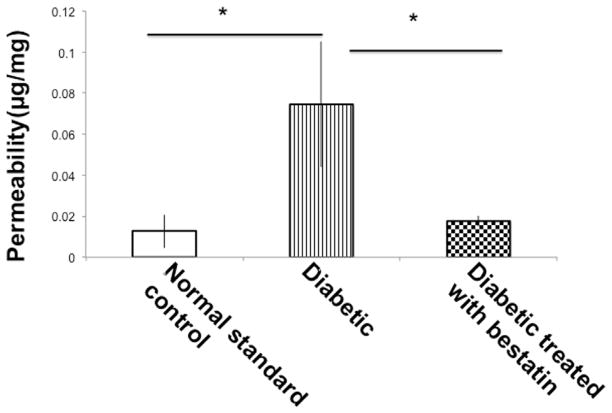
STZ-induced diabetes results blood capillary fenestration making it leaky and permeable. Blood retinal barrier breakdown is traced by Evans blue dye. Evans blue dye binds with plasma albumin and leaky capillaries with increased permeability allowing extravasation of albumin bound Evans blue in the surrounding tissue. In a non-leaky capillary, little or no extravasation of Evans blue occurs and the dye is removed through perfusion. As shown in Fig. 2, permeability in the retinal blood vessels was measured in vivo and retinal tissues of diabetic mice did yield significantly higher (*p ≤ 0.05) concentration of Evans blue in formamide extract. Intravitreal bestatin treatment significantly (*p ≤ 0.05) reduced Evans blue extravasation compared to diabetic mice.
Fig. 2.
Blood retinal barrier permeability was tested by Evans blue permeation assay. Diabetic mice retina leakage was significantly (*p ≤ 0.05) higher than normal non-diabetic mice retinas. Intravitreal bestatin treatment resulted significant (*p ≤ 0.05) reduction in the leakage of Evans blue dye. Error bar, SEM. Students’ t-test. Each value represents the mean ± SEM results in three independent experiments.
3.3. Intravitreal bestatin treatment inhibits retinal leukostasis as revealed in fluorescent retinal angiography
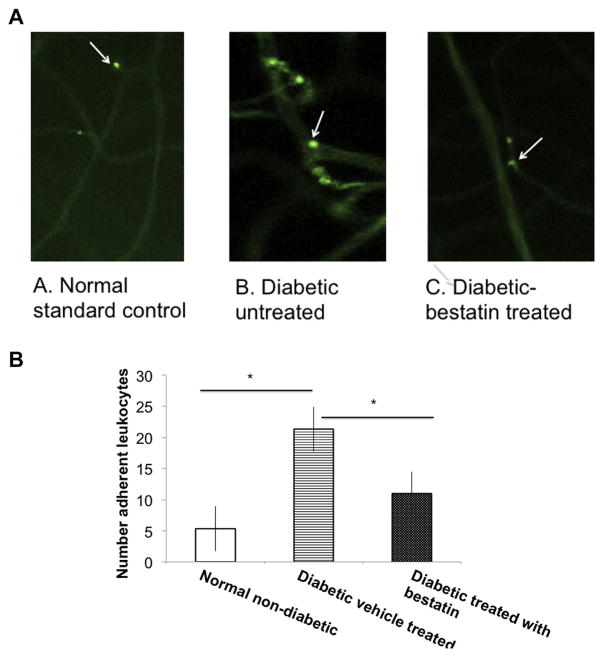
Intracardiac perfusion of Concanavalin-A lectin conjugated with FITC resulted in visualization of fluorescently stained adherent leukocytes in the intima of the retinal vasculature (Fig. 3A). Only whole retinae in which the entire vascular network was stained were used for analysis. The total number of adherent leukocytes per retina was counted. A significantly (*p ≤ 0.05) higher count of adherent leukocytes were seen in the flat mount retinas of diabetic mice compared to non-diabetic (Fig. 3B). A significant (*p ≤ 0.05) reduction in the count of leukocytes was found in the retinas of bestatin treated diabetic mice compared to vehicle treated diabetic mice (Fig. 3B).
Fig. 3.
Concanavalin-A is used to stain leukocytes. Following intracardiac perfusion of FITC-concanavalin lectin A and wash with PBS, flat-mount retinas were visualized and counted for the number of adherent leukocytes in the retinal vasculature. (A). A representative angiogram from each group of mice showing adherent leukocytes indicated by white arrows. (B). Only whole retinae in which the entire vascular network was stained were used for analysis. The total number of adherent leukocytes per retina was counted. Counts of adherent leukocytes were significantly higher in diabetic mice compared to normal non-diabetic and bestatin treated diabetic mice retinas. Error bar SEM, (*p ≤ 0.05) students’ t-test. Each value represents the mean ± SEM results in three independent experiments.
3.4. Intravitreal bestatin treatment inhibits inflammatory mediators of ECM degradation and angiogenesis
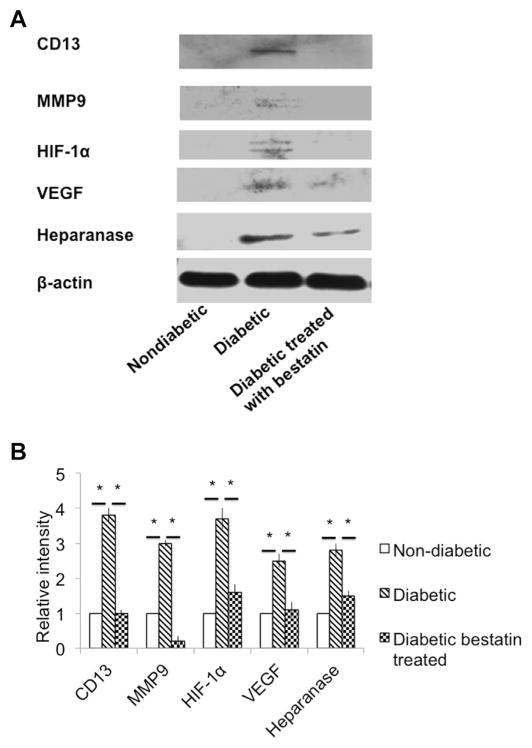
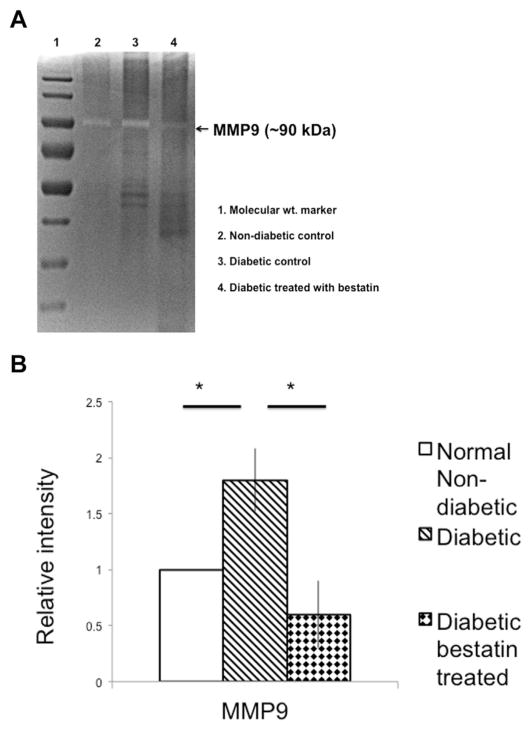
We previously reported (Bhattacharjee et al., 2012) that an ECM degrading enzyme heparanase is upregulated in the hyperglycemia-induced retinal endothelial cell with increased shedding of heparan sulfate (a breakdown product of heparanase enzyme substrate called heparan sulfate proteoglycan) and concomitant loss of tight junction protein occludin and ZO-1 (zona-occludin-1). We also reported that in vivo inhibition of heparanase prevents BRB breakdown in streptozotocin-induced diabetic mouse retina (Bhattacharjee et al., 2012). ECM degradation of retinal vasculature results in the release of angiogenic and inflammatory molecules. At 15th day of post-bestatin treatment, retinas of bestatin treated diabetic mice, vehicle treated diabetic mice and normal nondiabetic mice were harvested to extract proteins. Retinal protein extracts were Western blot analyzed to detect the expression of CD13, ECM degrading enzymes (heparanase, and MMP9) and angiogenic molecules (VEGF and HIF-1α). CD13 expression is significantly (*p ≤ 0.05) upregulated in the retinas of streptozotocin-induced diabetic mice retina compared to normal non-diabetic mice and bestatin is found to significantly (*p ≤ 0.05) reduce CD13 expression in diabetic mice (Fig. 4B). MMP9 plays a pivotal role in ECM degradation related to retinal neo-vascularization (Mohammad and Kowluru, 2012). In our study, we found diabetic mice retinas had significantly (*p ≤ 0.05) higher expression of MMP9 compared to non-diabetic mice retinas. Bestatin treatment inhibited MMP9 expression in the retinas of diabetic mice almost to baseline (*p ≤ 0.05) level ((Fig. 4B) compared to vehicle treated diabetic mice. The gelatinolytic activity of MMP9 was tested by gelatin zymography of retinal tissue homogenates and a representative gel image is shown as Fig. 5A. As shown in Fig. 5B, diabetic mice had significantly (*p ≤ 0.05) higher density of lytic clear band compared to non-diabetic control mice. Bestatin treatment resulted a significant (*p ≤ 0.05) inhibition of MMP-9 specific gelationolytic band densities compared to diabetic vehicle-treated mice. There was no MMP2 expression in the groups tested.
Fig. 4.
Intravitreal bestatin treatment inhibits the ECM degrading proteases and other angiogenic factors. (A). Western blot detection of CD13, ECM degrading proteases (MMP9 and heparanase) and angiogenic factors (HIF-1α and VEGF). (B) CD13 expression was seen in non-diabetic mice retinas, however, its expression is significantly (*p ≤ 0.05) higher in diabetic mice. A complete inhibition of CD13 was seen in bestatin treated mice. MMP9 expression was higher in vehicle treated diabetic mice and bestatin treatment resulted significant inhibition of MMP9 to almost baseline level. HIF-1α expression was significantly (*p ≤ 0.05) higher in diabetic mice than non-diabetic. Bestatin treatment resulted a significant inhibition of HIF-1α in diabetic mice. VEGF and heparanase expression was not seen in normal non-diabetic nice. Bestatin treatment significantly inhibited the expression of VEGF and heparanase in diabetic mice. The relative intensity values were normalized to control value set at 1.0. Each value represents the mean ± SEM results in three independent experiments, *p ≤ 0.05, students’ t-test.
Fig. 5.
Gelatinase activity of MMP9 was detected using commercial gelatin zymography gels. (A). Appearance of white lytic bands at ~90 kDa mark suggests gelatinase activity of MMP9. (B) A densitometry analysis of gelatinase activity of MMP9 suggests a significant increase in the intensity of lytic band in diabetic mice compared to non-diabetic. Gelatinase activity of MMP9 was found to have a significant drop in bestatin treated diabetic mice compared to diabetic control. The relative intensity values were normalized to control value set at 1.0. Each value represents the mean ± SEM, *p ≤ 0.05 student’s t-test.
Retinal leukostasis is known to mediate the capillary non-perfusion related to endothelial cell death and subsequent development of ischemia results in overproduction of HIF-1α (hypoxia inducible factor 1α) and VEGF (Lin et al., 2011). Both HIF-1α and VEGF are major contributors of retinal angiogenesis. In this study, we found a significant (*p ≤ 0.05) upregulation of both HIF-1α and VEGF in diabetic mice retinas compared to non-diabetic mice. Intravitreal bestatin treatment significantly (*p ≤ 0.05) downregulated the expression of both HIF-1α and VEGF in diabetic mice retinas (Fig. 5) compared to vehicle treated diabetic mice. Heparanase is an ECM degrading enzyme known to cleave heparan sulfate proteoglycan (HSPG) and release ECM-bound angiogenic and inflammatory molecules resulting retinal neovascularization and retinopathy (Bhattacharjee et al., 2012). We found no expression of heparanase in non-diabetic retinas (Fig. 4A). The upregulated expression of heparanase in diabetic mice retinas is significantly (p ≤ 0.05) inhibited by intravitreal bestatin treatment (Fig. 4B).
4. Discussion
CD13 is reported to play a role in ECM remodeling, tumor invasion and angiogenesis (Fujii et al., 1995). CD13 inhibitor bestatin is also reported to inhibit retinal neovascularization in a non-diabetic, hypoxia-induced murine model of retinopathy (Pasqualini et al., 2000). This is a first report that 6-months after the induction of diabetes by streptozotocin, BRB breakdown and inflammatory leukostasis is reversed through intravitreal bestatin treatment. Intravitreal bestatin treatment resulted in the inhibition of retinal permeability and leukocyte adhesion mediated through downregulation of CD13 and ECM degrading proteases (heparanase and MMP9). This is the first ever report about the CD13 functionality in a diabetic model of retinopathy.
Intravitreal bestatin treatment has never reported before. Systemic administration (i/v) of 200μg/mouse bestatin was used to inhibit retinal angiogenesis in a hypoxia-induced retinal neo-vascularization (Pasqualini et al., 2000). It is unknown whether systemic administration of bestatin results in toxicity. However, bestatin was reported to have irreversible inhibition of CD13/APN activity at an effective concentration of 10 μM (Shim et al., 2003). Our controlled release formulation contained 20 μM bestatin in 5 μL of vehicle for intravitreal administration. This formulation is novel and found safe and non-toxic following intravitreal injection of vehicle alone and shows no alteration in retinal deformity and angiograms.
In the sensory nervous system (Minami et al., 1990; Haeggström et al., 1990) in vitro and in the in vivo studies with mice retina (Husain et al., 2009) aminopeptidase activity of LTA4H is reported to include hydrolysis of short peptides including degradation of locally produced opioid peptide enkephalin (Griffin et al., 1992) and dynorphin (Nissen et al., 1995). In the retina, opioids and their receptor activation is important for cell survival following hypoxic or ischemic injury (Husain et al., 2009; Riazi-Esfahani et al., 2009). Degradation of opioids released in the inflammed tissue can be blocked by bestatin (Anja Schreiter et al., 2012). The anti-nociceptive function of bestatin through inhibition of opioid degradation may protect ischemia and non-perfusion induced upregulation of HIF and VEGF. Conflicting results exist about the LTA4H-aminopeptidase function in models other than retina. In tissues other than retina LTA4H-aminopeptidase is reported to have anti-inflammatory role through its ability to degrade tripeptide PGP(Pro-Gly-Pro) as antichemotactic (Snelgrove et al., 2010). In an in vitro study, a selective inhibitor (ARM1) of hydrolase function of LTA4 preventing the production of inflammatory LTB4 but retaining the anti-inflammatory aminopeptidase function is suggested to have a therapeutic potential (Stsiapanava et al., 2014). However, in the perspectives of retina biology, inhibition of both hydrolase and aminopeptidase activities of LTA4H appears necessary to prevent inflammation and to increase opioid-receptor activation. Bestatin treatment is also expected increase the production of lipoxin (LPX4) known to have an anti-inflammatory role (Rao et al., 2007). The anti-inflammatory molecule LPX4 is the product of 12-lipooxygenase using LTA4 as substrate. So, bestatin being the inhibitor of both hydrolase and aminopeptidase activity of LTA4H having protective functions through (1) downregulation inflammatory LTB4 expression, (2) upregulation of anti-inflammatory lipoxin (LXA4) production and (3) preventing the breakdown of opioids may thus have a therapeutic potential in the treatment of DR.
Our findings may be of further consideration that diabetes regulated CD13 expression in the retinas may be a therapeutic target to halt to the reversal of BRB loss, as well as to inhibit retinal vascular abnormalities. This study warrants further investigation into possible CD13 phenotypes of retina and the link between vascular remodeling and the LTA4H-aminopeptidase signaling pathway in the regulation of diabetic retinopathy. In summary, our studies suggest an innovative approach of therapeutic strategies for treatment of diabetic retinopathy.
Acknowledgments
Support is provided in part by grant number 2G12MD007595-06 (PSB) from the National Institute on Minority Health and Health Disparities (NIMHD), National Institutes of Health (NIH), Department of Health and Human Services (DHHS), Department of Defense (DoD) grant #W911NF1510059 (TH, PSB), Louis Stokes Louisiana Alliance for Minority Participation (LS-LAMP) – National Science Foundation (NSF) human resource development (HRD) award #1503226 (SM). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH, NIGMS, NSF or DoD. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Bhagwat SV, Lahdenranta J, Giordano R, Arap W, Pasqualini R, Shapiro LH. CD13/APN is activated by angiogenic signals and is essential for capillary tube formation. Blood. 2001;97(3):652–659. doi: 10.1182/blood.v97.3.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee PS, Huq TS, Potter V, Young A, Davenport IR, Graves R, Mandal TK, Clement C, McFerrin HE, Muniruzzaman S, Ireland SK, Hill JM. High-glucose-induced endothelial cell injury is inhibited by a Peptide derived from human apolipoprotein E. PLoS One. 2012;7(12):e52152. doi: 10.1371/journal.pone.0052152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comyn O, Lightman SL, Hykin PG. Corticosteroid intravitreal implants vs. ranibizumab for the treatment of vitreoretinal disease. Curr Opin Ophthalmol. 2013;24(3):248–254. doi: 10.1097/ICU.0b013e32835fab27. [DOI] [PubMed] [Google Scholar]
- Dorrell M, Uusitalo-Jarvinen H, Aguilar E, Friedlander M. Ocular neo-vascularization: basic mechanisms and therapeutic advances. Surv Ophthalmol. 2007;52:S3–S19. doi: 10.1016/j.survophthal.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Fujii H, Nakajima M, Saiki I, Yoneda J, Azuma I, Tsuruo T. Human melanoma invasion and metastasis enhancement by high expression of amino-peptidase N/CD13. Clin Exp Metastasis. 1995;13(5):337–344. doi: 10.1007/BF00121910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin KJ, Gierse J, Krivi G, Fitzpatrick FA. Opioid peptides are substrates for the bifunctional enzyme LTA4 hydrolase/aminopeptidase. Prostaglandins. 1992;44(3):251–257. doi: 10.1016/0090-6980(92)90018-o. [DOI] [PubMed] [Google Scholar]
- Gupta H, Aqil M, Khar RK, Ali A, Bhatnagar A, Mittal G. An alternative in situ gel-formulation of levofloxacin eye drops for prolong ocular retention. J Pharm Bioallied Sci. 2015;7(1):9–14. doi: 10.4103/0975-7406.149810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeggström JZ, Wetterholm A, Vallee BL, Samuelsson B. Leukotriene A4 hydrolase: an epoxide hydrolase with peptidase activity. Biochem Biophys Res Commun. 1990;173(1):431–437. doi: 10.1016/s0006-291x(05)81076-9. [DOI] [PubMed] [Google Scholar]
- Haeggström JZ, Kull F, Rudberg PC, Tholander F, Thunnissen MM. Leukotriene A4 hydrolase. Prostagl Other Lipid Mediat. 2002;68–69:495–510. doi: 10.1016/s0090-6980(02)00051-5. [DOI] [PubMed] [Google Scholar]
- Husain S, Potter DE, Crosson CE. Opioid receptor-activation: retina protected from ischemic injury. Investig Ophthalmol Vis Sci. 2009;50(8):3853–3859. doi: 10.1167/iovs.08-2907. [DOI] [PubMed] [Google Scholar]
- Ishida S, Usui T, Yamashiro K, Kaji Y, Ahmed E, Carrasquillo KG, Amano S, Hida T, Oguchi Y, Adamis AP. VEGF164 is proinflammatory in the diabetic retina. Investig Ophthalmol Vis Sci. 2003;44:2155–2162. doi: 10.1167/iovs.02-0807. [DOI] [PubMed] [Google Scholar]
- Lin M, Chen Y, Jin J, Hu Y, Zhou KK, Zhu M, Le YZ, Ge J, Johnson RS, Ma JX. Ischaemia-induced retinal neovascularisation and diabetic retinopathy in mice with conditional knockout of hypoxia-inducible factor-1 in retinal Müller cells. Diabetologia. 2011;54(6):1554–1566. doi: 10.1007/s00125-011-2081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Liu J, Zhang X, Zhang R, Huang Y, Wu C. In Situ gelling gelrite/alginate formulations as vehicles for ophthalmic drug delivery. AAPS PharmSciTech. 2010;11(2):610–620. doi: 10.1208/s12249-010-9413-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal S, Thimmasetty M, Prabhushankar G, Geetha M. Formulation and evaluation of an in situ gel-forming ophthalmic formulation of moxifloxacin hydrochloride. Int J Pharm Investig. 2012;2:78–82. doi: 10.4103/2230-973X.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami M, Ohishi N, Mutoh H, Izumi T, Bito H, Wada H, Seyama Y, Toh H, Shimizu T. Leukotriene A4 hydrolase is a zinc-containing aminopeptidase. Biochem Biophys Res Commun. 1990;173(2):620–626. doi: 10.1016/s0006-291x(05)80080-4. [DOI] [PubMed] [Google Scholar]
- Mohammad G, Kowluru RA. Diabetic retinopathy and signaling mechanism for activation of matrix metalloproteinase-9. J Cell Physiol. 2012;227(3):1052–1061. doi: 10.1002/jcp.22822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer AS, Ulbig MW. Laser treatment in diabetic retinopathy. Ophthalmologica. 2007;221(2):95–102. doi: 10.1159/000098254. [DOI] [PubMed] [Google Scholar]
- Nissen JB, Iversen L, Kragballe K. Characterization of the aminopeptidase activity of epidermal leukotriene A4 hydrolase against the opioid dynorphin fragment 1-7. Br J Dermatol. 1995;133(5):742–749. doi: 10.1111/j.1365-2133.1995.tb02749.x. [DOI] [PubMed] [Google Scholar]
- Orning L, Gierse JK, Fitzpatrick FA. The bifunctional enzyme leukotriene-A4 hydrolase is an arginine aminopeptidase of high efficiency and specificity. J Biol Chem. 1994;269(15):11269–11273. [PubMed] [Google Scholar]
- Pasqualini R, Koivunen E, Kain R, Lahdenranta J, Sakamoto M, Stryhn A, Ashmun RA, Shapiro LH, Arap W, Ruoslahti E. Aminopeptidase N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesis. Cancer Res. 2000;60(3):722–727. [PMC free article] [PubMed] [Google Scholar]
- Rao NL, Dunford PJ, Xue X, Jiang X, Lundeen KA, Coles F, Riley JP, Williams KN, Grice CA, Edwards JP, Karlsson L, Fourie AM. Anti-inflammatory activity of a potent, selective leukotriene A4 hydrolase inhibitor in comparison with the 5-lipoxygenase inhibitor zileuton. J Pharmacol Exp Ther. 2007;321(3):1154–1160. doi: 10.1124/jpet.106.115436. [DOI] [PubMed] [Google Scholar]
- Riazi-Esfahani M, Kiumehr S, Asadi-Amoli F, Dehpour AR. Effects of intravitreal morphine administered at different time points after reperfusion in a rabbit model of ischemic retinopathy. Retina. 2009;29(2):262–268. doi: 10.1097/IAE.0b013e31818a211d. [DOI] [PubMed] [Google Scholar]
- Ribeiro L, Bandello F, Tejerina AN, Vujosevic S, Varano M, Egan C, Sivaprasad S, Menon G, Massin P, Verbraak FD, Lund-Andersen H, Martinez JP, Jürgens I, Smets E, Coriat C, Wiedemann P, Ágoas V, Querques G, Holz FG, Nunes S, Neves C, Cunha-Vaz J. Characterization of retinal disease progression in a 1-year longitudinal study of eyes with mild nonproliferative retinopathy in diabetes type 2. Evicr Net Study Group Investig Ophthalmol Vis Sci. 2015;56(9):5698–5705. doi: 10.1167/iovs.15-16708. [DOI] [PubMed] [Google Scholar]
- Robinson R, Barathi VA, Chaurasia SS, Wong TY, Kern TS. Update on animal models of diabetic retinopathy: from molecular approaches to mice and higher mammals. Dis Model Mech. 2012;5(4):444–456. doi: 10.1242/dmm.009597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Iida T, Kano M, Itagaki K. Five-year results of photodynamic therapy with and without supplementary antivascular endothelial growth factor treatment for polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol. 2014;252(2):227–235. doi: 10.1007/s00417-013-2433-1. [DOI] [PubMed] [Google Scholar]
- Schreiter A, Gore C, Labuz D, Fournie-Zaluski MC, Roques BP, Stein C, Machelska H. Pain inhibition by blocking leukocytic and neuronal opioid peptidases in peripheral inflamed tissue. FASEB J. 2012;26(12):5161–5171. doi: 10.1096/fj.12-208678. [DOI] [PubMed] [Google Scholar]
- Shim JS, Kim JH, Cho HY, Yum YN, Kim SH, Park HJ, Shim BS, Choi SH, Kwon HJ. Irreversible inhibition of CD13/aminopeptidase N by the antiangiogenic agent curcumin. Chem Biol. 2003;10(8):695–704. doi: 10.1016/s1074-5521(03)00169-8. [DOI] [PubMed] [Google Scholar]
- Snelgrove RJ, Jackson PL, Hardison MT, Noerager BD, Kinloch A, Gaggar A, Shastry S, Rowe SM, Shim YM, Hussell T, Blalock JE. A critical role for LTA4H in limiting chronic pulmonary neutrophilic inflammation. Science. 2010;330(6000):90–94. doi: 10.1126/science.1190594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stsiapanava A, Olsson U, Wan M, Kleinschmidt T, Rutishauser D, Zubarev RA, Samuelsson B, Rinaldo-Matthis A, Haeggström JZ. Binding of Pro-Gly-Pro at the active site of leukotriene A4 hydrolase/aminopeptidase and development of an epoxide hydrolase selective inhibitor. Proc Natl Acad Sci U S A. 2014;111(11):4227–4232. doi: 10.1073/pnas.1402136111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong A, Wong TY, Khachigian LM. Emerging therapeutic approaches in the management of retinal angiogenesis and edema. J Mol Med Berl. 2011;89(4):343–361. doi: 10.1007/s00109-010-0709-z. [DOI] [PubMed] [Google Scholar]