Abstract
Transforming growth factor-beta (TGF-β) is a pleiotrophic cytokine that has been shown to influence the differentiation and function of T cells. As such, the role that TGF-β plays in immune-mediated disease, such as multiple sclerosis (MS), has become a major area of investigation since CD4 T cells appear to be a major mediator of the autoimmunity. This review analysis the literature on the role that TGF-β plays in the generation and regulation of encephalitogenic T cells in experimental autoimmune encephalomyelitis, an animal model of MS, as well as T cells of MS patients. Since TGF-β plays a major role in the development and function of CD4 regulatory T cells, which are defective in MS patients, recent studies have found potential mechanisms to explain the basis for these regulatory T cell defects to establish a foundation for potentially modulating TGF-β signaling to restore normal T cell function in MS patients.
Keywords: T cell, multiple sclerosis, TGF-β, experimental autoimmune encephalomyelitis, miRNA
Introduction
Transforming growth factor-β (TGF-β) has been found to play a diverse set of roles in development, cell differentiation, wound healing and immune regulation. As the name implies, it was originally described by its role in transforming non-malignant cells into neoplastic cells [1, 2]. Today, it is recognized that TGF-β is produced by many cell types, both immune and non-immune, and is capable of both positively and negatively regulating cell expansion and function. There are three TGF-β members, TGF-β1, TGF-β2, and TGF-β3. The expression of each isoform is spatially and temporally distinct. Although deletion of TGF-β2 and TGF-β3 in mice results in embryonic lethality [3–5], loss of TGF-β1 results in systemic inflammation, suggesting that TGF-β1 plays a role in limiting the immune response [6]. TGF-β1 is expressed abundantly in the immune system, whereas expression of TGF-β2 and TGF-β3 are minimal. Thus, TGF-β1 has been studied extensively in the immune system, particularly as a major contributor to immune regulation.
The relevance of TGF-β on T cells was recognized as early as 1986 when it was demonstrated that TGF-β inhibited IL-2 production, as well as proliferation, by T cells [7]. The pathology and severe wasting observed in TGF-β1-deficient mice was largely contributed to CD4+ T cells since deletion of CD4+ T cells protected TGF-β1-deficient mice from lethal inflammation [8]. The absence of TGF-β1 signaling resulted in spontaneous T cell differentiation and the onset of autoimmunity [9]. However, CD8+ T cell activation and proliferation was also shown to be inhibited by TGF-β1 and deletion of CD8+ T cells in TGF-β1-deficient mice reduces the pathology observed in TGF-β1-deficient mice [10, 11].
The immunosuppressive nature of TGF-β led to speculation that it may be useful in suppressing autoimmunity. Several studies in the early 1990’s demonstrated that treatment of experimental autoimmune encephalomyelitis (EAE), a rodent model for multiple sclerosis (MS), with TGF-β1 or TGF-β2 at the time of disease induction or during the course of disease resulted in reduced neurological damage and fewer CNS lesions [12–15]. In addition, TGF-β2 was beneficial in ameliorating a viral model of MS [16]. These early studies in EAE generated interest in pursuing TGF-β as a therapy for MS.
Role of TGF-β in the differentiation and function of encephalitogenic T cells
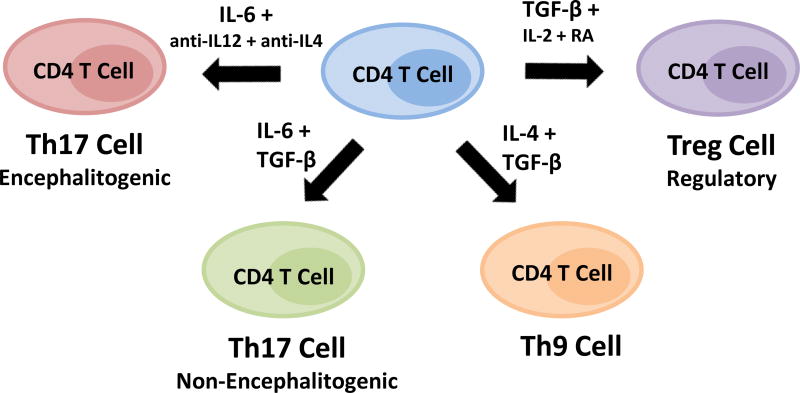
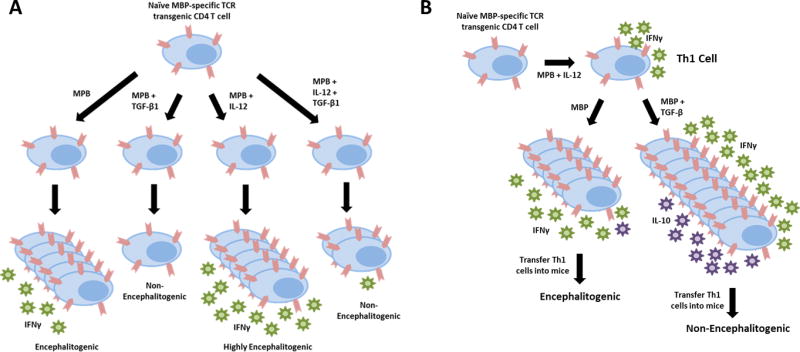
Since autoreactive T cells are present in all individuals, yet most individuals do not develop autoimmunity, TGF-β likely contributes to protecting us from these self-reactive T cells. It is well-documented that TGF-β can influence the differentiation of naïve CD4 T cells into effector T cells with different phenotypes. Most notably, TGF-β is known to promote the differentiation of CD4 regulatory T cells (Treg), with IL-2 and retinoic acid (RA) contributing to this process (Fig. 1) [17–20]. TGF-β + IL-6 have been shown to drive Th17 cell differentiation in vitro [21–23] and TGF-β + IL-4 have been shown to drive Th9 cell differentiation in vitro [24, 25]. In contrast, TGF-β is a negative regulatory for the differentiation of Th1 cells [26–28]. Using myelin-specific T cell receptor transgenic CD4 T cells, it was demonstrated that activation of naïve myelin-specific T cells in the presence of TGF-β1 results in reduced antigen-driven proliferation, failure to differentiate into effector T cells, and failure to induce experimental autoimmune encephalomyelitis (EAE) when adoptively transferred into mice [28]. Differentiation of myelin-specific T cell receptor transgenic CD4 T cells under Th1 cell conditions in the presence of TGF-β1 also resulted in T cells that had reduced IFNγ production and a reduced capacity to induce EAE (Fig. 2A). This is consistent with a previous study illustrating that TGF-β blocks IL-12-induced tyrosine phosphorylation, inhibiting the Jak-Stat pathway and differentiation of Th1 cells [26].
Figure 1. TGF-β influences the differentiation of subsets of CD4 T cells.
CD4 T cells can differentiate into several phenotypes. TGF-β in the presence of IL-6 promotes the differentiation of Th17 cells, but these cells are not highly encephalitogenic. TGF-β in the presence of IL-4 generated Th9 cells that have also been implicated in CNS autoimmunity, IL-9 can also have anti-inflammatory effects. TGF-β signaling is vital to the development and function of Tregs, which are necessary to prevent and control autoimmunity.
Figure 2. TGF-β negatively regulates naïve and effector CD4 T cells, but by distinct mechanisms.
TGF-β inhibits the proliferation and differentiation of naïve CD4 T cells, even under Th1 cell polarizing conditions. In contrast, TGF-β enhances cytokine production and proliferation of effector Th1 cells, but also upregulated the anti-inflammatory cytokine IL-10. Thus, TGF-β also alters myelin-specific effector Th1 cells such that they are no longer encephalitogenic.
Much less is known about how TGF-β affects effector T cells, particularly at sites of inflammation. Given that TGF-β is expressed in the central nervous system (CNS), understanding how TGF-β may alter the phenotype or function of effector T cells that infiltrate the CNS in the context of CNS infection and autoimmunity was important. To address this issue, Huss et al [28] differentiated myelin-specific T cell receptor transgenic CD4 T cells in vitro into Th1 cells which produced robust amounts of IFNγ and no IL-17, rested the Th1 cells, and then restimulated the myelin-specific Th1 cells in the presence of TGF-β1 or a TGF-β neutralizing antibody. Surprisingly, the Th1 cells activated with myelin peptide in the presence of TGF-β1 had an increase in proliferation, whereas the Th1 cells activated in the presence of α-TGF-β had reduced proliferation. Further analysis found that myelin-specific effector Th1 cells that were re-activated in the presence of TGF-β had increased activation markers and enhanced production of IFNγ. This indicated that TGF-β had the opposite effect on naïve and effector CD4 T cells with regard to activation and proliferation. Therefore, the presence of TGF-β in lymph nodes where naïve T cells typically encounter antigen would suppress T cell activation and differentiation, even if Th1-promoting cytokines, such as IL-12, were present. In contrast, TGF-β at the site of inflammation, such as the CNS in MS patients, may actually enhance proliferation and cytokine production of effector Th1 cells.
To further address this issue, myelin-specific T cell receptor transgenic Th1 cells were restimulated with antigen and TGF-β1 or α-TGF-β, and then transferred into naïve mice. Since TGF-β1 enhanced the activation and cytokine production by effector Th1 cells in vitro, it was anticipated that the TGF-β1-stimulated myelin-specific Th1 cells would be highly encephalitogenic. In contrast, these cells had a reduced capacity to cause CNS inflammation and demyelination (Fig. 2B) [28]. Furthermore, the myelin-specific Th1 cells cultured with α-TGF-β actually resulted in enhanced disease severity, suggesting that TGF-β was inducing a molecule or pathway in effector Th1 cells that negatively regulated their function, despite their increased activation. It was discovered that IL-10 was being induced in a dose-dependent manner in Th1 cells by TGF-β1. Transfecting the Th1 cells with a siRNA specific for IL-10, prior to activation with antigen plus TGF-β1, generated Th1 cells that had the same encephalitogenic potential as Th1 cells activated with antigen alone. This demonstrated that TGF-β1 induced robust IL-10 expression in effector Th1 cells that diminished the encephalitogenic capacity of myelin-specific Th1 cells. Since TGF-β, particularly TGF-β2 and TGF-β3, are expressed by glial and neuronal cells [29], infiltrating Th1 cells in the CNS of MS patients may be partially regulated by CNS-derived TGF-β.
In MS patients, inflammation in the CNS is somewhat cyclical with periods of significant inflammation, followed by periods of quiescence. However, it is clear that the number of inflammatory lesions in the CNS is significantly less than the number of clinical exacerbations [30]. Therefore, the exposure of effector T cells to the CNS environment is far more frequent than predicted by clinical indicators of disease. To explore how TGF-β may limit the function of chronically activated myelin-specific Th1 cells, as would occur in MS patients, Huss et al [31] analyzed the consequences of TGF-β1 exposure through multiple rounds of antigen activation of myelin-specific Th1 cells. The percentage of myelin-specific Th1 cells expressing IL-10, as well as the amount of IL-10, increased with each cycle of activation. This generated myelin-specific T cells that had a reduced capacity to induce EAE with each stimulation. TGF-β1 was shown to induce IL-10 via Smad4, and the encephalitogenicity could be restored by inhibiting IL-10. With each cycle of stimulation of myelin-specific Th1 cells with TGF-β1, the expression of IFNγ and T-bet were reduced, and the ability of these myelin-specific Th1 cells to migrate to the CNS was reduced. These studies illustrate that TGF-β1 can negatively regulate the function of effector Th1 cells, and this regulation is enhanced with each exposure of effector Th1 cells to the antigen in the presence of TGF-β1. These studies were done using TGF-β1, but since TGF-β2 and TGF-β3 also bind to the same receptor as TGF-β1, it is possible that CNS-produced TGF-β2 and TGF-β3 could regulate encephalitogenic Th1 cells in MS and contribute to remission. This is consistent with observations that IL-10 is elevated during remission [32]. However, this would also suggest that newly differentiated effector T cells would be needed to perpetuate CNS inflammation long-term.
Interest in TGF-β in MS increased over the past decade with the observation that IL-6 + TGF-β drove the differentiation of murine Th17 cells in vitro [21–23], and Th17 cells were identified as encephalitogenic in mice and humans [33–36]. The focus on the role of Th17 cells in MS began with the observation that IL-23 drove the expansion of murine myelin-specific Th17 cells and these cells were highly encephalitogenic when transferred into naïve mice [33]. In humans, it was found that IL-17 transcripts were present in CNS lesions [35], consistent with a previous observation that IL-17 mRNAwere elevated in the blood and CSF of MS patients [36]. Given that the data on the role of IFNγ and Th1 cells in EAE and MS was inconsistent, much of the focus shifted from Th1 to Th17 cells in the MS field.
Several studies demonstrated that IL-6 + TGF-β were sufficient to differentiate murine naïve CD4 T cells into Th17 cells in vitro [21–23]. To determine if these cytokines were sufficient to generate encephalitogenic Th17 cells, Yang et al [34] differentiated naïve myelin-specific T cell receptor transgenic CD4 T cells in vitro into Th17 cells with IL-6 + TGF-β and then transferred these effector Th17 cells into naïve mice. However, these myelin-specific Th17 cells failed to induce EAE. In contrast, transfer of myelin-specific Th17 cells differentiated with IL-6, while neutralizing the Th1 and Th2 pathways, were capable of inducing EAE when transferred into naïve mice. This data suggested that TGF-β negatively regulated the differentiation of encephalitogenic Th17 cells (Fig. 1). From studies investigating the effect of TGF-β on naïve T cell activation, it was found that TGF-β negatively regulates T-bet [27], a transcription factor that has been shown to be important in the differentiation and function of encephalitogenic Th1 and Th17 cells [37–39]. It was confirmed that myelin-specific Th17 cells differentiated with IL-6 + TGF-β lacked T-bet expression [34]. Perhaps, more importantly, these non-encephalitogenic Th17 cells expressed lower levels of GM-CSF and IL-23 receptor, two molecules known to be critical for encephalitogenicity [40].
The data between the EAE models varied regarding the contribution of TGF-β and Th17 cells in EAE. The studies by Huss et al [27, 31] utilized a T cell receptor transgenic mouse specific for myelin basic protein Ac1-11 (MBP Ac1-11) in which the naïve CD4 T cells can be differentiated and transferred following a single activation with antigen. Several studies have evaluated the role of IL-6 + TGF-β in Th17 cell differentiation using the widely used 2D2 mouse which has a transgenic T cell receptor specific for myelin oligodendrocyte glycoprotein 35–55 (MOG35-55). Using naïve 2D2 CD4 T cells, Jäger et al. [41] concluded that Th17 cells generated with IL-6 + TGF-β were encephalitogenic. However, the study used 2D2 Th17 cells that were transferred after two stimulations, the first activation included IL-6 + TGF-β, while the second activation lacked TGF-β. Yang et al [34] also found with the MBP Ac1-11-specific T cell receptor transgenic cells that primary stimulation with IL-6 + TGF-β, followed by a secondary stimulation with antigen only resulted in T cells that could transfer EAE, but these T cells expressed both IL-17 and IFNγ. The absence of TGF-β restored encephalitogenicity, and implied that the CD4+ T cells maintain plasticity and are not committed Th17 cells. In both models, secondary stimulation with IL-23 helps maintain IL-17 expression. It has previously been shown that TGF-β promotes the epigenetic modification of the Il17a-Il17f locus but this is reversible during restimulation of Th17 cells in the absence of TGF-β [42]. Overall, these studies would indicate that TGF-β negatively regulates the differentiation of encephalitogenic T cells, but that this can be overcome when myelin-specific T cells are reactivated in the absence of TGF-β.
Although IL-6 + TGF-β1 were insufficient to generate encephalitogenic T cells, the Kuchroo lab concluded that restimulation of Th17 cells generated with IL-6 + TGF-β1 in the presence of IL-23 produced stable Th17 cells that were highly pathogenic. They also discovered that these Th17 cells expressed TGF-β3 and hypothesized that the IL-23 induced TGF-β3 which stabilized the Th17 phenotype [43]. To address this hypothesis, naïve 2D2 T cells were initially differentiated with IL-6 + TGF-β1 or IL-6 + TGF-β3. What is not clear from this study is whether the T cells were re-stimulated in the absence of cytokines, as indicated in the publication that was cited for the methods, or transferred after primary stimulation. Regardless, transfer of Th17 cells generated with IL-6 + TGF-β3 were highly encephalitogenic compared to the Th17 cells generated with IL-6 + TGF-β1. This seemed somewhat surprising given that TGF-β1 and TGF-β3 signal through the same receptor. The study further demonstrated that the Th17 cells generated with IL-6 + TGF-β1 had a unique transcriptional profile compare to the Th17 cells generated with IL-6 + TGF-β3. A similar study was performed using the MBP Ac1-11-specific T cell receptor transgenic T cells and found that primary differentiation with IL-6 + TGF-β1 or IL-6 + TGF-β3 followed by adoptive transfer failed to induce EAE [40]. In addition, the phenotypic characterization of both Th17 cell populations found that these non-encephalitogenic Th17 cells produced robust amounts of IL-17, but failed to make GM-CSF or efficiently upregulate IL-23R expression. Thus, it remains unclear whether TGF-β3 has a differential role in Th17 cell differentiation than TGF-β1. However, a previous study proposed that TGF-β1 and TGF-β3 have opposing roles in CNS autoimmunity, due to the observation that TGF-β1 was elevated in the CNS of EAE mice, yet TGF-β3 was elevated in mice with ameliorated EAE due to 17β-estradiol treatment [44].
The cytokines that play a role in the differentiation of human Th17 cells remains controversial. It was initially published that IL-6 + IL-1β, but not TGF-β, were the critical cytokines for the generation of human Th17 cells [45]. However, another study could not replicate this data and found that IL-21 + TGF-β were the necessary cytokines for the differentiation of human Th17 cells [46]. It had also been shown that murine CD4 T cells could be differentiated into Th17 cells with IL-21 + TGF-β [47–49]. From these two studies, it was hypothesized that IL-6 + IL-1β enhanced IL-17 expression in memory cells that also expressed IL-21, and that IL-21 + TGF-β actually promoted the differentiation of Th17 cells from naïve CD4 T cells. These studies were all conducted in vitro, and thus, it is not possible to definitively determine what cytokines are critical for the development of human encephalitogenic Th17 cells in vivo, or the role that TGF-β may play in this process.
TGF-β has also been shown to play a role in the differentiation of Th9 cells which produce robust amounts of IL-9 [24, 25]. Using a two-step activation protocol with 2D2 T cells, it was shown that myelin-specific T cells differentiated in vitro with IL-4 + TGF-β, and then restimulated in the absence of cytokines, were capable of inducing EAE [41]. A pathogenic role for Th9 cells was a surprising outcome since it had previously been shown that IL-9 had regulatory function [50]. A recent study found that IL-9 negatively regulated Th17 cells [51]. In addition, IL-9 levels in the CSF of MS patients negatively correlated with inflammation, neurodegeneration and disease progression [51], supporting a regulatory role of IL-9 in MS. An in vitro study found that IL-9 in the presence of IFNγ promoted the proliferation of oligodendrocyte precursor cells, whereas IL-9 in the presence of IL-17 inhibited oligodendrocyte precursor cell proliferation, suggesting that Th9 cells may have inverse functions in the presence of Th1 or Th17 cells [52].
Understanding the role of TGF-β in defective regulatory T cells in MS
TGF-β plays a vital role in the development and function of CD4+ regulatory T cells (Tregs; Fig. 1). In mice with deficiencies in TGF-β signaling, the number of Tregs in adult mice appears normal, yet, the number of Tregs in the periphery during the first few days of life is significantly reduced [53–55]. It appears that a lack of TGF-β signaling inhibits the development of Tregs in the thymus. Since Tregs express CD25, the high affinity IL-2 receptor, this limited pool of Tregs likely proliferate in response to IL-2 resulting in normal numbers of Tregs in adult mice. Given that the Tregs in mice with impaired TGF-β signaling originate from a small pool of Tregs, there is a lack of diversity in the Treg repertoire [9, 54, 56]. Since Tregs function by recognizing the same antigen as the effector T cells that they regulate, lack of diversity would result in an inability to recognize and suppression an immune response to many antigens, particularly self-antigens. The inability of Tregs from TGFβ signaling-impaired mice to suppress autoimmunity has been attributed to a decreased repertoire of Tregs, as well as low Foxp3 expression. While it is clear that there is a Treg defect in MS patients, it is unclear whether this is due to a reduced number of Tregs or an impaired function of Tregs [57–62]. Similar to mice with impaired TGFβ signaling, Tregs of MS patients have a decreased T cell receptor repertoire [61], suggesting that they may be inherently prone to autoimmunity due to a lack of diversity in their Treg population. Thymic production of Tregs has been shown to be lower in MS patients, and the ability of Tregs to function properly appeared to be dependent on the number of new thymic emigrant Tregs, not the absolute number of Tregs [61, 63].
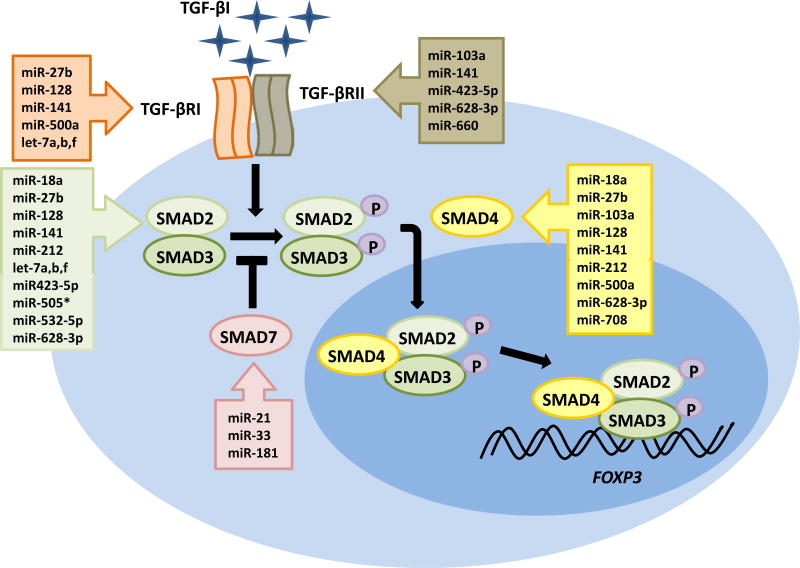
One leading hypothesis to understand the defect in Tregs in MS patients is based on the observation that miRNA, non-coding RNA that negatively regulate translation, play a vital role in Treg development [64–66]. Numerous studies have identified differential expression of miRNAs in MS patients [67–77]. It was shown that naïve CD4 T cells of MS patients have altered miRNA expression that influence their differentiation and favor the differentiation of pro-inflammatory Th1 cells [76]. Severin et al [78] identified 19 miRNAs that were differentially expressed in naïve CD4 T cells of MS patients that could potentially target components of the TGF-β signaling pathway (Fig. 3), including TGF-β receptor I (TGFβRI), TGF-β receptor II (TGFβRII), SMAD2, SMAD4 and SMAD7. In accordance with this observation, MS patients had reduced gene expression for TGFβRI and SMAD4 in their naïve CD4 T cells. Using naïve CD4 T cells from healthy individuals, it was shown that over-expression of the miRNAs found in MS patients that target TGFβRI and SMAD4 resulted in impaired Treg development in vitro. In a separate study, it was found that TGFβRII expression was reduced in MS patients’ CD4 T cells and this correlated with an increase in miR-17 [79], further supporting a role for miRNAs in the Treg defect observed in MS patients. These miRNAs may reduce natural Treg development in the thymus, similar to the limited production of Tregs in mice with impaired TGF-β signaling. In addition, inducible Treg (iTreg) differentiation may be reduced in the periphery since both types of Tregs are derived from naïve CD4 T cells. Ultimately, this could result in the generation of a less diverse population of Tregs that is incapable of suppressing self-reactive T cells and increasing the risk of autoimmunity. A clinical trial using TGF-β2 failed [80] which may now be explained by the observation the TGF-β signaling components are lower in CD4 T cells of MS patients, and thus, these cells would be less responsive to TGF-β.
Figure 3. Naïve CD4 T cells of MS patients have dysregulated miRNA expression which target the TGF-β signaling pathway and suppress Treg development and function.
Numerous miRNAs were found to be over-expressed in naïve CD4 T cells of MS patients that target TGF-βRI, TGF-βRII, SMAD2 and SMAD4. In contrast, 3 miRNA were down-regulated that were predicted to target SMAD7, a negative regulator of the TGF-β signaling pathway. Severin et al [78] found that these miRNAs reduced the ability of naïve CD4 T cells to differentiate into Tregs which may partially explain the Treg defect observed in MS patients.
Other studies have proposed that miRNAs that target the TGF-β pathway are critical regulators of Th17/Treg balance in MS. One study investigated if two miRNAs, miR-27a and miR-214 which had been found to be differentially expressed in MS patients, fluctuated with disease activity [81]. Analysis of the expression levels of these two miRNAs in CD4 T cells found that miR-27a was upregulated during relapse, in contrast to miR-214 which was upregulated during remission. Since miR-27a had previously been shown to target the TGF-β signaling pathway, they concluded that miR-27a may be regulating Treg function in MS patients. Naghavian et al [82] proposed that miR-141 and miR-200a may be regulating the balance between Th17 cells and Tregs. Both these miRNAs were upregulated during relapses in MS patients and down-regulated during remission. Given that they were predicted to target the TGF-β pathway, they proposed they the level of these miRNAs may influence the differentiation and/or function of Tregs. Both of these studies are predicated on the observation that TGF-β plays a critical role in both Th17 and Treg development. However, as stated above, it remains to be determined if TGF-β plays a vital role in encephalitogenic Th17 cells, particularly in humans.
Conclusion
The sum of the data would suggest that enhancement of TGF-β signaling in T cells of MS patients would be beneficial. Although TGF-β has been implicated in some potentially inflammatory T cell populations, such as Th9 and Th17 cells, the data is not compelling in human CD4 T cells. In contrast, there is a consensus that TGF-β enhances Treg development and function, and negatively regulates encephalitogenic Th1 cells. It is well-documented that there is a defect in the Treg population in MS patients, so modulation of TGF-β signaling may be used to correct this defect. This review focused on recent studies on T cells, but it is important to remember that MS is a complex disease and TGF-β signaling likely plays a vital role in other cells and tissues, particularly the gut where TGF-β expression is abundant and our immune system is tuned based on the interaction between the gut with the microbiota. And finally, TGF-β is highly expressed in the CNS and is known to play a vital role in CNS development and function. Thus, understanding the role that TGF-β signaling plays during CNS inflammation and repair is an area that is critical to understanding how TGF-β can be used or manipulated therapeutically in MS.
Acknowledgments
This work was supported by the National Multiple Sclerosis Society grants (RG 3812 and RG 5241) and the National Institutes of Health grants (RO1NS067441 and R21NS078390). P.W.L. is supported by award TL1TR000091-05 from Clinical Translational Science Award, funded by National Institutes of Health.
Abbreviations
- TGF-β
transforming growth factor-β
- MS
multiple sclerosis
- EAE
experimental autoimmune encephalomyelitis
- Treg
regulatory T cell
- RA
retinoic acid
- CNS
central nervous system
- MBP
myelin basic protein
Footnotes
Conflict of Interest
The authors declare no financial or commercial conflict of interest.
References
- 1.de Larco J, Todaro G. Growth factors from murine scarcoma virus-transformed cells. Proc. Natl. Acad. Sci. U.S.A. 1978;75:4001–4005. doi: 10.1073/pnas.75.8.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts A, Frolik C, Anzano M, Sporn M. Transforming growth factors from neoplastic and nonneoplastic tissues. Fed. Proc. 1983;42:2621–2626. [PubMed] [Google Scholar]
- 3.Sanford LP, Ormsby I, Gittenberger-de Groo A, Sariola H, Friedman R, Boivin GP, et al. TGFβ2 knockout mice have multiple developmental defects that are non-overlapping with other TGFβ knockout phenotypes. Development. 1997;127:2659–2670. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Proetzel G, Pawlowski SA, Wiles MV, et al. Transforming growth factor-beta 3 is required for secondary palate fusion. Nat. Genet. 1995;11:409–414. doi: 10.1038/ng1295-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaartinen V, Voncken J, Shuler C, Warburton D, Bu D, Heisterkamp N, Groffen J. Abnormal lung development and cleft palate in mice lacking TGF-beta 3 indicates defects of epithelial-mesenchymal interaction. Nat. Genet. 1995;11:415–421. doi: 10.1038/ng1295-415. [DOI] [PubMed] [Google Scholar]
- 6.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, et al. Targeted disruption of the mouse transforming growth factor-beta1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kehrl JH, Wakefield LM, Roberts AB, Jakowlew S, Alvarez-Mon M, Derynck R, Sporn MB, Fauci AS. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J. Exp. Med. 1986:1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Letterio JJ, Geiser AG, Kulkarni AB, Dang H, Kong L, Nakabayashi T, Mackall CL, Gress RE, Roberts AB. Autoimmunity associated with TGF-beta1-deficiency in mice is dependent on MHC class II antigen expression. J. Clin. Invest. 1996;98:2109–2119. doi: 10.1172/JCI119017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorelik L, Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 10.Ranges GE, Figari IS, Espevik T, Palladino MA., Jr Inhibition of cytotoxic T cell development by transforming growth factor beta and reversal by recombinant tumor necrosis factor alpha. J. Exp. Med. 1987;166:991–998. doi: 10.1084/jem.166.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi S, Yoshida K, Ward JM, Letterio JJ, Longenecker G, Yaswen L, Mittleman B, et al. Beta 2-microglobulin-deficient background ameliorates lethal phenotype of the TGF-beta 1 null mouse. J. Immunol. 1999;163:4013–4019. [PubMed] [Google Scholar]
- 12.Kuruvilla AP, Shah R, Hochwald GM, Liggitt HD, Palladino MA, Thorbecke GJ. Protective effect of transforming growth factor beta 1 on experimental autoimmune diseases in mice. Proc. Natl. Acad. Sci. USA. 1991;88:2918–2921. doi: 10.1073/pnas.88.7.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Racke MK, Dhib-Jalbut S, Cannella B, Albert PS, Raine CS, McFarlin DE. Prevention and treatment of chronic relapsing experimental allergic encephalomyelitis by transforming growth factor-beta 1. J. Immunol. 1991;146:3012–3017. [PubMed] [Google Scholar]
- 14.Johns LD, Flanders KC, Ranges GE, Sriram S. Successful treatment of experimental allergic encephalomyelitis with transforming growth factor-beta 1. J. Immunol. 1991;147:1792–1796. [PubMed] [Google Scholar]
- 15.Racke MK, Sriram S, Carlino J, Cannella B, Raine CS, McFarlin DE. Long-term treatment of chronic relapsing experimental allergic encephalomyelitis by transforming growth factor-beta 2. J. Neuroimmunol. 1993;46:175–183. doi: 10.1016/0165-5728(93)90247-v. [DOI] [PubMed] [Google Scholar]
- 16.Drescher KM, Murray PD, Lin X, Carlino JA, Rodriquez M. TGF-beta 2 reduces demyelination, virus antigen expression, and macrophage recruitment in a viral model of multiple sclerosis. J. Immunol. 2000;164:3207–3213. doi: 10.4049/jimmunol.164.6.3207. [DOI] [PubMed] [Google Scholar]
- 17.Chen W, Jin W, Hardegen N, Lei K, Li L, Marinos N, McGrady G, Wahl S. Conversion of peripheral CD4+CD25− naïve T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. J. Immunol. 2004;172:5149–5153. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 19.Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA. Natural and induced CD4+CD25+ cells educate CD4+CD25− cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J. Immunol. 2004;172:5213–5221. doi: 10.4049/jimmunol.172.9.5213. [DOI] [PubMed] [Google Scholar]
- 20.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 21.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 23.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 24.Veldhoen M, Uyttenhove C, van Snick J, et al. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat. Immunol. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 25.Dardalhon V, Awasthi A, Kwon H, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3()) effector T cells. Nat. Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bright JJ, Sriram S. TGF-beta inhibits IL-12-induced activation of Jak-STAT pathway in T lymphocytes. J. Immunol. 1998;161:1772–1777. [PubMed] [Google Scholar]
- 27.Gorelik L, Constant S, Flavell RA. Mechanism of transforming growth factor beta-induced inhibition of T helper type 1 differentiation. J. Exp. Med. 2002;195:1499–1505. doi: 10.1084/jem.20012076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huss DJ, Winger RC, Peng H, Yang Y, Racke MK, Lovett-Racke AE. TGF-beta enhances effector Th1 cell activation but promotes self-regulation via IL-10. J. Immunol. 2010;184:5628–5636. doi: 10.4049/jimmunol.1000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pelton RW, Saxena B, Jones M, Moses HL, Gold LI. Immunhistochemical localization of TGF-b1, TGF-b2 and TGFb3 in mouse embryo: Expression patterns suggest multiple roles during embryonic development. J. Cell Biol. 1991;115:1091–1105. doi: 10.1083/jcb.115.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stone LA, Smith ME, Albert PS, Bash CN, Maloni H, Frank JA, McFarland HF. Blood-brain barrier disruption on contrast-enhanced MRI in patients with mild relapsing-remitting multiple sclerosis: relationship to course, gender, and age. Neurology. 1995;45:1122–1126. doi: 10.1212/wnl.45.6.1122. [DOI] [PubMed] [Google Scholar]
- 31.Huss DJ, Winger RC, Cox GM, Guerau-de-Arellano M, Yang Y, Racke MK, Lovett-Racke AE. TGF-β signaling via Smad4 drives IL-10 production in effector Th1 cells and reduces T-cell trafficking in EAE. Eur. J. Immunol. 2011;41:2987–2996. doi: 10.1002/eji.201141666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carrieri PB, Provitera V, De Rosa T, Tartaglia G, Gorga F, Perrella O. Profile of cerebrospinal fluid and serum cytokines in patients with relapsing-remitting multiple sclerosis: a correlation with clinical activity. Immunopharmacol. Immunotoxicol. 1998;20:373–382. doi: 10.3109/08923979809034820. [DOI] [PubMed] [Google Scholar]
- 33.Langrish CL, Chen Y, Blumenschein M, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y, Weiner J, Liu Y, Smith AJ, Huss DJ, Winger R, Peng H, Cravens PD, Racke MK, Lovett-Racke AE. T-bet is essential for encephalitogenicity of both Th1 and Th17 cells. J. Exp. Med. 2009;206:1549–1564. doi: 10.1084/jem.20082584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat. Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 36.Matusevicius D, Kivisakk P, He B, Kostulas N, Ozenci V, Fredrikson S, Link H. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult. Scler. 1999;5:101–104. doi: 10.1177/135245859900500206. [DOI] [PubMed] [Google Scholar]
- 37.Lovett-Racke AE, Rocchini AE, Choy J, Northrop SC, Hussain RZ, Ratts RB, Sikder D, Racke MK. Silencing T-bet defines critical role in the differentiation of autoreactive T lymphocytes. Immunity. 2004;21:719–731. doi: 10.1016/j.immuni.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 38.Gocke AR, Cravens PD, Ben L, Hussain RZ, Northrop SC, Racke MK, Lovett-Racke AE. T-bet regulates the fate of Th1 and Th17 lymphocytes in autoimmunity. J. Immunol. 2007;178:1341–1348. doi: 10.4049/jimmunol.178.3.1341. [DOI] [PubMed] [Google Scholar]
- 39.Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, Ramos HL, Wei L, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-β signaling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee PW, Yang Y, Racke MK, Lovett-Racke AE. Analysis of TGF-β1 and TGF-β3 as regulators of encephalitogenic Th17 cells: Implications for multiple sclerosis. Brain Behav. Immun. 2015;46:44–49. doi: 10.1016/j.bbi.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jäger A, Dardalhon V, Sobel RA, Bettelli E, Kuchroo VK. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J. Immunol. 2009;183:7169–7177. doi: 10.4049/jimmunol.0901906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mukasa R, Balasubramani A, Lee YK, Whitley SK, Weaver BT, Shibata Y, Crawford GE, Hatton RD, Weaver CT. Epigenetic instability of cytokine and transcription factor gene loci underlies plasticity of the T helper 17 cell lineage. Immunity. 2010;32:616–627. doi: 10.1016/j.immuni.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, Peters A, Wu C, et al. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol. 2012;13:991–999. doi: 10.1038/ni.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matejuk A, Dwyer J, Hopke C, Vandenbark AA, Offner H. Opposing roles for TGF-beta1 and TGF-beta3 isoforms in experimental autoimmune encephalomyelitis. Cytokine. 2004;25:45–51. doi: 10.1016/j.cyto.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 45.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 46.Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, Kuchroo VK, Hafler DA. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 49.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, et al. IL-6 programs TH-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nature Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 50.Schmitt E, Bopp T. Discovery and initial characterization of Th9 cells: the early years. Semin. Immunopathol. 2016 Nov 28; doi: 10.1007/s00281-016-0610-0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 51.Ruocco G, Rossi S, Motta C, Macchiarulo G, Barbieri F, De Bardi M, Borsellino G, et al. T helper 9 cells induced by plasmacytoid dendritic cells regulate interleukin-17 in multiple sclerosis. Clin. Sci. (Lond) 2015;129:291–303. doi: 10.1042/CS20140608. [DOI] [PubMed] [Google Scholar]
- 52.Ding X, Cao F, Cui L, Ciric B, Zhang GX, Rostami A. IL-9 signaling affects central nervous system resident cells during inflammatory stimuli. Exp. Mol. Pathol. 2015;99:570–574. doi: 10.1016/j.yexmp.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061–7. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Y, Zhang P, Li J, Kulkarni AB, Perruche S, Chen W. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol. 2008;9:632–40. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- 55.Ouyang W, Beckett O, Ma Q, Li MO. Transforming growth factor-beta signaling curbs thymic negative selection promoting regulatory T cell development. Immunity. 2010;32:642–53. doi: 10.1016/j.immuni.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marie JC, Liggitt D, Rudensky AY. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity. 2006;25:441–54. doi: 10.1016/j.immuni.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 57.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–9. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haas J, Hug A, Viehöver A, Fritzsching B, Falk CS, Filser A, et al. Reduced suppressive effect of CD4+CD25 high regulatory T cells on the T cell immune response against myelin oligodendrocyte glycoprotein in patients with multiple sclerosis. Eur J Immunol. 2005;35:3343–52. doi: 10.1002/eji.200526065. [DOI] [PubMed] [Google Scholar]
- 59.Huan J, Culbertson N, Spencer L, Bartholomew R, Burrows GG, Chou YK, et al. Decreased FOXP3 levels in multiple sclerosis patients. J Neurosci Res. 2005;81:45–52. doi: 10.1002/jnr.20522. [DOI] [PubMed] [Google Scholar]
- 60.Kumar M, Putzki N, Limmroth V, Remus R, Lindemann M, Knop D, et al. CD4+CD25+FoxP3+ T lymphocytes fail to suppress myelin basic protein-induced proliferation in patients with multiple sclerosis. J Neuroimmunol. 2006;180:178–84. doi: 10.1016/j.jneuroim.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 61.Haas J, Fritzsching B, Trübswetter P, Korporal M, Milkova L, Fritz B, et al. Prevalence of newly generated naive regulatory T cells (Treg) is critical for Treg suppressive function and determines Treg dysfunction in multiple sclerosis. J Immunol. 2007;179:1322–30. doi: 10.4049/jimmunol.179.2.1322. [DOI] [PubMed] [Google Scholar]
- 62.Venken K, Hellings N, Thewissen M, Somers V, Hensen K, Rummens JL, et al. Compromised CD4+ CD25(high) regulatory T-cell function in patients with relapsing-remitting multiple sclerosis is correlated with a reduced frequency of FOXP3-positive cells and reduced FOXP3 expression at the single-cell level. Immunology. 2008;123:79–89. doi: 10.1111/j.1365-2567.2007.02690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Balint B, Haas J, Schwarz A, Jarius S, Fürwentsches A, Engelhardt K, et al. T-cell homeostasis in pediatric multiple sclerosis: old cells in young patients. Neurology. 2013;81:784–92. doi: 10.1212/WNL.0b013e3182a2ce0e. [DOI] [PubMed] [Google Scholar]
- 64.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–64. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gao Y, Lin F, Su J, Gao Z, Li Y, Yang J, et al. Molecular mechanisms underlying the regulation and functional plasticity of FOXP3(+) regulatory T cells. Genes Immun. 2012;13:1–13. doi: 10.1038/gene.2011.77. [DOI] [PubMed] [Google Scholar]
- 66.Dooley J, Linterman MA, Liston A. MicroRNA regulation of T-cell development. Immunol Rev. 2013;253:53–64. doi: 10.1111/imr.12049. [DOI] [PubMed] [Google Scholar]
- 67.De Santis G, Ferracin M, Biondani A, Caniatti L, Rosaria Tola M, Castellazzi M, et al. Altered miRNA expression in T regulatory cells in course of multiple sclerosis. J Neuroimmunol. 2010;226:165–71. doi: 10.1016/j.jneuroim.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 68.Gandhi R, Healy B, Gholipour T, Egorova S, Musallam A, Hussain MS, et al. Circulating microRNAs as biomarkers for disease staging in multiple sclerosis. Ann Neurol. 2013;73:729–40. doi: 10.1002/ana.23880. [DOI] [PubMed] [Google Scholar]
- 69.Otaegui D, Baranzini SE, Armañanzas R, Calvo B, Muñoz-Culla M, Khankhanian P, et al. Differential micro RNA expression in PBMC from multiple sclerosis patients. PLoS One. 2009;4:e6309. doi: 10.1371/journal.pone.0006309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Keller A, Leidinger P, Lange J, Borries A, Schroers H, Scheffler M, et al. Multiple sclerosis: microRNA expression profiles accurately differentiate patients with relapsing-remitting disease from healthy controls. PLoS One. 2009;4:e7440. doi: 10.1371/journal.pone.0007440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Du C, Liu C, Kang J, Zhao G, Ye Z, Huang S, et al. MicroRNA miR-326 regulates TH-17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nat Immunol. 2009;10:1252–9. doi: 10.1038/ni.1798. [DOI] [PubMed] [Google Scholar]
- 72.Noorbakhsh F, Ellestad KK, Maingat F, Warren KG, Han MH, Steinman L, et al. Impaired neurosteroid synthesis in multiple sclerosis. Brain. 2011;134:2703–21. doi: 10.1093/brain/awr200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Søndergaard HB, Hesse D, Krakauer M, Sørensen PS, Sellebjerg F. Differential microRNA expression in blood in multiple sclerosis. Mult Scler. 2013;19:1849–57. doi: 10.1177/1352458513490542. [DOI] [PubMed] [Google Scholar]
- 74.Ridolfi E, Fenoglio C, Cantoni C, Calvi A, De Riz M, Pietroboni A, et al. Expression and Genetic Analysis of MicroRNAs Involved in Multiple Sclerosis. Int J Mol Sci. 2013;14:4375–84. doi: 10.3390/ijms14034375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith KM, Guerau-de-Arellano M, Costinean S, Williams JL, Bottoni A, Mavrikis Cox G, et al. miR-29ab1 deficiency identifies a negative feedback loop controlling Th1 bias that is dysregulated in multiple sclerosis. J Immunol. 2012;189:1567–76. doi: 10.4049/jimmunol.1103171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guerau-de-Arellano M, Smith KM, Godlewski J, Liu Y, Winger R, Lawler SE, et al. Micro-RNA dysregulation in multiple sclerosis favours pro-inflammatory T-cell-mediated autoimmunity. Brain. 2011;134:3578–89. doi: 10.1093/brain/awr262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oe F, Jr, Moore CS, Kennedy TE, Antel JP, Bar-Or A, Dhaunchak AS. MicroRNA dysregulation in multiple sclerosis. Front Genet. 2012;3:311. doi: 10.3389/fgene.2012.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Severin ME, Lee PW, Liu Y, Gormley MG, Pei W, Yang Y, Guerau-de-Arellano M, Racke MK, Lovett-Racke AE. MicroRNAs targeting TGFβ signaling underlie the regulatory T cell defect in multiple sclerosis. Brain. 2016;139:1747–1761. doi: 10.1093/brain/aww084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meira M, Sievers C, Hoffmann F, Rasenack M, Kuhle J, Derfuss T, Kappos L, Lindberg RLP. Unraveling Natalizumab effects on deregulated miR-17 expression in CD4+ T cells of patients with relapsing-remitting multiple sclerosis. J. Immunol. Res. 2014 doi: 10.1155/2014/897249. Article ID 897249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Calabresi PA, Fields NS, Maloni HW, Hanham A, Carlino J, Moore J, Levin MC, Dhib-Jalbut S, Tranquill LR, Austin H, McFarland HF, Racke MK. Phase 1 trial of transforming growth factor beta 2 in chronic progressive MS. Neurology. 1998 Jul;51(1):289–92. doi: 10.1212/wnl.51.1.289. [DOI] [PubMed] [Google Scholar]
- 81.Ahmadian-Elmi M, Bidmeshki Pour A, Naghavian R, Ghaedi K, Tanhaei S, Izadi T, Nasr-Esfahani MH. miR-27a and miR-214 exert opposite regulatory roles in Th17 differentiation via mediating different signaling pathways in peripheral blood CD4+ T lymphocytes of patients with relapsing-remitting multiple sclerosis. Immunogenetics. 2016;68:43–54. doi: 10.1007/s00251-015-0881-y. [DOI] [PubMed] [Google Scholar]
- 82.Naghavian R, Ghaedi K, Kiani-Esfahani A, Ganjalikhani-Hakemi M, Etemadifar M, Nasr-Esfahani MH. miR-141 and miR-200a, revelation of new possible players in modulation of Th17/Treg differentiation and pathogenesis of multiple sclerosis. PLoS One. 2015;10:e0124555. doi: 10.1371/journal.pone.0124555. [DOI] [PMC free article] [PubMed] [Google Scholar]