Abstract
Parkinson’s disease (PD) is the second most common age-related neurodegenerative disease. 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is a prototypical neurotoxicant used in mice to mimic primary features of PD pathology including striatal dopamine depletion and dopamine neuron loss in the substantia nigra pars compacta (SNc). In the literature, there are several experimental paradigms involving multiple doses of MPTP that are used to elicit dopamine neuron loss. However, a recent study reported that a single low dose caused significant loss of dopamine neurons. Here, we determined the effect of a single intraperitoneal injection of one of three doses of MPTP (0.1, 2 and 20 mg/kg) on dopamine neurons, labeled by tyrosine hydroxylase (TH+), and total neuron number (Nissl+) in the SNc using unbiased stereological counting. Data reveal a significant loss of neurons in the SNc (TH+ and Nissl+−) only in the group treated with 20 mg/kg MPTP. Groups treated with lower dose of MPTP (0.1 and 2 mg/kg) only showed significant loss of TH+ neurons rather than TH+ and Nissl+. Striatal dopamine levels were decreased in the groups treated with 2 and 20 mg/kg MPTP and striatal terminal markers including, TH and the dopamine transporter (DAT), were only decreased in the groups treated with 20 mg/kg MPTP. These data demonstrate that lower doses of MPTP likely result in loss of TH expression rather than actual dopamine neuron loss in the SN. This finding reinforces the need to measure both total neuron number along with TH+ cells in determining dopamine neuron loss.
Keywords: MPTP, stereology, tyrosine hydroxylase, dopamine transporter, vesicular monoamine transporter, dopamine
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease among the elderly in which the individual suffers from motor impairments. Nearly 1% of the world population suffers from PD, clinically diagnosed with rigidity, resting tremor, and bradykinesia [1, 2]. Pathophysiological features of PD consist of dopaminergic cell degeneration in the substantia nigra pars compacta (SNc), striatal dopamine (DA) depletion and protein aggregates compromised of alpha-synuclein and ubiquitin [2–4].
Multiple genetic and toxin animal models of PD have been developed to study the underlying mechanisms of this complex neurodegenerative disease. Transgenic mouse models of multiple genes involved in the development of PD such as alpha synuclein, Parkin, PINK, LRRK2, DJ-1 have been proven to be beneficial in the study of PD, but typically do not reproduce the full manifestation of PD phenotypes [5–11]. The case of frozen addicts and the discovery of the involvement of MPTP in the development of Parkinson like symptoms in these individuals triggered the research using MPTP as a neurotoxicant to mimic PD in animal models such as nonhuman primates and mice [12]. MPTP is the most widely used prototypical neurotoxicant to study the underlying mechanisms of PD [13–15]. High doses of MPTP result in DA neuron loss in the SNc and striatal DA depletion. Thus, MPTP animal models are valuable tools to study PD, as they mimic many aspects of the pathophysiology observed in PD.
Numerous MPTP models incorporating cumulative MPTP doses ranging between 20–250mg/kg, either as several doses in one day or over the course of several days to a week, have been developed in order to study the neurodegeneration of the nigrostriatal pathway [13, 14, 16]. Although multiple high doses (10–30 mg/kg) of MPTP are generally required to cause DA neuron loss, a recent study reported that single low doses of MPTP (0.1 – 2 mg/kg) caused a loss of tyrosine hydroxylase positive (TH+) cells in young OF1 mice [16]. The purpose of this study was to determine the effects of lower single MPTP doses on dopamine neuron loss and dopaminergic neurochemistry and assess whether similar results are obtained in C57BL/6J mice, the best-characterized and most commonly used strain of mice used for MPTP studies.
Materials and Methods
Chemicals
MPTP-HCl was purchased from Sigma Aldrich (Milwaukee, WI, USA). Polyclonal rabbit anti-TH (cat#AB152) was purchased from EMD Millipore (Billerica, MA, USA). Monoclonal rat anti-DAT (cat# AB5990) and α-tubulin antibody (cat#AB80799) were purchased from Abcam (Cambridge, UK) and polyclonal rabbit anti-VMAT2 was a gift from Dr. Gary Miller at Emory University. Alexafluor secondary antibodies (anti-rabbit and anti-mouse) were purchased from Thermo-Fisher Scientific (Waltham, MA). Horseradish peroxidase conjugated secondary antibodies were purchased from Bio-Rad (Hercules CA). Biotinylated secondary antibody and 3,3-diaminobenzidine–peroxidase (DAB) including nickel enhancer for immunohistochemistry were purchased from Vector laboratories (PK-6100, SK-4100, Burlingame, CA). Monoamine standards for high performance liquid chromatography- electrochemical detection (HPLC-ECD) analysis for dopamine (DA), 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) were purchased from Sigma-Aldrich (Milwaukee, WI, USA). All other chemicals were purchased from Sigma Aldrich or Thermo-Fisher unless specifically noted.
Animals
C57BL/6J male mice (3–4 months old) were obtained from Jackson laboratories (Bar Harbor, ME). Animals were group housed with a maximum of three animals per cage and acclimated to the vivarium for a week prior to initiation of the study. Animals were kept in a normal 12:12 hour light/dark cycle with free access to food and water. All procedures were conducted in accordance with the National Institutes of Health’s Guide for Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the Northeast Ohio Medical University.
Experimental Design and Treatment
Mice (n = 5–6 per group) received a single intraperitoneal (i.p) injection of either saline or MPTP in a total volume of 100 μl. The three MPTP doses of 0.1, 2 and 20 mg/kg were selected based on the study conducted by Dovero and colleagues [16]. Seven days after dosing, mice were euthanized. The whole midbrain and right hemisphere of the striatum were harvested and immersion fixed in 4% paraformaldehyde (PFA) for 72 hours. After fixation, the brain samples were placed in 30% sucrose and stored at 4°C until immunohistochemical processing. Striatum from the left hemisphere was dissected out from the forebrain, frozen in liquid nitrogen and stored at −80 °C until HPLC and western blotting was performed. During the course of study, all animals were weighed twice, once before dosing and a week after dosing (before they were euthanized).
Sample Preparation
The dissected left striatum of each animal was sonicated in 500ul of homogenizing buffer (320 mM sucrose, 5 mM HEPES and 1μl/ml protease inhibitor cocktail; pH 7.4). Each sample was then aliquoted into two different tubes of 200 and 300 μl to be used in HPLC and western blot analysis, respectively.
HPLC with Electrochemical Detection of Striatal Neurochemistry
The aliquoted homogenates for HPLC were further diluted with 0.2 N perchloric acid at a 1:1 ratio and centrifuged at 14000 × rpm for 20 min at 4°C; supernatants were collected and filtered using a 0.45 micron filtered tube. The pellets were dried and kept for protein assay. HPLC analysis for catecholamines was performed essentially as described by our previous work [17]. Briefly, an aliquot of 10 μl of the supernatant was injected into the HPLC (Waters, Milford, MA, USA) for neurochemical analysis of DA and its metabolites, DOPAC and HVA. A cation exchange reverse column (MD-150 x 3.2 column, ESA Biosciences Inc) was used to separate the components. Isocratic mobile phase (MD-TM mobile phase, Thermo-Fisher Scientific, Waltham, MA) containing 2.2 mM filtered NaCl adjusted to pH 3.3 pumped at a constant flow rate of 0.4 ml/min was used. The compounds were quantified by electrochemical detection using glossy carbon working electrode, 2.0 mm diameter in situ silver reference electrode (Flow cell, 2mm GC, ISAC, waters, Milford, MA, USA). Quantification of DA and metabolites was made with reference to the standard curves obtained from individual standards (DA, DOPAC, and HVA) and were normalized to protein concentration measured by BCA kit (Thermo-Fisher Scientific, Waltham, MA).
Western Immunoblots
Western blots were performed as described previously [18, 19]. Briefly, the homogenates were centrifuged at 3500 × rpm for 5 minutes at 4° C. The supernatant was collected and centrifuged at 14000 × rpm for 45 minutes at 4° C. The pellets were re-suspended in 100 μl of homogenization buffer supplemented with 0.1% protease inhibitor. Following protein quantification, 20 μg of protein sample was loaded per lane on 4–12 % Tris-Bis NuPage gels (Invitrogen, Carlsbad, CA). After electrophoresis, proteins from the gels were transferred to a polyvinylidene difluoride membrane, using the iBlot transfer machine. The nonspecific protein-binding sites on the membrane were blocked with 7.5% nonfat dry milk in Tris-buffered saline (135mM NaCl, 2.5mM KCl, 50mM Tris, and 0.1% Tween 20, pH 7.4). After Blocking, the membrane was incubated in a rabbit polyclonal TH antibody (1:2000) in Tris-buffered saline with 2% nonfat dry milk overnight at 4°C. The membrane was washed three times and incubated in a goat anti-rabbit horseradish peroxidase secondary antibody (1:20,000) for an hour at room temp. The specific antibody-bound protein was detected by chemiluminescence using an Alpha Innotech Fluorochem (San Leandro, CA) imaging and stored as a digital image. The membrane was then stripped for 15 min at 25°C in Stripping Buffer (Thermo fisher Scientific, cat# P146430) and reprobed sequentially with a monoclonal antibody to the N-terminus of DAT (1:1,000) and a polyclonal VMAT2 (1:1000) antibody. A monoclonal α-tubulin antibody (1:1,000) was used to confirm equal loading of samples and the densitometric value for each sample was normalized to its associated α-tubulin value for statistical analysis.
Immunohistochemistry and Unbiased Stereology
Midbrains were coronally sectioned (40 μM) on a freezing, sliding microtome (HM 450 microtome, Thermo Fisher Scientific, Waltham, MA). The sections containing the substantia nigra were collected and kept in a cryoprotective solution (25% sucrose and 25% ethylene glycol in 1 x PBS) at −20° C until processed for immunohistochemical staining.
For stereology purposes, every 6th section through the substantia nigra with a total of 6 sections per animal was processed for Nissl staining (0.5% cresyl violet) and subsequently for TH immunohistochemistry. Nissl staining was used to identify the region of interest (SN) and for total cell counts in the SN. For immunohistochemistry, the sections were pre-treated with 75% methanol and 2.5% H2o2 to quench the endogenous peroxidase and then pre-blocked in dilution buffer (4% normal serum, 0.6%triton x-100 and 5% BSA in 1x-PBS) and incubated with rabbit anti-TH polyclonal antibody (Millipore, Bedford, MA, AB152, RRID AB_390204) at a dilution of 1:1,000 at 4°C for 48 hours. The sections were then incubated with the biotinylated secondary antibody of goat anti rabbit (Vector laboratory, Burlingame, CA) for 1 hr at a 1:200 dilution followed by the avidin-peroxidase complex. DAB substrate with a nickel enhancer was used as the chromogen.
Unbiased stereological counting was performed using Leica DM 2500 LED microscope. Unilateral counts of TH+ and Nissl+ cells in the SNc were performed on every sixth section throughout the SN. Borders of the SN were determined using a 5x objective and then the cells were counted at 63x magnification. A counting frame of 50 μm × 50 μm with framing space of 200 μm and a height of 10 μm was chosen. Only the cells that came into focus within the counting frame height were counted. The coefficient error for all the animals counted was equal or below 0.1 and a minimum of 100 markers were counted within 40–60 framing sites for each animal.
Immunofluorescent Staining for Terminal Markers
For immunofluorescent staining, striatal sections were rinsed, blocked in 10% goat serum in 0.3% Triton X 100 in 1x PBS for 1 hour to prevent the nonspecific bindings. The sections were then incubated in rabbit polyclonal TH (1:2000 in 5% goat serum) and rat monoclonal DAT (1:1000 in 5% goat serum) antibodies at 4°C overnight and then incubated in Alexa fluor anti-rabbit and anti-rat (1:250, in 5% goat serum) secondary antibodies. Sections were rinsed and mounted on slides for imaging processes with an Olympus FXS-100 fluorescent microscope under identical settings. Fluorescent intensity of the striatal images were quantified using image J software.
Statistical analysis
Data are reported as means ± SEM. Data were analyzed using one-way ANOVA with Fisher’s LSD post hoc test for comparisons of each treatment group with the control. Graphs and statistical analyses were performed using GraphPad prism (version 6.07). Significance is reported at p<0.05.
Results
2.1. Effects of MPTP treatment on dopamine neuron loss and TH expression
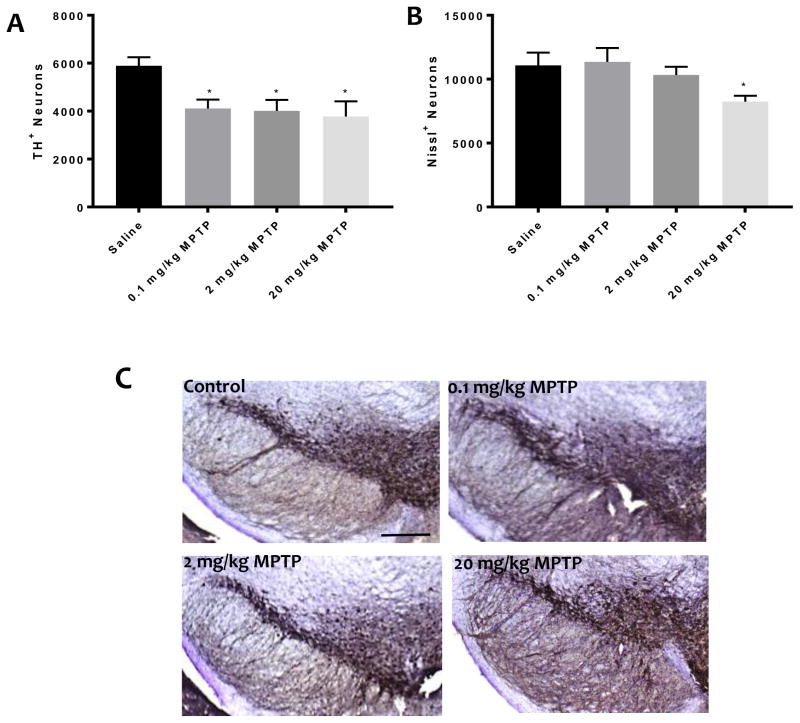
Unbiased stereological counts of TH+ cells in the SNc revealed significant reduction in the number of dopamine neurons (TH+) in all the MPTP treated groups compared to control (F(3,18)= 4.385, p<0.05) (Fig. 1). Nissl+ cells were also counted to determine whether or not this loss of TH+ represented a true loss of dopamine neurons (Fig. 1). Significant Nissl+ cell loss was only observed in the highest MPTP (20 mg/kg) treatment group (F(3,18)= 3.02, p<0.05).
Fig. 1. Effect of MPTP on dopamine neuron loss in the substantia nigra.
Unbiased stereology counts of (A) TH+ neurons and (B) Nissl stained neurons of one brain hemisphere in mice treated with 0.1, 2 or 20 mg/kg.* indicates significantly different from control (p<0.05, n=5–6). (C) Representative TH immunostaining of saline and MPTP treatment groups, scale bar shows 250 μm.
2.2. Effects of MPTP on striatal catecholamine levels
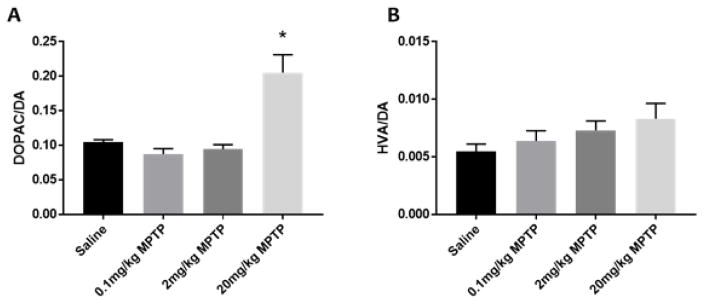
Striatal DA was reduced in a dose-related manner by MPTP (Fig. 2). However, significant reduction in the striatal DA was observed in the groups treated with 2 and 20 mg/kg MPTP compared to controls (F(3,13)=22.36, p<0.05). DOPAC was significantly reduced in all MPTP treated groups compared to controls (F(3,13)= 9.28, p<0.05). Striatal HVA concentration was significantly reduced only in the high dose MPTP (20 mg/kg) treated group (F(3,14)= 9.34, p<0.05). MPTP also resulted in a significant increase of the ratio of DOPAC/DA only in the group treated with 20 mg/kg MPTP (F(3,11) = 14. 42, p<0.05, Fig. 3). MPTP treatment did not result in a significant change in the HVA/DA turnover ratio (p>0.05, Fig. 3).
Fig. 2. Effect of MPTP on dopaminergic neurochemistry in the striatum of C57BL/6J mice.
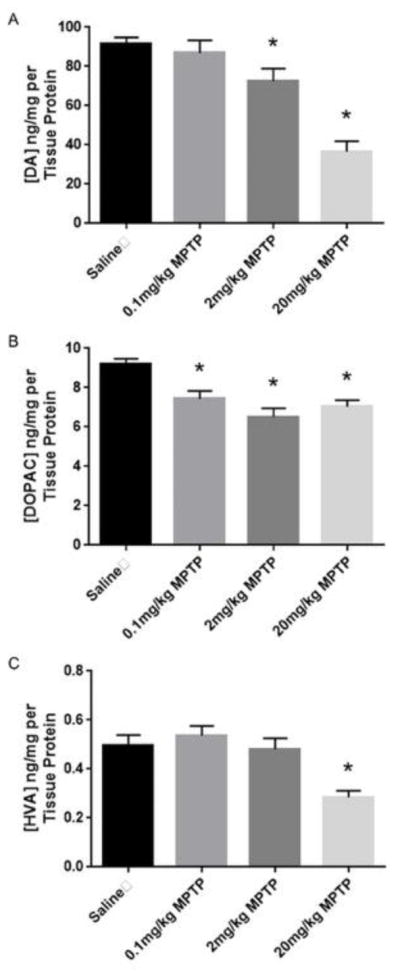
(A) MPTP treatment reduced striatal DA levels in a dose response manner. (B) MPTP treatment had a significant effect on DOPAC in all the MPTP treatment groups. (C) MPTP treatment (20 mg/kg) significantly reduced the striatal level of HVA. * indicates significantly different from control (p<0.05, n=5–6).
Fig. 3. Effect of MPTP on DA turnover as determined by DOPAC/DA and HVA/DA ratios in the striatum of C57BL/6J mice.
High dose of MPTP treatment significantly increased (A) DOPAC/DA ratio, but no significant effect on the (B) HVA/DA ratio was observed. * indicates significantly different from control (p<0.05, n=5–6).
2.3. Effects of MPTP on the levels of striatal DA terminal markers
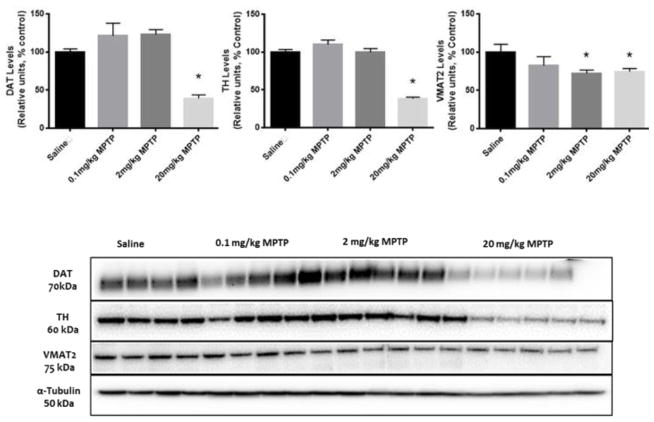
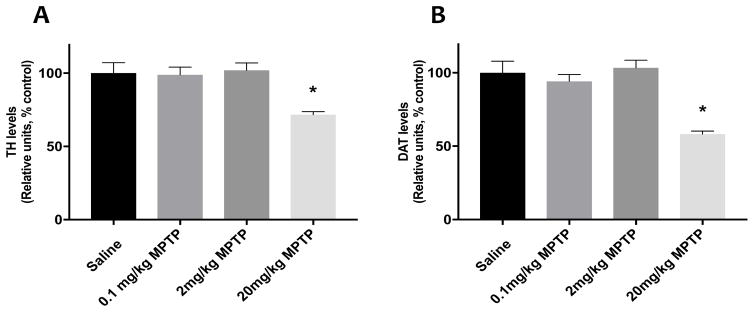
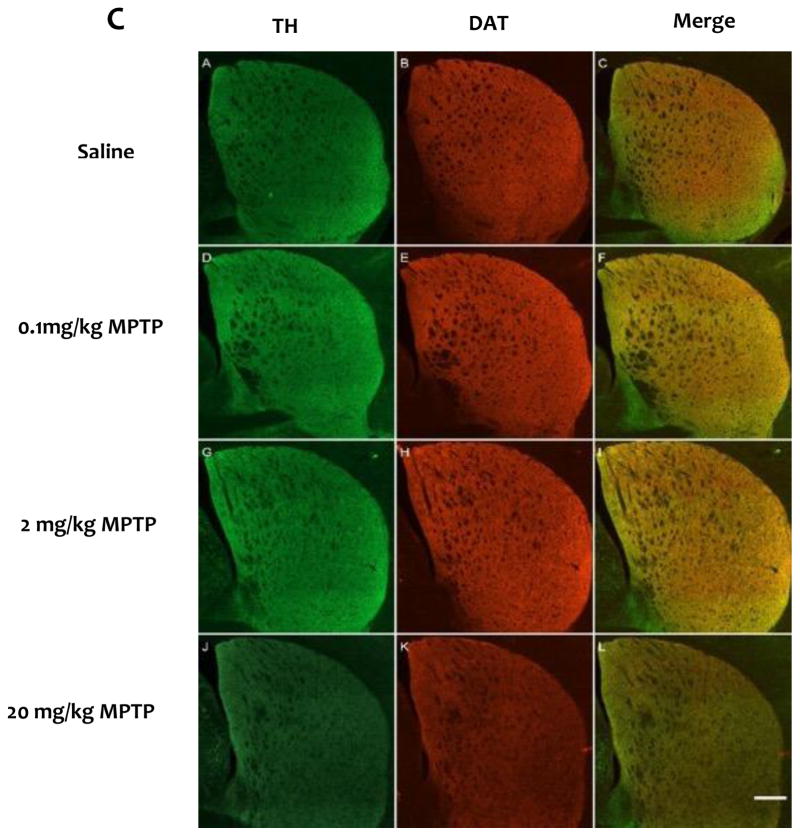
Using western immunoblots, we observed significantly lower striatal levels of TH (F(3, 14) = 22.27, p<0.05) and DAT (F(3, 14) = 69.56, p<0.05) only in the group treated with 20mg/kg MPTP (Fig. 4). MPTP treatments (2 and 20 mg/kg) significantly changed the striatal levels of VMAT2 (F(3,14)= 2.66, p<0.05) compared to controls. (Fig. 4). Immunofluorescent analysis revealed a significant reduction in TH and DAT immunoreactivity (F (3,18) = 9.12, F(3,18) =17.7, respectively, p<0.05 for both) in the striatum of the mice treated with 20 mg/kg MPTP compared to controls (Fig. 5).
Fig. 4. Effect of MPTP treatment on dopaminergic terminal markers.
Upper panel: Quantification of the WB for DAT, TH and VMAT2 in the striatum. Lower panel: Striatal levels of the dopaminergic terminal markers (DAT, TH, VMAT2) measured by western blot assay. Tubulin levels were used to ensure equal loading. * indicates significantly different from control (p<0.05, n=5–6).
Fig. 5.
Quantification of the striatal TH (A) and DAT (B) immunofluorescent images. C) Representative Immunostaining of TH and DAT in the striatum. Scale bar represents 500 μm.
Discussion
The goal of this study was to identify the effects of single low doses of MPTP on DA neuron loss and neurochemical markers of DA terminal integrity. Another goal was an attempt to replicate the results of the Dovero study [16], using C57BL/6J mice, the best characterized and most often used mouse strain for assessing MPTP neurotoxicity. Our data demonstrate that a single dose of 20 mg/kg MPTP causes overt nigral dopamine neuron loss that is accompanied by loss of striatal dopamine and terminal markers. In contrast, lower single doses only reduce TH+ cell counts with no significant effect on neuron number and limited or no effects on striatal dopamine levels or dopamine terminal markers. Taken together, these data demonstrate the importance of counting total neurons in addition to TH+ neurons to determine cell loss in the SNc.
As mentioned above, the study by Dovero and colleagues reported the intriguing finding that very low doses of MPTP (0.1 mg/kg) not normally associated with neurotoxicity caused loss of TH+ neurons. Unfortunately, comparing the result of this study to the Dovero study is challenging as that manuscript lacked information on the total cell counts in the SNc and no other measures of dopaminergic neurodegeneration. Nigrostriatal degeneration leading to PD is a long progressive process which develops over the course of decades and a significant loss of DA neurons and striatal DA is required before clinical symptoms are apparent [20]. Similarly, MPTP models demonstrate time-related loss of striatal DA and DA neuron loss, which typically peak 2–7 days after administration of MPTP [13, 21]. Here, we observed a significant loss of TH expression in the SNc 7 days after a single dose of 0.1, 2 and 2 mg/kg MPTP. In contrast to the study by Dovero, the two lower doses did not display overt DA neuron loss nor striatal loss of the terminal markers of TH and DAT, and striatal DA loss was not observed at the lowest dose. Although it is not clear why a loss of TH+ cells was observed in mice that did not display loss of Nissl+ neurons, it has been reported that there is a reduction of TH protein levels and TH mRNA levels in the surviving dopamine neurons in PD patients [22]. A study conducted by Kastner and colleagues also showed a reduction in the TH content in surviving dopaminergic neurons after MPTP treatment in monkeys [23] and reduction of TH protein levels and TH mRNA levels in the SNc and striatum of mice treated with MPTP has also been reported [24, 25]. TH+ cell loss was also found to occur significantly prior to Nissl+ neuron loss in the 4×16 mg/kg MPTP model, which was maximal at 4–7 days after the last MPTP injection [13]. Taken together, these studies suggest MPTP has direct transcriptional and/or translational effects on TH. Additional studies incorporating determination of TH mRNA levels in the DA neurons of the SNc coupled with measurement of TH enzymatic activity will be needed to accurately assess the alteration of TH early in the process of progressive dopaminergic degeneration or with doses of neurotoxicants that do not lead to overt loss of neurons.
To further to examine the effects of single low doses of MPTP on the nigrostriatal system, we assessed striatal dopamine levels along with markers of dopamine neuron integrity, including the dopamine transporter (DAT), vesicular monoamine transporter 2 (VMAT2) and TH. In contrast to the effects in the SNc, doses as low as 2 mg/kg MPTP resulted in a small, but significant loss of striatal DA and the lowest MPTP dose (0.1 mg/kg) resulted in decreased DOPAC levels. The reduction in striatal dopamine at 2 mg/kg MPTP was accompanied by decreased VMAT2 levels in the striatum. No reductions in DAT or TH levels were observed except for the 20 mg/kg dose. DA levels are determined by a variety of factors, including TH, the rate limiting enzyme involved in DA synthesis [26], monoamine oxidase, which metabolizes DA to DOPAC [27] and, to a lesser extent, catecholamine-o-methyltransferase that converts DA to HVA through the intermediate 3-methoxytyramine [28]. Additionally, DAT and VMAT2 contribute significantly to the regulation of DA levels through control of release and re-uptake [29]. In the literature, the lowest single doses of MPTP found to be given to C57BL/6J mice was 6.25–12.5 mg/kg, the lower of which resulted in significant striatal dopamine loss, but no effect on TH levels [21]. Low (2×5 mg/kg) and high (3×10 mg/kg) doses of MPTP were reported to have differential effects on retinal DA, DOPAC and HVA levels, with low dose effects on DOPAC levels not observed at the higher dose, which was hypothesized to be the result of inhibition of MAO at low MPTP doses [30]. With regard to loss of DA with low dose MPTP, there are multiple potential mechanisms that may contribute to this loss that occurs without corresponding dopamine neuron loss. First, MPTP treatment can result in direct nitration of tyrosine hydroxylase resulting in the inactivation of enzyme activity and reduction in dopamine levels [31]. Second, VMAT2 levels are known to highly correlate with loss of striatal DA in MPTP models [32] and has been shown to be a reliable indicator of dopamine terminal damage in PD [29, 33]. Clearly, there is need for additional studies on the differential effects of low and high dose regimens of MPTP as it relates to dopamine metabolism and handling.
Another reason that comparison of our work to that of Dovero and co-workers is difficult relates to strain differences in the mice chosen for study. Here, we used young male C57BL/6J mice compared to OF1 mice used in the Dovero study. OF1 mice used in the Dovero studies are an albino outbred stock of mice and the phenotypic heterogeneity in outbred stocks may obscure any existing differences between groups [34]. Host susceptibility is one of the factors influencing how individuals respond to an insult; just like humans not all strains of mice are equally susceptible to MPTP neurotoxicity; for instance, C57BL/6J, which are broadly used in toxin animal models to mimic aspects of PD, are one of the most sensitive strains to MPTP [35–37]. Concerning other neurotoxicants such as paraquat, C57BL/6J mice have been reported to be more susceptible to paraquat neurotoxicity compared to DBA/2J mice, losing more DA cells in the SNc after paraquat exposure [38]. Various strains of BXD recombinant Inbred (RI) (an inbred of C57BL/6J and DBA/2J) mice demonstrate a broad response variation to MPTP neurotoxicity both in males and females [39–41]. Each of these BXD RI strains have a unique genetic composition exclusive to the strain, representing a genetically diverse population which is suitable to study the significance of genetic predisposition regarding the effect of multiple neurotoxicants. The accessibility of comprehensive genetic information regarding different strains of mice and mouse genome have provided researchers with unique opportunities to study the role of genetic predisposition in response to an insult. Quantitative Trait Loci (QTL) analyses have been performed to pinpoint candidate genes that may be involved in response variation regarding multiple neurotoxicants including MPTP and paraquat [38, 40]. Thus, similar analyses in the OF1 mice may provide unique insight into the mechanisms of MPTP, and perhaps PD, susceptibility, if indeed they are shown to lose DA neurons to very low doses of MPTP.
In summary, our data clearly demonstrate that the lower single doses of MPTP used here only result in loss of TH+ cells in the SNc, rather than actual neuronal loss in C57/BL6J mice. This underscores the importance of counting total neuron number along with phenotypic neuron counts to ensure accurate interpretation of dopaminergic neurodegeneration. Additionally, our data and that from the literature highlight the need for additional studies with expanded MPTP regimens in other strains of mice, including the OF-1, which may ultimately aid in identifying underlying genetic susceptibilities to dopaminergic neurotoxicity. In addition to strain and dose differences, there is emerging evidence regarding differential sensitivity to neurotoxicity in subpopulations of DA neurons [42]. Therefore, determining DA cell populations in the SNc, which are the most susceptible may aid in better understanding the underlying susceptibility of dopamine neurons to neurotoxicants.
Highlights.
Low doses of MPTP cause loss of TH expression rather than actual loss of neurons
Higher doses of MPTP cause both loss of TH expression and neurons
Striatal dopamine levels and terminal marker loss are consistent with cell loss
Acknowledgments
This study was supported in part by grants from the NIH, R01ES021800, and the Michael J Fox Foundation for Parkinson’s Disease Research. Additional support was provided through generous donations from the Glenn and Karen Leppo, the Richard Nicely and the Allan and Janice Woll Parkinson’s Research Funds. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of NIH or any other funding source.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cannon JR, Greenamyre JT. Gene–environment interactions in Parkinson’s disease: Specific evidence in humans and mammalian models. Neurobiology of Disease. 2013;57:38–46. doi: 10.1016/j.nbd.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klingelhoefer L, Reichmann H. Pathogenesis of Parkinson disease[mdash]the gut-brain axis and environmental factors. Nat Rev Neurol. 2015;11(11):625–636. doi: 10.1038/nrneurol.2015.197. [DOI] [PubMed] [Google Scholar]
- 3.Gibb WR, Lees AJ. Anatomy, pigmentation, ventral and dorsal subpopulations of the substantia nigra, and differential cell death in Parkinson’s disease. Journal of Neurology, Neurosurgery, and Psychiatry. 1991;54(5):388–396. doi: 10.1136/jnnp.54.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez-Oroz MC, et al. Initial clinical manifestations of Parkinson’s disease: features and pathophysiological mechanisms. The Lancet Neurology. 2009;8(12):1128–1139. doi: 10.1016/S1474-4422(09)70293-5. [DOI] [PubMed] [Google Scholar]
- 5.Masliah E, et al. Dopaminergic Loss and Inclusion Body Formation in α-Synuclein Mice: Implications for Neurodegenerative Disorders. Science. 2000;287(5456):1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- 6.Yacoubian TA, et al. Transcriptional dysregulation in a transgenic model of Parkinson disease. Neurobiology of Disease. 2008;29(3):515–528. doi: 10.1016/j.nbd.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sommer B, et al. Mouse models of α-synucleinopathy and Lewy pathology. Experimental Gerontology. 2000;35(9–10):1389–1403. doi: 10.1016/s0531-5565(00)00181-9. [DOI] [PubMed] [Google Scholar]
- 8.Goldberg MS, et al. Parkin-deficient Mice Exhibit Nigrostriatal Deficits but Not Loss of Dopaminergic Neurons. Journal of Biological Chemistry. 2003;278(44):43628–43635. doi: 10.1074/jbc.M308947200. [DOI] [PubMed] [Google Scholar]
- 9.Gispert S, et al. Parkinson Phenotype in Aged PINK1-Deficient Mice Is Accompanied by Progressive Mitochondrial Dysfunction in Absence of Neurodegeneration. PLoS ONE. 2009;4(6):e5777. doi: 10.1371/journal.pone.0005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tong Y, et al. R1441C mutation in LRRK2 impairs dopaminergic neurotransmission in mice. Proceedings of the National Academy of Sciences. 2009;106(34):14622–14627. doi: 10.1073/pnas.0906334106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andres-Mateos E, et al. DJ-1 gene deletion reveals that DJ-1 is an atypical peroxiredoxin-like peroxidase. Proceedings of the National Academy of Sciences. 2007;104(37):14807–14812. doi: 10.1073/pnas.0703219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langston JW, Langston EB, Irwin I. MPTP-induced parkinsonism in human and non-human primates--clinical and experimental aspects. Acta neurologica Scandinavica Supplementum. 1984;100:49–54. [PubMed] [Google Scholar]
- 13.Jackson-Lewis V, et al. Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurodegeneration. 1995;4(3):257–269. doi: 10.1016/1055-8330(95)90015-2. [DOI] [PubMed] [Google Scholar]
- 14.Bezard E, et al. Effects of Different Schedules of MPTP Administration on Dopaminergic Neurodegeneration in Mice. Experimental Neurology. 1997;148(1):288–292. doi: 10.1006/exnr.1997.6648. [DOI] [PubMed] [Google Scholar]
- 15.Petroske E, et al. Mouse model of Parkinsonism: a comparison between subacute MPTP and chronic MPTP/probenecid treatment. Neuroscience. 2001;106(3):589–601. doi: 10.1016/s0306-4522(01)00295-0. [DOI] [PubMed] [Google Scholar]
- 16.Dovero S, Gross C, Bezard E. Unexpected toxicity of very low dose MPTP in mice: A clue to the etiology of Parkinson’s disease? Synapse. 2016;70(2):49–51. doi: 10.1002/syn.21875. [DOI] [PubMed] [Google Scholar]
- 17.Yochum C, et al. Prenatal Cigarette Smoke Exposure Causes Hyperactivity and Agressive Behavior: Role of Altered Catcholamines and BDNF. Experimental neurology. 2014;254:145–152. doi: 10.1016/j.expneurol.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hossain MM, Richardson JR. Mechanism of pyrethroid pesticide-induced apoptosis: role of calpain and the ER stress pathway. Toxicol Sci. 2011;122(2):512–25. doi: 10.1093/toxsci/kfr111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hossain MM, DiCicco-Bloom E, Richardson JR. Hippocampal ER stress and learning deficits following repeated pyrethroid exposure. Toxicol Sci. 2015;143(1):220–8. doi: 10.1093/toxsci/kfu226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halliday GM, McCann H. The progression of pathology in Parkinson’s disease. Annals of the New York Academy of Sciences. 2010;1184(1):188–195. doi: 10.1111/j.1749-6632.2009.05118.x. [DOI] [PubMed] [Google Scholar]
- 21.O’Callaghan JP, Miller DB, Reinhard JF. Characterization of the origins of astrocyte response to injury using the dopaminergic neurotoxicant, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Brain Res. 1990;521(1–2):73–80. doi: 10.1016/0006-8993(90)91526-m. [DOI] [PubMed] [Google Scholar]
- 22.Javoy-Agid F, et al. Decreased tyrosine hydroxylase messenger RNA in the surviving dopamine neurons of the substantia nigra in Parkinson’s disease: an in situ hybridization study. Neuroscience. 1990;38(1):245–53. doi: 10.1016/0306-4522(90)90389-l. [DOI] [PubMed] [Google Scholar]
- 23.Kastner A, et al. Decreased tyrosine hydroxylase content in the dopaminergic neurons of MPTP-intoxicated monkeys: effect of levodopa and GM1 ganglioside therapy. Ann Neurol. 1994;36(2):206–14. doi: 10.1002/ana.410360213. [DOI] [PubMed] [Google Scholar]
- 24.Kozina EA, et al. Tyrosine hydroxylase expression and activity in nigrostriatal dopaminergic neurons of MPTP-treated mice at the presymptomatic and symptomatic stages of parkinsonism. Journal of the Neurological Sciences. 340(1):198–207. doi: 10.1016/j.jns.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 25.Xu Z, et al. Selective alterations of gene expression in mice induced by MPTP. Synapse. 2005;55(1):45–51. doi: 10.1002/syn.20089. [DOI] [PubMed] [Google Scholar]
- 26.Tabrez S, et al. A synopsis on the role of tyrosine hydroxylase in Parkinson’s disease. CNS Neurol Disord Drug Targets. 2012;11(4):395–409. doi: 10.2174/187152712800792785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Youdim MB, Sourkes TL. Properties of purified, soluble monoamine oxidase. Can J Biochem. 1966;44(10):1397–400. doi: 10.1139/o66-158. [DOI] [PubMed] [Google Scholar]
- 28.Rivett AJ, Eddy BJ, Roth JA. Contribution of sulfate conjugation, deamination, and O-methylation to metabolism of dopamine and norepinephrine in human brain. J Neurochem. 1982;39(4):1009–16. doi: 10.1111/j.1471-4159.1982.tb11490.x. [DOI] [PubMed] [Google Scholar]
- 29.Miller GW, et al. Dopamine transporters and neuronal injury. Trends Pharmacol Sci. 1999;20(10):424–9. doi: 10.1016/s0165-6147(99)01379-6. [DOI] [PubMed] [Google Scholar]
- 30.Hamilton WR, et al. Effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) on retinal dopaminergic system in mice. Neurosci Lett. 2012;515(2):107–10. doi: 10.1016/j.neulet.2012.02.085. [DOI] [PubMed] [Google Scholar]
- 31.Ara J, et al. Inactivation of tyrosine hydroxylase by nitration following exposure to peroxynitrite and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Proc Natl Acad Sci U S A. 1998;95(13):7659–63. doi: 10.1073/pnas.95.13.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tillerson JL, et al. Detection of behavioral impairments correlated to neurochemical deficits in mice treated with moderate doses of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Exp Neurol. 2002;178(1):80–90. doi: 10.1006/exnr.2002.8021. [DOI] [PubMed] [Google Scholar]
- 33.Miller GW, et al. Immunochemical analysis of vesicular monoamine transporter (VMAT2) protein in Parkinson’s disease. Exp Neurol. 1999;156(1):138–48. doi: 10.1006/exnr.1998.7008. [DOI] [PubMed] [Google Scholar]
- 34.Chia R, et al. The origins and uses of mouse outbred stocks. Nat Genet. 2005;37(11):1181–1186. doi: 10.1038/ng1665. [DOI] [PubMed] [Google Scholar]
- 35.Hamre K, et al. Differential strain susceptibility following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) administration acts in an autosomal dominant fashion: quantitative analysis in seven strains of Mus musculus. Brain Res. 1999;828(1–2):91–103. doi: 10.1016/s0006-8993(99)01273-1. [DOI] [PubMed] [Google Scholar]
- 36.Rodríguez S, et al. Resistance of the golden hamster to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-neurotoxicity is not only related with low levels of cerebral monoamine oxidase-B. Experimental and toxicologic pathology: official journal of the Gesellschaft fur Toxikologische Pathologie. 2013;65(1–2):127–133. doi: 10.1016/j.etp.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 37.Riachi NJ, Harik SI. Strain differences in systemic 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity in mice correlate best with monoamine oxidase activity at the blood-brain barrier. Life sciences. 1988;42(23):2359–2363. doi: 10.1016/0024-3205(88)90189-0. [DOI] [PubMed] [Google Scholar]
- 38.Yin L, et al. Genetic-based, differential susceptibility to paraquat neurotoxicity in mice. Neurotoxicol Teratol. 2011;33(3):415–21. doi: 10.1016/j.ntt.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 39.Cook R, et al. Identification of a single QTL, Mptp1, for susceptibility to MPTP-induced substantia nigra pars compacta neuron loss in mice. Brain research Molecular brain research. 2003;110(2):279–288. doi: 10.1016/s0169-328x(02)00659-9. [DOI] [PubMed] [Google Scholar]
- 40.Alam G, et al. MPTP Neurotoxicity is Highly Concordant Between the Sexes Among BXD Recombinant Inbred Mouse Strains. NeuroToxicology. doi: 10.1016/j.neuro.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones BC, et al. Systems analysis of genetic variation in MPTP neurotoxicity in mice. Neurotoxicology. 2013;37:26–34. doi: 10.1016/j.neuro.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.German DC, et al. The neurotoxin MPTP causes degeneration of specific nucleus A8, A9 and A10 dopaminergic neurons in the mouse. Neurodegeneration. 1996;5(4):299–312. doi: 10.1006/neur.1996.0041. [DOI] [PubMed] [Google Scholar]