Abstract
Capillary electrophoresis coupled with mass spectrometry (CE-MS) has been used as a platform for discovery and validation of urinary peptides associated with chronic kidney disease (CKD). CKD affects ∼ 10% of the population, with high associated costs for treatments. A urinary proteome-based classifier (CKD273) has been discovered and validated in cross-sectional and longitudinal studies to assess and predict the progression of CKD. It has been implemented in studies employing cohorts of > 1000 patients. CKD273 is commercially available as an in vitro diagnostic test for early detection of CKD and is currently being used for patient stratification in a multicentre randomized clinical trial (PRIORITY). The validity of the CKD273 classifier has recently been evaluated applying the Oxford Evidence-Based Medicine and Southampton Oxford Retrieval Team guidelines and a letter of support for CKD273 was issued by the US Food and Drug Administration. In this article we review the current evidence published on CKD273 and the challenges associated with implementation. Definition of a possible surrogate early endpoint combined with CKD273 as a biomarker for patient stratification currently appears as the most promising strategy to enable the development of effective drugs to be used at an early time point when intervention can still be effective.
Keywords: chronic kidney disease (CKD), CKD progression, peptides-based classifiers, proteomics, urinary biomarkers
Introduction
Chronic diseases are one of the biggest burdens of developed societies as a result of the increase in life expectancy. Among these chronic diseases, chronic kidney disease (CKD) is affects ∼ 10% of the population [1]. CKD is defined as a heterogeneous disease generally characterized by abnormalities in kidney structure and function lasting >3 months [2]. CKD and its final stage, end-stage renal disease (ESRD), represent a huge economic and personal burden. CKD has a major impact on the quality of life and life expectancy of patients affected by this disease, especially when reaching ESRD. Based on data published on the cost of CKD in the USA [3], it appears reasonable to estimate that the total annual costs of CKD in Europe are >€ 100 billion. Considering the increase in life expectancy, these costs are estimated to further increase by 5–10% per year.
CKD is, by definition, a chronic disease and incurable. Intervention strategies are mostly aimed at slowing progression and preserving kidney function as long as possible. Multiple studies have demonstrated that early intervention is the most effective approach in managing CKD [4].
Onset and progression of CKD is currently assessed based on the estimated glomerular filtration rate (eGFR) calculated from serum creatinine [5] and/or urinary albumin excretion (UAE) [6]. These biomarkers have certain shortcomings that have been discussed in detail, including substantial variability and lack of accuracy [7–9]. A major shortcoming, especially when considering that early intervention is the most effective approach, is that both markers are indicative of a late stage of disease. These markers are not connected with molecular pathophysiology, but a consequence of substantial damage to the kidney. Therefore, there is a need for new biomarkers for detecting pre-clinical pathophysiological alterations at an early stage of the disease to guide individual therapeutic interventions for prevention of disease progression.
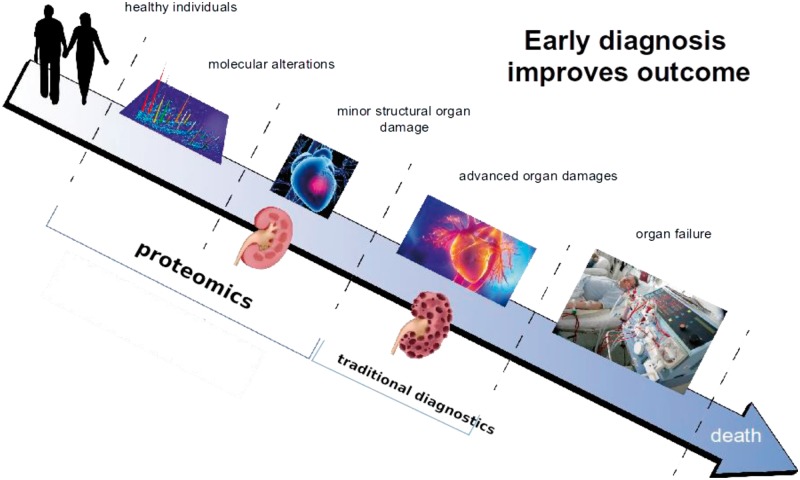
Proteins are responsible for structure and signalling in living organisms and in every organ. Not surprisingly, proteins are also the general target for drug-based intervention. Based on these considerations, it appears only logical investigate proteomic changes in the context of CKD, aiming at identifying molecular changes associated with CKD onset and progression that can be linked to molecular pathophysiology and that could serve as more appropriate biomarkers or even as therapeutic targets. Figure 1 illustrates this concept of how early diagnosis and/or prognosis of diseases, based on proteomic changes involved in pathology, improves chances for better outcomes for patients.
Fig. 1.
Early diagnosis and/or prognosis of diseases improves chances for a better outcome for the patient. The initiation of molecular processes that result in (chronic) diseases can be detected based on molecular changes, using proteomic technologies, prior to advanced organ damage. This could allow earlier intervention where drugs are most effective. The figure is adapted from Stepczynska et al. [10].
Clinical proteomics
The general idea that proteins are the key molecules in disease processes has led to development of the clinical proteomics field. After an initial period of ‘trial and error’ [11], the field has now matured, suggestions for appropriate study designs have been established [12] and clinical proteomics has started delivering results that can be employed to the benefit of patients.
Mass spectrometry (MS) technologies are typically used in clinical proteomics. Different MS technologies exist, with specific advantages and disadvantages as outlined in, for example, Frantzi et al. [13]. The technologies enable the identification and quantification of proteins and peptides in body fluids, tissues or cells, hence enabling the identification of proteins that could reflect human pathology. Especially in the context of kidney disease, one particular technology, capillary electrophoresis coupled with mass spectrometry (CE-MS), has been optimized and applied in multiple studies [14]. This development was also driven by specific qualities of urine: urine can be collected non-invasively, in large quantities, and it reflects input mostly from the kidney (∼ 70% of the urine proteome is thought to originate from the kidney) [15]. In addition, the urine proteome is quite stable and a standard sample as well as reference values are available [16]. The CE-MS technology consists of separation of urinary proteins and peptides in a capillary with a high-voltage field (CE) coupled with MS, which determines the protein molecular mass and abundance. Reproducible sample preparation protocols, the high accuracy of MS and software development have made the CE-MS platform highly suitable for clinical purposes [17].
Initial studies on a smaller scale demonstrated that single biomarkers are insufficient to display pathophysiology in a comprehensive way that would allow accurate assessment of disease [18–20]. To compensate for this, and also for the high variability of single biomarkers, the concept of multimarker approaches was introduced. High-dimensional algorithms, such as support vector machines (SVMs), allow combining multiple biomarkers into a classifier, and have proven to outperform other methods for combining biomarkers into a classifier [21]. However, this approach may result in significant overfitting [22], generating classifiers that ultimately have no value in independent samples/cohort. This is especially an issue in small cohorts and, as such, verifying the multimarker classifier in an independent cohort has been highlighted as a major, mandatory, central effort in any multimarker study [23, 24].
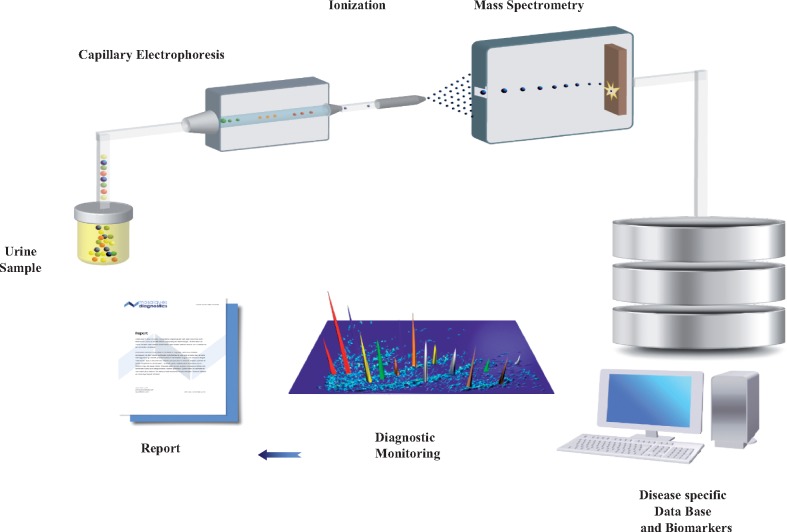
Following the above-mentioned considerations, CE-MS (as shown in Figure 2) has been used in several studies in the context of kidney diseases to identify urinary peptides that can be associated with diseases and/or their progression. Urine proteomics employing CE-MS can be considered as a ‘liquid biopsy’ of the kidney and urogenital tract [25]. The kidney is a delicate and sophisticated structure containing > 1 million multicellular filtration units, the glomeruli. Glomeruli filter the blood and produce the primary urine, which is further concentrated in the tubuli. Under normal conditions, low molecular weight proteins and only a small fraction of proteins of middle molecular weight pass through the glomerular filtration barriers and reach the tubules. Because of the high efficacy of the reabsorption process by proximal tubular epithelial cells, under physiological conditions only small amounts of protein are secreted into the urine [26]. However, under pathological conditions, initiation of molecular changes in the protein content can be detected in urine in a non-invasive way, thus giving rise to protein/peptide biomarkers for CKD management. In the following paragraphs we will discuss CE-MS application for the diagnosis and prognosis of CKD, ongoing studies and the current regulatory status for clinical implementation.
Fig. 2.
Representation of urinary CE-MS workflow. Urinary proteins are detected by CE-MS, where the mass and relative abundance of each protein is analysed. All the detected peptides are stored in a database, which allows further evaluation of the peptides to be used for diagnostic purposes.
The story of the CKD273 classifier
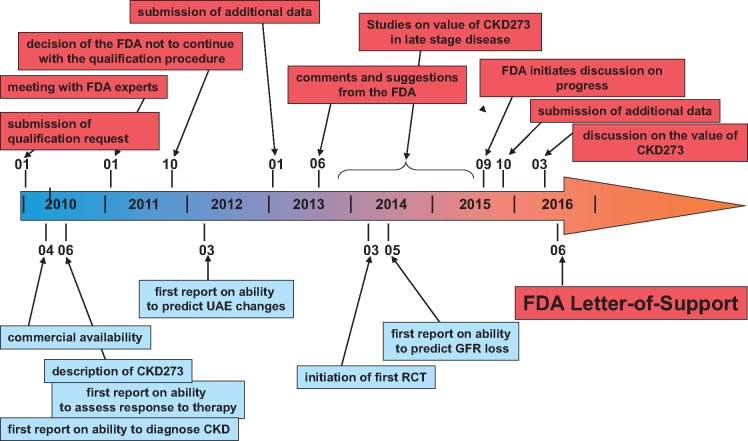
A graphic depiction of major milestones towards implementation of CKD273 is presented in Figure 3. Based on the above-mentioned considerations, several studies were initiated to enable detection of CKD based on urinary proteomic changes [15, 27–30], ideally at an early point in time when the kidney is not yet irreversibly damaged. In one of the first larger studies involving 379 healthy subjects and 230 patients suffering from various kidney diseases, Good et al. [31] used CE-MS to identify urinary peptides significantly different between patients with CKD of different aetiologies and controls with intact kidney function. The rational was to identify biomarkers associated with CKD in general and, by combining these, enable the early detection of molecular changes that predict the development or progression of CKD. In the above study, 273 urinary peptides were identified that significantly differed between CKD and healthy controls (all listed as supplementary information, available at www.mcponline.org/content/9/11/2424/suppl/DC1). These 273 potential biomarkers were combined into one classifier based on urinary peptides, termed CKD273, which was initially tested for accurate diagnosis of CKD [31]. In the same study, CKD273 was validated in an independent test set of 110 individuals with CKD and 34 healthy individuals, showing a sensitivity of 85% and specificity of 100% with an area under the curve (AUC) of 0.96 for the diagnosis of CKD. In a later study, Molin et al. [32] investigated the performance of CKD237 in 137 urine samples (62 patients and 75 controls). This study resulted in a similar accuracy in the diagnosis of CKD (AUC = 0.96) as in the initial study.
Fig. 3.
Major milestones on the path towards implementation of CKD273. Shown are the main milestones and developments from the initial discovery until the issuing of the FDA Letter of Support.
Major components of CKD273 are diverse collagen fragments, reduced in CKD, and fragments of abundant blood-derived proteins and/or proteins involved in inflammation (e.g. fibrinogen, apolipoproteins, haemoglobin, alpha-1 antitrypsin etc.), increased in CKD. Peptides in CKD273 can be linked to the kidney, and to CKD pathophysiology, as also indicated in Figure 4. The parental proteins (giving rise to the peptides) are present in the kidney, some (e.g. uromodulin or type I collagen) in high quantities [33]. As such, it is reasonable to assume that the peptide biomarkers were generated in the kidney, reflecting CKD-associated molecular changes, and the peptide changes observed in CKD urine can be associated with biological processes intimately linked to CKD. The reduction of collagen fragments indicates attenuation of collagen degradation, which results in increased deposits of collagen in the kidney tissue, in fibrosis. In fact, fibrosis is a key element of CKD, and fibrosis is tightly associated with CKD progression [34]. An increase in alpha-1 antitrypsin degradation, and consequently a reduction of alpha-1 antitrypsin protein levels, may support inflammation and could also promote fibrosis: alpha-1 antitrypsin was found to attenuate renal fibrosis [35].
Fig. 4.
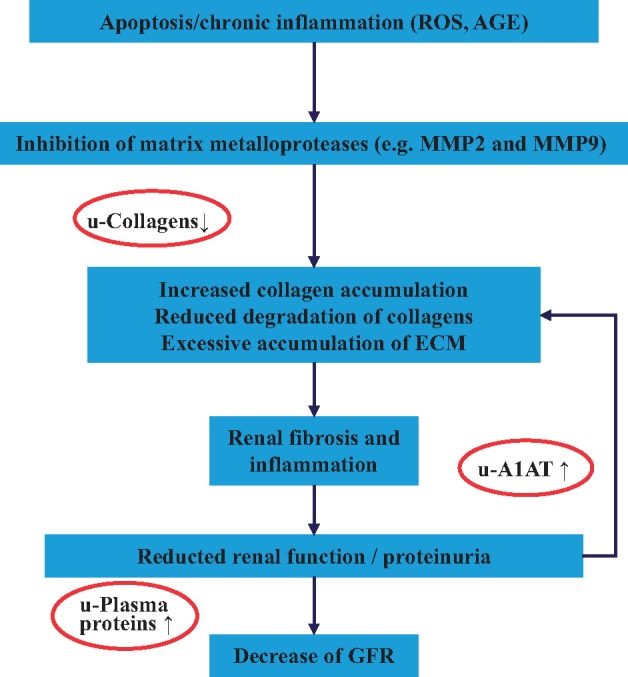
Biological processes generally accepted to be involved in CKD onset and progression. The processes are indicated in the boxes. Outside, peptides found significantly changes in CKD are indicated, at the respective step in the disease development. [36].
Assessment and prediction of CKD using the CKD273 classifier
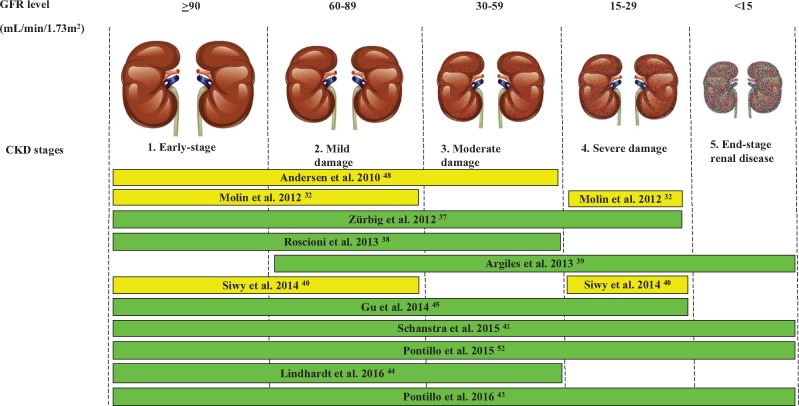
A graphic depiction of the studies published to date using CKD273 is presented in Figure 5 and a short description of each of these studies is listed in Table 1. To establish an added value in patient management, CKD273 was assessed in several studies. The classifier demonstrated applicability in a longitudinal study including samples from type 1 and 2 diabetic patients observed over an average of 10 years. In this study, CKD273 outperformed UAE in predicting diabetic kidney disease (AUC of CKD273 0.93 versus AUC of UAE 0.67) [37]. Following these positive results, CKD273 was further evaluated for its ability to predict change from normoalbuminuria to microalbuminuria and from microalbuminuria to macroalbuminuria using samples from a prospective study over 3 years. Investigating 88 samples from patients with type 2 diabetes mellitus, superior performance of CKD273 in predicting class changes in albuminuria over UAE was demonstrated [38]. Next, the CKD273 classifier was tested in 53 patients at various CKD stages, demonstrating value in the prediction of CKD progression to ESRD [39]. The prediction of CKD progression was also confirmed in a large population-based cohort of 797 individuals over an average of 4.8 years [45]. In a large multicentric cohort of 1990 individuals, which included 522 subjects where 3-year follow-up data were available, the prognostic value of CKD273 was assessed. The findings verified CKD273 to be a better predictor of CKD progression than UAE [41]. In an additional very recent study, the superiority of CKD273 over albuminuria in predicting CKD progression was demonstrated in 737 samples from the Diabetic Retinopathy Candesartan Trials (DIRECT) 2 cohort [44]. Furthermore, the prediction of CKD progression was investigated in detail in a large cohort of 2672 individuals at various CKD stages, aimed at specifying the value of CKD273 in comparison with the state of the art to predict eGFR loss at different baseline glomerular filtration rate (GFR) strata [43]. The results demonstrated that CKD273 performed much better than albuminuria in predicting loss of GFR at an early CKD stage (eGFR >70 mL/min/1.73 m2), but provided no advantage in patients with advanced-stage disease (eGFe <50 mL/min/1.73 m2), where albuminuria appears to have better performance. The application of CKD273 to predict risk of death or development of vascular complications on top of kidney diseases or to assess the aetiology of kidney diseases [46] should be further investigated.
Fig. 5.
Representation of the studies evaluating the performance of the CKD273-classifier in the diagnosis and prognosis of CKD according to disease stage. The bars show the CKD stages in which the classifier was used. The Figure is adapted from Critselis and Lambers Heerspink [36].
Table 1.
List of studies published that support the benefit of CKD273
| Study background | Study information | Number of samples | Study type | Performance (CKD273) | Comparator | Performance (comparator) | Reference |
|---|---|---|---|---|---|---|---|
| Diagnosis of DN in T2D patients | Diagnosis: macroalbuminuria | 137 | Cross-sectional |
|
— | — | Molin et al. [32] |
| Prediction of DN in T2D patients | Normalbuminuric patients transition to macroalbuminuria | 316 (35 patients) | Longitudinal |
|
UAE |
|
Zürbig et al. [37] |
| Prediction of transition to higher albuminuria stages | Transition from normo- to microalbuminuria, transition from micro- to macroalbuminuria | 88 | Longitudinal |
|
eGFR + UAE | AUC 0.91 | Roscioni et al. [38] |
| Association with renal hard endpoints |
|
53 | Longitudinal | — | UAE | — | Argiles et al. [39] |
| Multicentric diagnosis of DN in T2D patients | Diagnosis: macroalbuminuria | 167 | Cross-sectional | AUC 0.95–1.00 | — | — | Siwy et al. [40] |
| Diagnosis and prediction of CKD progression | Prognosis: eGFR decline >5% per year |
|
Cross-sectional Longitudinal | AUC 0.821 | eGFR + UAE | AUC 0.758 | Schanstra et al. [41] |
| Diagnosis and risk of progression to CKD | Prognosis: eGFR decline >4 mL/min/ 1.73 m2/year | 35 | Longitudinal |
|
UAE (dipstick) | AUC 0.85 | Ovrehus et al. [42] |
| Prediction of decline in glomerular filtration |
|
2627 | Longitudinal |
|
UAE |
|
Pontillo et al. [43] |
| Post hoc analysis of the DIRECT-Protect 2 trial |
|
737 | Longitudinal |
|
|
|
Lindhardt et al. [44] |
| Prediction of CKD stage 3 | Longitudinal study, prediction of CKD stage 3 | 2087 | Longitudinal | HR 1.23 |
|
|
Pontillo et al., submitted for publication [52] |
DN, ; HR, hazard ratio; T2D, ; Sens., sensitivity; Spec., specificity.
Treatment and ongoing research exploring the CKD273 classifier
Beyond prognostic value, CKD273 also depicts the impact of treatment, showing its potential in assessing therapeutic interventions, as has also been previously shown for other urinary peptides [47]. CKD273 scores were significantly altered after treatment with irbesartan (an antihypertensive and antiproteinuric drug): specifically, a change towards values of healthy individuals was detectable after 2 years of treatment with irbesartan [48]. Of note, an immediate impact of irbesartan treatment on CKD273 was not detectable, indicating that the drug does not act on molecular targets directly involved in CKD. In a very recent study, the value of CKD273 in predicting response to spironolactone was investigated [49]. The results of the study demonstrated that a higher baseline score in CKD273 was significantly associated with a response to spironolactone (as assessed via albuminuria reduction).
The above results have also initiated the implementation of CKD273 in a large multicentric randomized clinical trial stratifying patients based on risk of CKD progression. In the PRIORITY (Proteomic prediction and Renin angiotensin aldosterone system Inhibition prevention Of early diabetic nephRopathy in TYpe 2 diabetic patients with normoalbuminuria) trial, CKD273 is employed to select a population at high risk of progression towards CKD to assess if spironolactone prevents early diabetic nephropathy (using microalbuminuria as the endpoint) in diabetic patients without pathological albuminuria [50]. Preliminary results of this PRIORITY trial further confirmed the validity of CKD273 as a clinical assay for patient risk stratification in interventional trials [40, 44].
Current regulatory status of the CKD273 classifier
The performance of CKD273 as a biomarker for clinical studies was further investigated in a recent systematic review by Critselis and Lambers Heerspink [36]. The Oxford Evidence-Based Medicine (EBM) and Southampton Oxford Retrieval Team (SORT) guidelines for the prognostic value of tests were applied to CKD273. The authors identified several studies that confirmed the validity and value of the classifier for predicting CKD progression (Table 2). The CKD273 classifier obtained a high score at level Ib to be employed in randomized controlled trials according to the Oxford EBM.
Table 2.
Oxford EBM [50] and SORT [40] evidence level score regarding the validity of the CKD273 classifier in CKD prediction
| Methodology | Samples collection at a common point | Sufficient follow-up time | Blind outcome criteria | Adjustment for prognostic factors | EBM score | SORT score | |
|---|---|---|---|---|---|---|---|
| Argiles et al. [39] | Prospective cohort | No | Yes | Yes | No | Ib | 1 |
| Gu et al. [45] | Prospective cohort | No | Yes | Yes | Yes | −* | 2 |
| Roscioni et al. [38] | Prospective cohort | Yes | Yes | Yes | Yes | Ib | 4 |
| Schanstra et al. [41] | Prospective cohort | Yes | Yes | Yes | Yes | Ib | 4 |
Not calculated (follow-up time insufficient). The table was adapted from Critselis and Lambers [36].
The US Food and Drug Administration (FDA) has recently issued a letter of support for CKD273 (http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/UCM508790.pdf). The letter aims to encourage the visibility of CKD273 and to support further evaluation and studies to establish its clinical utility in ‘prognostic enrichment, drug development and study design considerations’. In particular, the FDA affirmed that the proposed use of the classifier for prognosis of early diabetic kidney disease is consistent with the FDA’s guidance ‘Enrichment Strategies for Clinical Trials to Support Approval of Human Drugs and Biological Products’. This is an important achievement: to date, the FDA has only issued 12 letters of support in total; the one for CKD273 is the only one in the context of CKD.
To further confirm the prognostic utility of the classifier, surrogates and earlier endpoints for CKD are being investigated. In 2014, the European Medicines Agency (EMA) [51] suggested that primary efficacy endpoints for compound testing should be prevention or delay of renal function decline, defined as either time to occurrence or incidence rate of CKD stage 3, with or without prevention of proteinuria/albuminuria. Therefore, CKD273 was employed in a study to predict early CKD progression, as suggested by EMA, comparing its performance with clinical parameters using CE-MS data from 2087 subjects with baseline eGFR ≥60 mL/min/1.73 m2. The results clearly demonstrate that CKD273 enables prediction of progression to CKD stage 3 with significantly superior performance than any other variables generally employed (age, gender, body mass index, blood pressure, eGFR and UAE) (unpublished data, submitted as abstract) [52].
Based on the above-mentioned data and evaluation results, CKD273 is now available as an in vitro diagnostic device in Germany for the early detection of CKD in diabetic patients. The procedure has been standardized, the urine sample is shipped overnight to the analytical laboratory, and the results are reported back to the sender within 5 days. However, as outlined below, currently this service is only available for privately insured patients.
Future perspectives
While CKD273 is available for early detection of CKD for individual patients in Europe, implementation for the publicly insured population is still being debated. Discussions are ongoing with the G-BA, the German authority regulating reimbursement in the health care system. It is very concerning that these discussions were initiated in 2011, yet no definitive conclusion has been reached.
A major argument against implementation of the classifier towards clinical use is the lack of studies demonstrating a significant positive effect on the accepted hard endpoint (ESRD or death). While such a demand may appear sensible at first sight, it is at the same time unrealistic, as it would require a study over a period of 15–20 years (from early, subclinical disease to endpoint), likely including enormous numbers of patients (several hundred thousand), and prohibiting intervention with any new drug (that was not available at the start of the study). It is obvious that such a study cannot be performed and that it would not receive ethics approval. Demands for such studies effectively prohibit bringing benefits to patients and hinder the development of therapeutic drugs. The demand for a trial on a hard endpoint rests on the argument of the absence of a proven benefit of early intervention and the hypothesis that molecular changes may be different in early CKD and are not targeted by the currently used drugs. However, such arguments contradict current literature and knowledge.
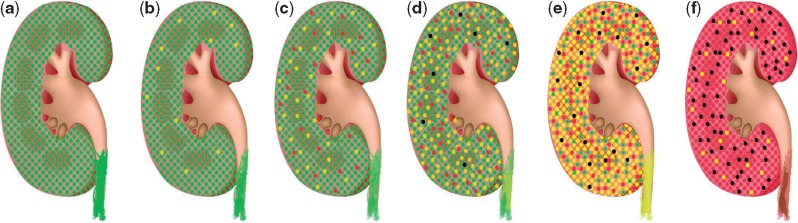
As recently demonstrated in a comprehensive analysis by Schievink et al. [4], intervention at the earliest time point shows the greatest benefit. This is also evident when evaluating CKD on a mechanistic level. As depicted in Figure 6, from the initially healthy status (a), CKD generally starts with reversible damage in some glomeruli (b); at this point in time, these damages may be reversed. Without treatment, additional glomeruli will be damaged (c), a few beyond the point where effective treatment may be possible. At the same time, pressure on the remaining intact glomeruli increases and damage of individual glomeruli is further accelerated (d), with subsequent detectable impact on total kidney functional parameters (albuminuria and/or eGFR). The molecular mechanisms addressable by therapeutic intervention (indicated by the yellow glomeruli in Figure 6) are the same at this later stage than at the beginning. However, the irreversible damage has occurred to multiple glomeruli (indicated by red and black in Figure 6), and glomeruli lost cannot be replaced. While progression from (d) to (e) and (f) cannot be prevented, as a result of too high a burden on the few remaining glomeruli, it could likely be prevented with intervention at (b) or (c), at a stage where molecular changes displayed by CKD273 are evident but functional parameters are not yet affected.
Fig. 6.
Schematic concept of CKD onset and progression. The kidney contains ∼1 million filtration units (glomeruli). From the initially healthy status (a), some glomeruli experience pathological molecular changes (yellow) reversibly compromising their function. Without treatment, additional glomeruli will be damaged (c), a few also beyond the point where effective treatment may be possible (red). As pressure on the remaining intact glomeruli increases, damage of individual glomeruli is further accelerated (d), with now detectable impact on kidney functional parameters (albuminuria and/or eGFR), and the first glomeruli are lost (black). The molecular mechanisms are identical at this later stage to the beginning, but irreversible damage has occurred to multiple glomeruli, which cannot be repaired. Progression from (d) to (e) (established CKD, most glomeruli damaged, many beyond repair or completely lost) and then to (f) (ESRD, very few glomeruli still functional) cannot be prevented because of the too high burden on the few remaining glomeruli. Prevention would have been possible with intervention at (b) or (c), stages where molecular changes displayed by CKD273 are evident but functional parameters are not yet affected.
Prevention of implementation of potential benefits for patient management is even more puzzling when considering that all studies to date have shown a positive result, and especially also in the light of the societal challenges associated with CKD (see above) and the evidence for a health economic profit in several assessments [33, 53]. It is unclear why, in the light of all the evidence, implementation of novel beneficial approaches meet such substantial resistance (possibly to not endanger business models resting on the status quo). Ultimately, the current situation indicates that precision medicine, as exemplified by the application of CKD273, may only be available for the wealthy, but not for the general society.
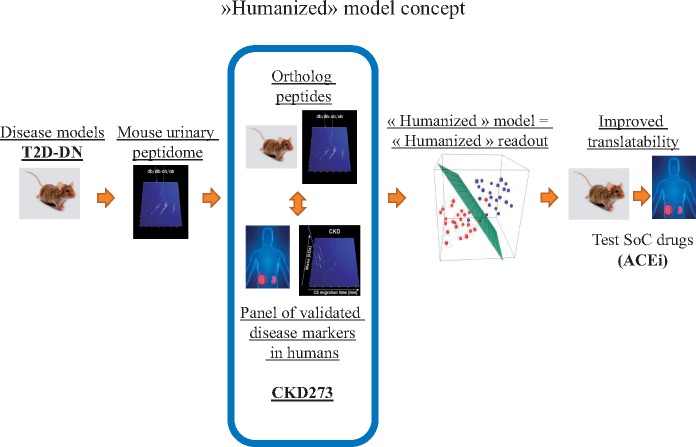
A further future perspective is application in the development of ‘humanized’ animal models [54]. It has become evident that animal models do not well-reflect human disease on a molecular level [55]. Using CKD273, the animal model most closely resembling human disease (based on similarity in molecular changes) could be identified, thereby generating a ‘humanized model’ that will enable substantial improvement in preclinical testing, increasing the chances of success in human subjects (Figure 7) [54].
Fig. 7.
Humanized model concept. Improving the translatability of animal models of disease with the development of a multimolecular humanized readout. The analysis of multiple urinary proteomic changes in the animal model allows identifying, similar (ortholog) changes in human disease and animal models, leading to a humanized readout, which will more efficiently translate the effects of new drugs in preclinical models to the clinic.
CKD273 has demonstrated a significant improvement over the current state of the art in detecting CKD at a very early point in time, when intervention is more likely to be successful and prevention of CKD progression appears feasible. A further area of application is supporting the development of therapeutic drugs that interfere with CKD progression, as CKD273 predicts the risk of CKD progression and can be used as an inclusion criterion in clinical trials. However, as outlined above, the development of such treatments and design of interventional clinical trials are often prevented by the demand to address the long-term renal endpoint of the disease, ESRD. In this regards, efforts towards defining earlier endpoints and to early predict progression of CKD, where drug intervention would be effective, would be beneficial. The definition of an early surrogate endpoint (e.g. CKD stage 3) combined with CKD273 as a biomarker for patient stratification would give a substantial boost to the development of new drugs to prevent CKD.
Supplementary Material
Acknowledgements
The research presented here was supported in part by the FP7 programmes ‘Clinical and system–omics for the identification of the Molecular De-terminants of established Chronic Kidney Disease’ (iMODE-CKD, PEOPLE-ITN-GA-2013-608332), ‘Molecular Medicine’ and ‘Systems Biology to Identify Molecular Targets for Vascular Disease Treatment’ (SysVasc, HEALTH-2013 603288).
Conflict of interest statement
H.M. is the founder and co-owner of Mosaiques Diagnostics, which developed the CE-MS technology and the MosaiquesVisu software. C.P. is employed by Mosaiques Diagnostics.
References
- 1. Jha V, Garcia-Garcia G, Iseki K. et al. Chronic kidney disease: global dimension and perspectives. Lancet 2013; 382: 260–272 [DOI] [PubMed] [Google Scholar]
- 2. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39: S1–S266 [PubMed] [Google Scholar]
- 3. Honeycutt AA, Segel JE, Zhuo X. et al. Medical costs of CKD in the Medicare population. J Am Soc Nephrol 2013; 24: 1478–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schievink B, Kropelin T, Mulder S. et al. Early renin-angiotensin system intervention is more beneficial than late intervention in delaying end-stage renal disease in patients with type 2 diabetes. Diabetes Obes Metab 2016; 18: 64–71 [DOI] [PubMed] [Google Scholar]
- 5. Coresh J, Turin TC, Matsushita K. et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 2014; 311: 2518–2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Parving HH, Persson F, Rossing P.. Microalbuminuria: a parameter that has changed diabetes care. Diabetes Res Clin Pract 2015; 107: 1–8 [DOI] [PubMed] [Google Scholar]
- 7. Brenner BM, Cooper ME, de Zeeuw D. et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861–869 [DOI] [PubMed] [Google Scholar]
- 8. Miller WG, Bruns DE, Hortin GL. et al. Current issues in measurement and reporting of urinary albumin excretion. Clin Chem 2009; 55: 24–38 [DOI] [PubMed] [Google Scholar]
- 9. Perkins BA, Ficociello LH, Roshan B. et al. In patients with type 1 diabetes and new-onset microalbuminuria the development of advanced chronic kidney disease may not require progression to proteinuria. Kidney Int 2010; 77: 57–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stepczynska A, Schanstra JP, Mischak H.. Implementation of CE-MS-identified proteome-based biomarker panels in drug development and patient management. Bioanalysis 2016; 8: 439–455 [DOI] [PubMed] [Google Scholar]
- 11. Check E. Running before we can walk. Nature 2004; 429: 496–497 [DOI] [PubMed] [Google Scholar]
- 12. Mischak H, Ioannidis JP, Argiles A. et al. Implementation of proteomic biomarkers: making it work. Eur J Clin Invest 2012; 42: 1027–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frantzi M, Latosinska A, Fluhe L. et al. Developing proteomic biomarkers for bladder cancer: towards clinical application. Nat Rev Urol 2015; 12: 317–330 [DOI] [PubMed] [Google Scholar]
- 14. Jankowski J, Schanstra JP, Mischak H.. Body fluid peptide and protein signatures in diabetic kidney diseases. Nephrol Dial Transplant 2015; 30(Suppl 4): iv43–iv53 [DOI] [PubMed] [Google Scholar]
- 15. Rossing K, Mischak H, Rossing P. et al. The urinary proteome in diabetes and diabetes-associated complications: new ways to assess disease progression and evaluate therapy. Proteomics Clin Appl 2008; 2: 997–1007 [DOI] [PubMed] [Google Scholar]
- 16. Mischak H, Kolch W, Aivalotis M. et al. Comprehensive human urine standards for comparability and standardization in clinical proteome analysis. Proteomics Clin Appl 2010; 4: 464–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mischak H, Vlahou A, Ioannidis JP.. Technical aspects and inter-laboratory variability in native peptide profiling: the CE-MS experience. Clin Biochem 2013; 46: 432–443 [DOI] [PubMed] [Google Scholar]
- 18. Kaiser T, Hermann A, Kielstein JT. et al. Capillary electrophoresis coupled to mass spectrometry to establish polypeptide patterns in dialysis fluids. J Chromatogr A 2003; 1013: 157–171 [DOI] [PubMed] [Google Scholar]
- 19. Wittke S, Fliser D, Haubitz M. et al. Determination of peptides and proteins in human urine with capillary electrophoresis–mass spectrometry, a suitable tool for the establishment of new diagnostic markers. J Chromatogr A 2003; 1013: 173–181 [DOI] [PubMed] [Google Scholar]
- 20. Weissinger EM, Wittke S, Kaiser T. et al. Proteomic patterns established with capillary electrophoresis and mass spectrometry for diagnostic purposes. Kidney Int 2004; 65: 2426–2434 [DOI] [PubMed] [Google Scholar]
- 21. Dakna M, Harris K, Kalousis A. et al. Addressing the challenge of defining valid proteomic biomarkers and classifiers. BMC Bioinformatics 2010; 11: 594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Subramanian J, Simon R.. Overfitting in prediction models – is it a problem only in high dimensions? Contemp Clin Trials 2013; 36: 636–641 [DOI] [PubMed] [Google Scholar]
- 23. Mischak H, Apweiler R, Banks RE. et al. Clinical proteomics: a need to define the field and to begin to set adequate standards. Proteomics Clin Appl 2007; 1: 148–156 [DOI] [PubMed] [Google Scholar]
- 24. Mischak H, Allmaier G, Apweiler R. et al. Recommendations for biomarker identification and qualification in clinical proteomics. Sci Transl Med 2010; 2: 46ps42. [DOI] [PubMed] [Google Scholar]
- 25. Mischak H. Pro: urine proteomics as a liquid kidney biopsy: no more kidney punctures! Nephrol Dial Transplant 2015; 30: 532–537 [DOI] [PubMed] [Google Scholar]
- 26. Thongboonkerd V. Recent progress in urinary proteomics. Proteomics Clin Appl 2007; 1: 780–791 [DOI] [PubMed] [Google Scholar]
- 27. Rossing K, Mischak H, Dakna M. et al. Urinary proteomics in diabetes and CKD. J Am Soc Nephrol 2008; 19: 1283–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Snell-Bergeon JK, Maahs DM, Ogden LG. et al. Evaluation of urinary biomarkers for coronary artery disease, diabetes, and diabetic kidney disease. Diabetes Technol Ther 2009; 11: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jantos-Siwy J, Schiffer E, Brand K. et al. Quantitative urinary proteome analysis for biomarker evaluation in chronic kidney disease. J Proteome Res 2009; 8: 268–281 [DOI] [PubMed] [Google Scholar]
- 30. Merchant ML, Perkins BA, Boratyn GM. et al. Urinary peptidome may predict renal function decline in type 1 diabetes and microalbuminuria. J Am Soc Nephrol 2009; 20: 2065–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Good DM, Zürbig P, Argiles A. et al. Naturally occurring human urinary peptides for use in diagnosis of chronic kidney disease. Mol Cell Proteomics 2010; 9: 2424–2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Molin L, Seraglia R, Lapolla A. et al. A comparison between MALDI-MS and CE-MS data for biomarker assessment in chronic kidney diseases. J Proteomics 2012; 75: 5888–5897 [DOI] [PubMed] [Google Scholar]
- 33. Mischak H, Delles C, Klein J. et al. Urinary proteomics based on capillary electrophoresis-coupled mass spectrometry in kidney disease: discovery and validation of biomarkers, and clinical application. Adv Chronic Kidney Dis 2010; 17: 493–506 [DOI] [PubMed] [Google Scholar]
- 34. Duffield JS. Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest 2014; 124: 2299–2306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cho JH, Ryu HM, Oh EJ. et al. Alpha1-antitrypsin attenuates renal fibrosis by inhibiting TGF-beta1-induced epithelial mesenchymal transition. PLoS One 2016; 11: e0162186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Critselis E, Lambers HH.. Utility of the CKD273 peptide classifier in predicting chronic kidney disease progression. Nephrol Dial Transplant 2016; 31: 249–254 [DOI] [PubMed] [Google Scholar]
- 37. Zürbig P, Jerums G, Hovind P. et al. Urinary proteomics for early diagnosis in diabetic nephropathy. Diabetes 2012; 61: 3304–3313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roscioni SS, de Zeeuw D, Hellemons ME. et al. A urinary peptide biomarker set predicts worsening of albuminuria in type 2 diabetes mellitus. Diabetologia 2012; 56: 259–267 [DOI] [PubMed] [Google Scholar]
- 39. Argiles A, Siwy J, Duranton F. et al. CKD273, a new proteomics classifier assessing CKD and its prognosis. PLoS One 2013; 8: e62837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Siwy J, Schanstra JP, Argiles A. et al. Multicentre prospective validation of a urinary peptidome-based classifier for the diagnosis of type 2 diabetic nephropathy. Nephrol Dial Transplant 2014; 29: 1563–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schanstra JP, Zurbig P, Alkhalaf A. et al. Diagnosis and prediction of CKD progression by assessment of urinary peptides. J Am Soc Nephrol 2015; 26: 1999–2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ovrehus MA, Zurbig P, Vikse BE, Hallan SI.. Urinary proteomics in chronic kidney disease: diagnosis and risk of progression beyond albuminuria. Clin Proteomics 2015; 12: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pontillo C, Jacobs L, Staessen JA. et al. A urinary proteome-based classifier for the early detection of decline in glomerular filtration. Nephrol Dial Transplant 2016; pii: gfw23 [DOI] [PubMed] [Google Scholar]
- 44. Lindhardt M, Persson F, Zurbig P. et al. Urinary proteomics predict onset of microalbuminuria in normoalbuminuric type 2 diabetic patients, a sub-study of the DIRECT-Protect 2 study. Nephrol Dial Transplant 2016; pii: gfw292 [DOI] [PubMed] [Google Scholar]
- 45. Gu YM, Thijs L, Liu YP. et al. The urinary proteome as correlate and predictor of renal function in a population study. Nephrol Dial Transplant 2014; 29: 2260–2268 [DOI] [PubMed] [Google Scholar]
- 46. Siwy J, Zürbig P, Argiles A. et al. Non-invasive diagnosis of chronic kidney diseases using urinary proteome analysis. Nephrol Dial Transplant 2016; pii: gfw337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Haubitz M, Good DM, Woywodt A. et al. Identification and validation of urinary biomarkers for differential diagnosis and evaluation of therapeutic intervention in ANCA associated vasculitis. Mol Cell Proteomics 2009; 8: 2296–2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Andersen S, Mischak H, Zürbig P. et al. Urinary proteome analysis enables assessment of renoprotective treatment in type 2 diabetic patients with microalbuminuria. BMC Nephrol 2010; 11: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lindhardt M, Persson FI, Oxlund C. et al. Predicting albuminuria response to spironolactone treatment with urinary proteomics in patients with type 2 diabetes and hypertension. Nephrol Dial Transplant 2017; pii: gfw406. doi: 10.1093/ndt/gfw406 [DOI] [PubMed] [Google Scholar]
- 50. Lindhardt M, Persson F, Currie G. et al. Proteomic prediction and Renin angiotensin aldosterone system Inhibition prevention Of early diabetic nephRopathy in TYpe 2 diabetic patients with normoalbuminuria (PRIORITY): essential study design and rationale of a randomised clinical multicentre trial. BMJ Open 2016; 6: e010310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. EMA. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/06/WC500169469.pdf. 2014
- 52. Pontillo C, Zurbig P, Schanstra JP. et al. Urinary peptide-based prediction of progression from chronic kidney disease stage 2 to 3. Abstract [Google Scholar]
- 53. Bahlmann FH, Critselis E, Pontillo C. et al. Comparison of the cost-effectiveness of the urinary based CKD273 biomarker panel and current clinical practices in the management of chronic kidney disease progression. Nephrol Dial Transplant 2015; 30: iii305 [Google Scholar]
- 54. Klein J, Ramirez-Torres A, Ericsson A. et al. Urinary peptidomics provides a noninvasive humanized readout of diabetic nephropathy in mice. Kidney Int 2016; 90: 1045–1055 [DOI] [PubMed] [Google Scholar]
- 55. Siwy J, Zoja C, Klein J. et al. Evaluation of the Zucker Diabetic Fatty (ZDF) rat as a model for human disease based on urinary peptidomic profiles. PLoS One 2012; 7: e51334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.