Abstract
Sizeable disparities exist in breast cancer outcomes, both between Black and White patients in the United States, and between patients in the US and other high-income countries compared to low- and middle-income countries (LMICs). In both settings, health system factors are key drivers of disparities. In the US, Black women are more likely to die of breast cancer than Whites, and have poorer outcomes even among patients with similar stage and tumor subtype. Over-representation of higher-risk “triple negative” breast cancers contributes to breast cancer mortality in Black women; however, the greatest survival disparities occur within the good-prognosis hormone-receptor positive (HR+) subtypes. Disparities in access to treatment within the complex US health system may be responsible for a substantial portion of these differences in survival. In LMICs, breast cancer mortality rates are substantially higher than in the US, while incidence continues to rise. This mortality burden is largely attributable to health system factors, including late-stage presentation at diagnosis and lack of availability of systemic therapy. This article will review the existing evidence for how health-system factors in the United States contribute to breast cancer disparities, discuss methods for studying the relationship of health system factors to racial disparities, and provide examples of health system interventions that show promise for mitigating breast cancer disparities. We will then review evidence of global breast cancer disparities in low and middle income countries, the treatment factors that contribute to these disparities, and actions being taken to combat breast cancer disparities around the world.
Keywords: Breast Cancer, Disparities, Access to Care, Early Detection, Resource-Stratified Guidelines
Introduction: Variation in Breast Cancer Outcomes at Home and Around the Globe
At first glance, the issues of breast cancer disparities in the United States and around the world appear to be quite distinct. In the US, a complex health care system offers widespread breast cancer screening and multidisciplinary breast cancer care including surgery, radiation, chemotherapy and targeted therapies. When appropriately delivered, this combination of screening and treatment has been highly effective in reducing mortality over time. In contrast, in low and middle income countries (LMICs), screening and multi-modality treatment are not widely accessible or affordable, with correspondingly higher mortality rates. Thus, when viewed through a global lens, breast cancer disparities are a complex series of comparisons: between more and less advantaged patients within each setting, and between the more favorable range of outcomes in high-income countries compared to the worsened spectrum in LMICs. In this review, we discuss how health system factors in both settings impact breast cancer disparities. In the US, we consider how the complexities of the health care system create disparities in access to care, at individual points on the cancer care continuum and cumulatively, that drive differences in outcome, focusing on the Black-White disparity. In the global context, we consider how gaps in the health care systems impact overall outcomes in LMICs compared to high income countries (HICs), as well as variation in outcomes for patients with better or worse access to care within LMICs. In both contexts, we discuss the promise of interventions at the health system level to improve outcomes for disadvantaged patients with breast cancer.
Disparities in the U.S
Epidemiology of Breast Cancer Race Disparities in the U.S
It is well-recognized that Black women in the United States are more likely to die of their disease than Whites, a survival gap that has persisted for more than three decades even as overall mortality rates have improved by 36%.(1) In 2012, Black women were 42% more likely to die of breast cancer than non-Hispanic White women, with a breast cancer death rate of 31 per 100,000 among Blacks compared to 22 per 100,000 among Whites.(2) Meanwhile, breast cancer incidence among Black women continues to rise and has recently converged with the stable incidence rate of non-Hispanic White women, with incidence rates of 128/100,000 for Whites and 124/100,000 for Blacks in 2008–2012. This trend appears to be driven by increases in ER+ breast cancers (Fig 1a).(2)
Figure 1.
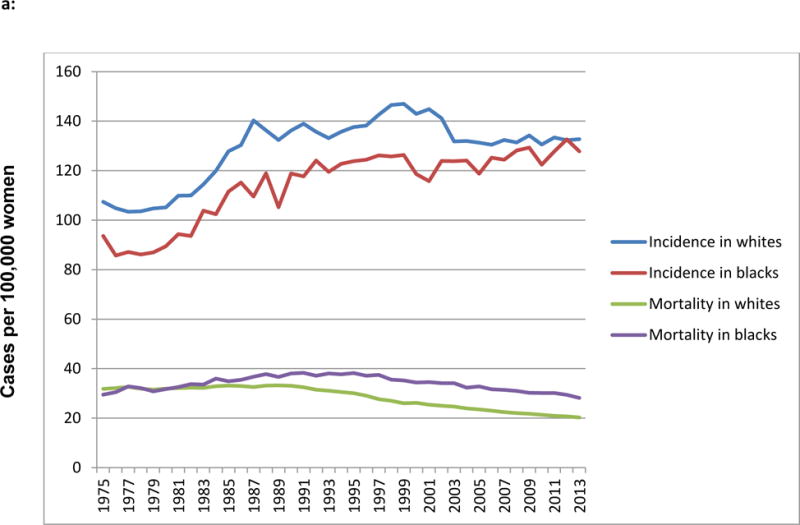
a: Trends in age-adjusted incidence and mortality of breast cancer in US black and white women, 1975–2013 (data from Surveillance Epidemiology and End Results (SEER) 9 Sites)
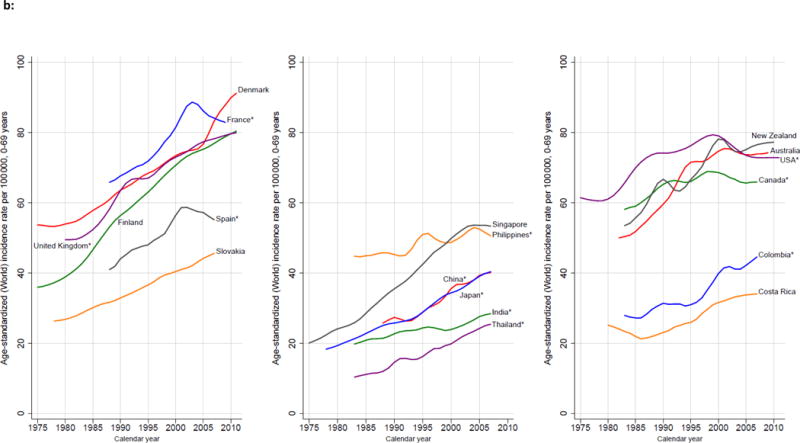
b: Trends in Age-Standardized Breast Cancer Incidence, Selected Countries, 1975–2010. Source: Ferlay and others 2013; WHO Mortality Database (http://www.who.int/healthinfo/statistics/mortality_rawdata/en/index.html), previously published.(107)
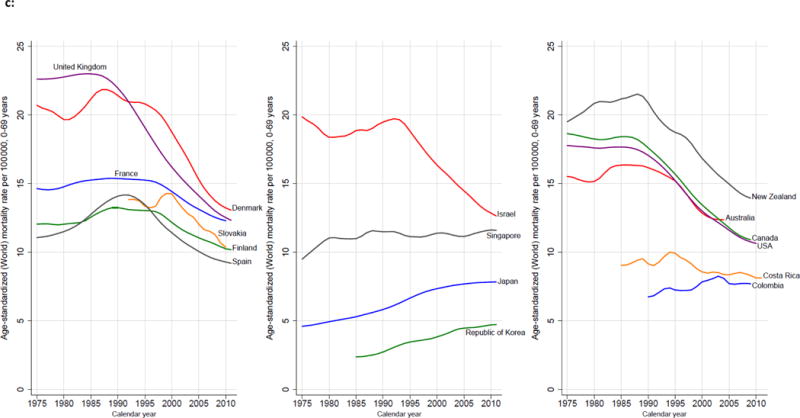
c: Trends in Age-Standardized Breast Cancer Mortality, Selected Countries, 1975–2010. Source: Ferlay and others 2013; WHO Mortality Database (http://www.who.int/healthinfo/statistics/mortality_rawdata/en/index.html), previously published.(107)
Several recognized biological and clinical difference contribute to the breast cancer mortality gap, including higher incidence of hormone receptor (HR) and HER2 receptor-negative or “triple negative” tumor subtype in young Black women with correspondingly lower incidence of HR+/HER2− cancers (Figure 1a),(1,3) more advanced stage at presentation, and higher grade and other adverse pathologic features in Black women with hormone receptor-positive tumor types.(4,5) As discussed elsewhere in this issue by Desmedt and colleagues, considerable genomic variability exists among HR+/HER2− cancers, as well as other clinical subtypes, with regard to both pre-treatment mutational status and acquired mutations associated with treatment resistance.(6) With the plethora of recent sequencing data, it is argued that sequencing further primary breast tumors is unlikely to discover new, common mutational signatures.(7) However, we posit that further characterization of racial differences in within-subtype biology, made possible by sequencing of tumors from large minority-enriched cohorts such as the Carolina Breast Cancer Study, may yield novel information regarding the biologic underpinnings of racial disparities.(8) Once tumor biology is accounted for, however, the presence of disparities within similar stage and subtype cancers, the unusual prominence of disparities in the highly treatable HR+/HER2− subtype relative to others, and the widening of the racial disparity in mortality over time (Figure 1) also suggest an influence of post-diagnosis factors on differences in outcome.(9) (10) This history and pattern of breast cancer disparity exemplifies the fundamental cause theory, which posits that disparities in health outcomes are related to differential access to and utilization of a complex variety of resources, and thus will persist despite treatment advances and be widest in groups where the most effective treatments exist.(11) It is possible that if novel, effective and expensive therapies emerge for triple negative breast cancer, such as the immunotherapy approaches discussed elsewhere in this issue,(12) disparities in the mortality of triple negative disease will paradoxically widen even as overall outcomes improve, if we do not solve the underlying problem of racial differences in access to cancer care.
The Health System and Breast Cancer Disparities
If we accept that differential access to healthcare resources is a key contributor to breast cancer disparities in the US and around the world, we must understand not only person-level but provider, institution and health system-level drivers of inequities in breast cancer outcomes in order to bring about change. Racial disparities in treatment exist across the cancer care continuum from diagnosis through surgery, chemotherapy, radiation and targeted therapies, are known to impact survival, and have been extensively described elsewhere.(13,14) Figure 2 illustrates how observed treatment disparities in the US add to biologic and stage differences to compound disparities in outcome.
Figure 2.
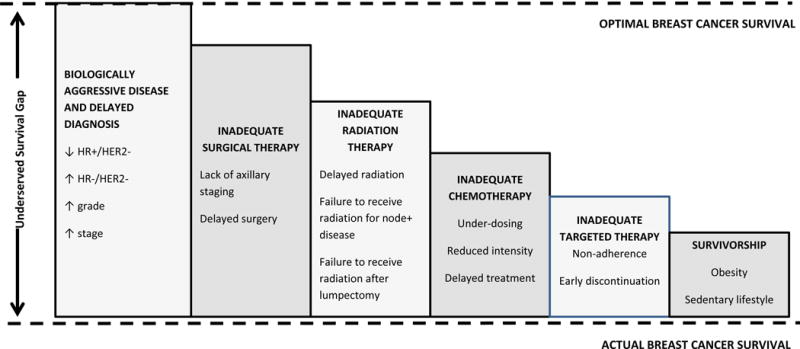
Contributors to US and global outcome disparities in breast cancer across the cancer care continuum. In addition to lack of any treatment (e.g. lack of radiation, lack of chemotherapy), specific treatment gaps are listed in each bar.
Disparities in US Breast Cancer Diagnosis, Surgery and Adjuvant Therapy
At the entry point of the cancer care continuum, differences in access to diagnostic testing and surgical care may impact outcomes. For the most part, these disparities relate to delayed care, limited ability to pay for care, or inability to complete treatment. Uninsured women are more likely to have delays in resolution of abnormal mammographic findings,(15) and Black women in Medicaid or Medicare are more likely to experience delays in surgical treatment than Whites. Further, surgical delays are more common in states with less generous Medicaid reimbursement for breast surgery and more restrictive re-enrollment policies.(16,17) Black race and insurance type have been noted to be independent predictors of delays in surgical treatment in studies where both factors are considered.(18,19) However, among uniformly insured women, delays in surgery are more common among Blacks after controlling for clinical factors.(20) Thus, access to care may be particularly problematic for publically insured minority women, but racial disparities in surgical timeliness persist despite access to insurance.
The quality of surgical therapy also differs by race, and these differences are linked to the institutions where minority patients seek care. We and others have noted slower adoption and lower rates of sentinel lymph node biopsy, an innovative morbidity-sparing surgical procedure, among eligible Black women compared to Whites,(21,22) and these differences are partially explained by the concentration of White patients within National Cancer Institute (NCI)-designated comprehensive cancer centers, hospitals with high breast cancer volume, greater affiliation with research cooperative groups and the American College of Surgeons Oncology Group (ACOSOG), and the NCI’s Comprehensive Community Oncology Program (CCOP).(23,24) Breast reconstruction is also less utilized by minority women, which appears to be related to lower access to plastic surgeons.(25) Interestingly, one large study showed that Black women were less actively involved in choosing their surgeon or hospital for breast surgery.(26)
Similar disparities are seen in radiation therapy. In two national studies, racial disparities in receipt and timeliness of adjuvant radiation were largely explained by differences in the distance to radiation care and the type of facility where patients received surgery.(27,28) Another study found that in California, racial disparities in radiation were found only in the most urban regions, and low socio-economic status was an independent risk factor.(29) A third study found that among low-income women, Blacks remained less likely than Whites to receive radiation.(30) Together, these studies suggest that racial disparities in radiation treatment differ based on where patients live, and that both race and poverty affect access to treatment.
Disparities in chemotherapy have been more difficult to pinpoint, with some studies showing Black women no less likely than Whites to receive chemotherapy for clinically similar disease.(31) However, multiple challenges exist in how chemotherapy is delivered. Black and Hispanic patients are more likely to have delays in initiating chemotherapy, which are associated with decrements in survival in multiple studies.(14,32,33) Black patients more often receive reduced-intensity adjuvant regimens and under-dosing of chemotherapy, particularly with respect to inappropriate “capping” of doses for overweight and obese patients.(34,35) Freedman and colleagues offer a more comprehensive discussion regarding the impacts of obesity, which is more common in Black breast cancer patients, on breast cancer treatment and outcomes elsewhere in this issue.(36) Regarding targeted therapies, Black women are less likely to receive trastuzumab and are more likely to receive non-standard chemotherapy in combination with trastuzumab within the Medicare program (37,38) and less likely to complete a full course of adjuvant trastuzumab even within National Comprehensive Cancer Network (NCCN) hospitals,(39) suggesting that insurance and site of care are not necessarily protective in the setting of very expensive treatments and those of long duration. Black women are less likely to initiate endocrine therapy than Whites, (40) and more likely to have difficulty with adherence.(41–43) Although not specific to Black women, multiple studies suggest that policy factors such as co-payment amount, the availability of generic alternatives, and Medicare low-income subsidies enhance adherence to endocrine therapy.(44–46)
Methods for Observational Study of Health System Contributors to Disparities
The Institute of Medicine (IOM) has defined disparities as “the difference in treatment or access not justified by the differences in health status or preferences of the groups.”.(47,48) In practical terms, the IOM framework has been interpreted to mean that researchers should not adjust for factors such as education or poverty as separate covariates in models of racial disparities, because inequity in these factors is part of the social construct of race, and models that treat them as separate may give a distorted picture of the experience of minority patients. In other words, unequal treatment offered to clinically similar patients of different races constitutes a racial disparity, even if the basis of said unequal treatment was a socioeconomic factor such as lack of ability to pay for care. However, other researchers present models that examine the “residual direct effect” of race, or the remaining independent association between race and an outcome after adjustment for a wider variety of potential confounders including factors such as income and education.(49) Unadjusted differences between racial groups may also be informative and descriptive, as for instance when we examine the percentage of Black and White patients treated in a certain hospital who receive mastectomy. All three approaches may be valuable, but the investigator should consider the purpose of the research. Where the intent is to generate hypotheses about an unmeasured or unknown characteristic of a given racial group that might explain worse survival outcomes, such as biologically aggressive disease, a model that adjusts for all measurable confounders including clinical and socioeconomic factors may help support or refute the hypothesis of an as-yet-unmeasured factor in the disparity (the residual direct effect of race). However, if the purpose is to point out an area in which a racial group is receiving sub-standard care, and for which an intervention may improve delivery of appropriate care, a model that adjusts only for clinical need and treatment appropriateness (IOM model) will likely give a more interpretable picture of the extent of the disparity. Further, if the purpose is to understand the treatment experience of patients at a given point of care, descriptive estimates of treatment differences by race, without adjusting for other factors, may give an early indicator of whether there is cause for concern for a disparity, and are straightforward for most audiences to understand.
System-Level Actions to Reduce US Breast Cancer Disparities
Interventions at the level of individual institutions, health systems or insurance payers are an attractive avenue to narrow disparities for several reasons. They can reach a large number of patients, can be aimed at sites or geographic areas serving disproportionately minority patients, or where disparities are known to be largest. They may rely to some extent on tested strategies for organizational change and adoption of innovations. Finally, they may be attractive to funders and policy-makers when framed as improvements to quality of care, which can benefit patients broadly, but will be expected to accrue more benefit to patients currently receiving sub-standard care, which usually includes vulnerable populations. In their landmark “Unequal Treatment” report, the Institute of Medicine highlighted this need for interventions focused on the health services drivers of disparities.(47) Unfortunately, more than a decade later, research in this area of cancer care delivery is still under-developed. Examples of successful health-system interventions are highlighted in the following paragraphs.
Access to mammography and timely surgical care after mammography can reduce disparities by removing access barriers that contribute to advanced stage at diagnosis. The Centers for Disease Control Breast and Cervical Cancer Early Detection Program (BCCEDP) is a national program offering free or low cost screening to uninsured women, as well as Medicaid waivers and case management for women with abnormal screening results. Evaluations of this program have demonstrated that 90% of enrollees with abnormal results completed diagnostic workup and initiated treatment within 30 days of their abnormal finding.(50) There is also some evidence of decreases in breast cancer mortality related to uptake of BCCEDP screening.(51) Although not yet evaluated, Medicaid expansion and other provisions of the Affordable Care Act that lowered uninsured rates may also act to close disparities in access to breast screening and surgical treatment. This hypothesis is indirectly supported by data that ACA policies disproportionately increased insurance rates among lower-income and non-white patients.(52) However, the narrow care networks of ACA plans may paradoxically limit access to high quality cancer care; a recent study found that only 41% of ACA networks included an NCI-designated comprehensive cancer center.(53)
Patient navigation is an established intervention that may disproportionately benefit minority patients by mitigating differences in health literacy, access to resources, and self-advocacy. Navigators improve timeliness of diagnostic breast cancer care in vulnerable populations.(54) The Patient Care Connect Program, which provides lay navigation in rural and under-resourced community cancer centers in the network of the University of Alabama at Birmingham (UAB), shows promise as a cost-effective intervention in minority and low patients of lower socioeconomic status to enhance access and possibly increase enrollment to clinical trials, but requires intensive training and program support to be successful.(55–58) Navigation has been associated with high patient satisfaction, particularly among minority and elderly patients,(59) but more work on how to implement navigation in vulnerable populations and low resource settings is needed. At the provider and practice level, programs that engage practices in collaborative quality improvement work, such as the American Society of Clinical Oncology’s (QOPI) program and the MUSIC (Michigan Urological Surgical Improvement Collaborative) program for urology providers in Blue Cross Blue Shield’s Michigan network, hold promise to engage providers, identify high-value targets for quality improvement and promote adoption of guideline-concordant care by providers.(60–62) To date, no such program has been tested in breast cancer specifically, or as a direct intervention on treatment disparities. Payer-level interventions may also be vehicles to raise care to desired standards or to reach out directly to patients at risk of sub-optimal outcomes. Within integrated health systems, oncology clinical care pathways have been demonstrated to lower ER and hospital utilization and cost during breast cancer treatment;(63) they may also may offer a way to mitigate treatment disparities, but this application has not been evaluated.
Audit and feedback interventions offer potential to improve cancer care delivery to minority patients by using automated functions, such as the electronic health record (EHR), to alert providers or researchers when gaps in care occur, and putting systems in place to correct gaps. One such intervention in safety-net hospital sites in New York consists of a registry that interacts with EHRs across institutions to ensure that handoffs from surgery to oncology providers occur seamlessly. Many technical barriers to implementation and competing priorities have been noted in this low-resource setting.(64) A similar intervention, the Accountability for Cancer Care Through Undoing Racism and Equity (ACCURE) study, is underway to improve delivery of adjuvant breast and lung cancer care in North Carolina.(65) Neither project has reported long term results at this time.
Disparities around the Globe
Epidemiology of Breast Cancer as a Global Epidemic
Breast cancer is the most common cause of cancer deaths among women worldwide, representing 25–35% of all female cancer cases.(66) Breast cancer incidence rates are higher in more developed than less developed regions, with 2012 incidence rates ranging from 18.9 cases/100,000 in Eastern Africa compared to 166.9 cases/100,000 in Western Europe. However, breast cancer incidence is increasing most rapidly in LMICs, which are poorly equipped to deal with this burden and are disproportionately affected by the rising incidence and mortality. Breast cancer fatality rates are inversely correlated with per capita gross domestic product (GDP).(67,68) Once considered primarily a disease of women in HICs, over half (52%) of new breast cancer cases and 62% of deaths occur in LMICs.(69) In 2012, breast cancer incidence in the U.S. and Canada combined accounted for only 15% of new breast cancer cases worldwide. In contrast, countries in Asia, Latin America and Africa, which include the majority of LMICs around the world, accounted for 54% of new cases.(68) The disproportionate number of young lives lost to breast cancer is especially concerning; among the 94,000 women ages 15–49 years who died of breast cancer around the world in 2010, 68,000 (72%) were in LMICs.(70) The impressive progress made in HICs to improve breast cancer outcomes has not been mirrored in LMICs. Breast cancer mortality rates in the U.S. and Europe have dropped nearly 2% each year since 1990.(71,72) In HICs, implementation of breast cancer early detection programs (including, but not limited to, population-based mammographic screening) together with locoregional treatment (management of the breast and axillary nodes using surgery and radiotherapy) (73–76) and systemic treatments (pharmacologic therapy that reduces the risk of metastatic spread) (77–80) has resulted in impressive reductions in national breast cancer mortality in HICs.(81–84) By contrast, breast cancer incidence rates have historically been low in LMICs, but these rates are rising disproportionately at the same time that mortality rates are continuing to rise or remain high. The aging of the current global population means that nearly 50 percent more women will develop and die from breast cancer in 2020 than in 2002.(85)
There are many parallels between the domestic breast cancer disparities issues and global breast cancer, especially in comparing underserved communities in the U.S. with populations from LMICs. However, the global breast cancer questions have to consider a broader set of questions that relate to cancer treatment access overall. In much of the world, countries are just beginning to consider making cancer treatment available at a population level. Thus the most critical research questions have some fundamental differences comparing the U.S. and global communities. In the U.S., we view cancer early detection, diagnosis and treatment as a standard component of healthcare delivery and therefore focus on biological questions related to incidence and treatment. In LMICs by contrast, cancer treatment is often considered beyond what is feasible for healthcare delivery outside of private healthcare facilities treating the minority with adequate financial means to pay independently for care, making the most important questions related to access and resource-appropriate treatments with therapies already proven to be effective in HICs. In Table 1, we compare and contrast key contributors to racial disparities in the US and LMICs, along with examples of system-level interventions that may be effective for each.
Table 1.
Summary of cancer care delivery gaps and possible system-level interventions in the US and low and middle income countries (LMICs)
| Care Gap | United States | LMIC | US System Interventions | LMIC System Interventions |
|---|---|---|---|---|
| Advanced stage at diagnosis | Yes | Yes | Free screening programs Medicaid expansion Increased insurance access |
Breast health awareness education and improved early diagnosis strategies |
| Delayed or Inadequate surgery | Yes | Yes | Insurance access Medicaid policy change Patient navigation QI programs EHR alerts |
Address timely access to cancer surgery without requiring patients to pay privately out of pocket |
| Delayed or No radiation | Yes | Yes | Patient navigation Audit and Feedback QI programs EHR alerts |
Determine if radiation therapy is available in country and then properly triage patients for timely treatment |
| Lack of chemo therapy/biologics | No | Yes | – | Assess access to drugs on WHO’s Essential Medicine List and improve availability at decreased cost through collective bargaining |
| Chemo under-dosing or non-standard agents; early discontinuation | Yes | Yes | Audit and Feedback Clinical Pathways QI Programs |
Assess proper drug utilization and delivery in existing systems and evaluate quality control |
| Lack of endocrine therapy | Yes | Yes | Pharmacy system alerts Patient navigation Co-pay assistance |
Establish estrogen-receptor testing availability and secure tamoxifen and generic aromatase inhibitor access for properly selected patients |
Breast Cancer Early Detection – A Prerequisite for Improving Global Breast Cancer Outcomes
The case for improved breast cancer early detection, i.e., making diagnoses at an earlier phase of malignant progression, to improve breast cancer survival is supported by evidence from randomized controlled trials (RCTs) and meta-analyses, which demonstrate better prognosis with incrementally smaller tumors.(86,87) A study examining breast cancers diagnosed between 1975–1999 reported that stage migration – as assessed by a reduction in tumor size in the period – accounted for a significant proportion of the survival benefit during that time in the U.S.(88) While screening mammography has been shown to be effective at reducing breast cancer mortality,(89,90) clinical breast examination (CBE) has been shown to effectively downstage breast cancer in LMICs where women are commonly diagnosed with advanced stage cancer at first presentation.(91,92) The 25-year update of the Canadian National Breast Screening Study was widely publicized for finding no survival benefit for women who underwent screening mammography.(93) Largely overlooked, however, were the highly successful early detection rates achieved in the study’s control group receiving clinical evaluation without mammographic screening, where the median invasive tumor size at diagnosis approximated 2cm for the first 10 years of the study, which is significantly better than LMICs like India where 75% of patients present with locally advanced (stage III) or metastatic (stage IV) cancer. The highly favorable early detection outcomes of the Canadian trial control group were achieved through breast health education combined with annual CBE but without mammographic screening, suggest that the study’s control group methodology could be a model for early detection program development. These findings indicate that countries unable to afford or implement mammographic screening should begin with the development of breast cancer awareness and CBE programs as a foundation for establishing breast cancer early detection.(94)
Systemic Adjuvant Therapy – An Essential Element for Improving Breast Cancer Survival
Early detection and adjuvant systemic therapy are synergistic and mutually dependent for improving breast cancer outcomes, since early detection only works if it can be followed by prompt, effective therapy.(95) Mathematical modeling suggests that between 28% and 65% of breast cancer mortality reduction can be attributed to early detection; the balance is due to pharmacotherapy with endocrine therapy, cytotoxic chemotherapy and targeted biologic treatments.(77,79,80,96,97) Unfortunately, many LMICs have limited resources to allocate to early breast-cancer detection, as well as cultural and social barriers, resulting in late diagnosis, which is more difficult to treat effectively, and is associated with increased morbidity and mortality.(98) Multiple barriers to breast healthcare exist in LMICs including lack of resources, inequitable distribution of services in urban vs. rural areas, and poverty. Total expenditure on health per capita (US$) in 2013 in counties classified by the World Bank as low income averaged US$36, compared to $277 in middle-income countries, and $4,687 in HICs.(99) Despite the increase in breast cancer incidence and the concomitant increase in breast-cancer related mortality, cancer is often a low priority in many LMICs, with spending on all cancers averaging 5% of the total expenditure on health.(100) In the past, the primary focus of healthcare dollars was for infectious diseases. However, rising rates of mortality related to the major noncommunicable diseases (NCD) led the United Nations to hold a high level General meeting in September 2011, heightening the awareness of member states to focus on the importance of leading non-communicable disease killers including cancer, heart disease, diabetes, and chronic respiratory disease.(101)
At the present time, access to effective treatment is limited or unavailable in many or most LMICs. A recent report reviewing national essential medicines lists (NEMLs) from LMICs found significant variation in available treatments for different types of early breast cancer: over 80% of the countries in the American hemisphere included all therapy components for all types of early breast cancer (except for HER2-overexpressing tumors). By comparison, over 40% of the countries in the Eastern Mediterranean and African regions did not have all treatment components for any subtype, and guideline-recommended treatments were less frequently included in the NEMLs of low-income than in middle income countries.(102) Even when included in NEMLs, actual availability of and access to many anti-cancer therapies can fall far short in many countries. As a result, cancer early detection and treatment disparities are reflected in worsened survival rates for women in LMICs versus HICs: five-year survival following breast cancer diagnosis is greater than 80% in the United States compared to only 32% in in sub-Saharan Africa.(98,103) Effective solutions are thus urgently needed at the global level to improve breast cancer outcomes and make measureable improvements in women’s health and well-being. Tools that have proven effective in the U.S., such as patient navigation, could play a valuable role in improving patient coordination in LMICs. There may also be opportunities for collective bargaining to achieve drug access at affordable rates in LMICs.
Call to Action to Reduce Global Breast Cancer Disparities
In 2014, a call for action to reduce disparities in breast cancer outcomes around the world by the American Cancer Society, Susan G. Komen for the Cure® and the Union for International Cancer Control (UICC), led to the formation of the Breast Cancer Initiative 2.5 (www.bci25.org), co-organized by the Breast Health Global Initiative (BHGI) and WE-CAN. BCI2.5 is a global campaign to unite the global breast cancer community behind a common goal to make breast health a global health priority and to reduce disparities in breast cancer outcomes for 2.5 million women by 2025. BCI2.5 explores innovative ways to implement affordable, appropriate, acceptable and feasible evidence-based strategies. A key element of this initiative is identifying, documenting and fostering dissemination of innovative approaches to the delivery of breast healthcare developed in low-resource settings.(104) This demands a collaborative effort that draws on the collective expertise and resources of individuals and institutions engaged in breast cancer care.(105) Future directions will hinge on identifying individualized approaches based upon building functional systems that provide effective early detection, diagnosis and treatment approaches based upon the implementation of existing resources.(106)
Conclusion
The issue of breast cancer disparities can be seen from many perspectives, domestically and globally. We have demonstrated that in both high-resource settings such as the United States, as well as the resource-constrained settings of LMICs, the outcome of breast cancer is often determined not only by its inherent biological and clinical characteristics, but by modifiable treatment factors highly influenced by access to care within a given health system. Across these diverse settings, a common thread is the need for innovative, flexible methods of delivering cancer care that make the best use of resources and eliminate barriers to optimal care for the most vulnerable patients. A true cancer care continuum links resources from early detection through timely and effective treatment in a robust chain leading to the best possible outcome for each individual patient. Only through strong health systems can we see the amazing innovations of breast cancer research in the 21st century reach their full potential as we reach the right patients at the right time with the right treatment.
Acknowledgments
Funding support
Dr. Reeder-Hayes receives grant support from a Conquer Cancer Foundation Career Development Award, a Susan G Komen Foundation Career Catalyst Award, and the Alliance for Clinical Trials in Oncology Alliance Scholar Award, and is also supported in part by career development and project funds from the University of North Carolina Breast Cancer Specialized Programs of Research Excellence grant P50CA058223-20. Dr. Anderson receives salary support from Susan G. Komen for the Cure (Leadership Grant #SAC110001) and has received unrestricted educational grants from Pfizer and Roche.
Footnotes
Dr. Reeder-Hayes has no conflicts of interest to disclose.
Dr. Anderson has no conflicts of interest to disclose.
References
- 1.Howlader N, Altekruse SF, Li CI, Chen VW, Clarke CA, Ries LA, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106(5) doi: 10.1093/jnci/dju055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, Jemal A. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA: a cancer journal for clinicians. 2016;66(1):31–42. doi: 10.3322/caac.21320. [DOI] [PubMed] [Google Scholar]
- 3.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295(21):2492–502. doi: 10.1001/jama.295.21.2492. doi 295/21/2492 [pii] [DOI] [PubMed] [Google Scholar]
- 4.Chen VW, Correa P, Kurman RJ, Wu XC, Eley JW, Austin D, et al. Histological characteristics of breast carcinoma in blacks and whites. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 1994;3(2):127–35. [PubMed] [Google Scholar]
- 5.Porter PL, Lund MJ, Lin MG, Yuan X, Liff JM, Flagg EW, et al. Racial differences in the expression of cell cycle-regulatory proteins in breast carcinoma. Cancer. 2004;100(12):2533–42. doi: 10.1002/cncr.20279. [DOI] [PubMed] [Google Scholar]
- 6.Yates L, Desmedt C. Translational Genomics: Practical Applications of the Genomic Revolution. Clin Can Res. 2017 doi: 10.1158/1078-0432.CCR-16-2548. in press;23. [DOI] [PubMed] [Google Scholar]
- 7.Nik-Zainal S. Mutational Signatures in Breast Cancer: The Problem at the DNA Level. Clin Can Res. 2017 doi: 10.1158/1078-0432.CCR-16-2810. in press;23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Troester MA, Sun X, Allot EH, Geradts J, Cohen SM, Tse CH, et al. Racial Differences in PAM50 Subtypes in the Carolina Breast Cancer Study. J Natl Cancer Inst. 2017 doi: 10.1093/jnci/djx135. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curtis E, Quale C, Haggstrom D, Smith-Bindman R. Racial and ethnic differences in breast cancer survival: how much is explained by screening, tumor severity, biology, treatment, comorbidities, and demographics? Cancer. 2008;112(1):171–80. doi: 10.1002/cncr.23131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Brien KM, Cole SR, Tse CK, Perou CM, Carey LA, Foulkes WD, et al. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clin Cancer Res. 2010;16(24):6100–10. doi: 10.1158/1078-0432.CCR-10-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phelan JC, Link BG, Tehranifar P. Social conditions as fundamental causes of health inequalities: theory, evidence, and policy implications. Journal of health and social behavior. 2010;51(Suppl):S28–40. doi: 10.1177/0022146510383498. [DOI] [PubMed] [Google Scholar]
- 12.Vonderheide RH, Domchek SM, Clark AS. Immunotherapy for breast cancer: what are we missing? Clin Can Res. 2017 doi: 10.1158/1078-0432.CCR-16-2569. in press;23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wheeler SB, Reeder-Hayes KE, Carey LA. Disparities in breast cancer treatment and outcomes: biological, social, and health system determinants and opportunities for research. Oncologist. 2013;18(9):986–93. doi: 10.1634/theoncologist.2013-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hershman D, McBride R, Jacobson JS, Lamerato L, Roberts K, Grann VR, et al. Racial disparities in treatment and survival among women with early-stage breast cancer. J Clin Oncol. 2005;23(27):6639–46. doi: 10.1200/JCO.2005.12.633. doi 23/27/6639 [pii] [DOI] [PubMed] [Google Scholar]
- 15.Durham DD, Robinson WR, Lee SS, Wheeler SB, Reeder-Hayes KE, Bowling JM, et al. Insurance-Based Differences in Time to Diagnostic Follow-up after Positive Screening Mammography. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2016;25(11):1474–82. doi: 10.1158/1055-9965.epi-16-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halpern MT, Schrag D. Effects of state-level medicaid policies and patient characteristics on time to breast cancer surgery among medicaid beneficiaries. Breast Cancer Res Treat. 2016;158(3):573–81. doi: 10.1007/s10549-016-3879-8. [DOI] [PubMed] [Google Scholar]
- 17.George P, Chandwani S, Gabel M, Ambrosone CB, Rhoads G, Bandera EV, et al. Diagnosis and surgical delays in African American and white women with early-stage breast cancer. Journal of women’s health (2002) 2015;24(3):209–17. doi: 10.1089/jwh.2014.4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polverini AC, Nelson RA, Marcinkowski E, Jones VC, Lai L, Mortimer JE, et al. Time to Treatment: Measuring Quality Breast Cancer Care. Ann Surg Oncol. 2016;23(10):3392–402. doi: 10.1245/s10434-016-5486-7. [DOI] [PubMed] [Google Scholar]
- 19.Smith EC, Ziogas A, Anton-Culver H. Delay in surgical treatment and survival after breast cancer diagnosis in young women by race/ethnicity. JAMA Surg. 2013;148(6):516–23. doi: 10.1001/jamasurg.2013.1680. [DOI] [PubMed] [Google Scholar]
- 20.Sheppard VB, Oppong BA, Hampton R, Snead F, Horton S, Hirpa F, et al. Disparities in breast cancer surgery delay: the lingering effect of race. Ann Surg Oncol. 2015;22(9):2902–11. doi: 10.1245/s10434-015-4397-3. [DOI] [PubMed] [Google Scholar]
- 21.Reeder-Hayes KE, Bainbridge J, Meyer AM, Amos KD, Weiner BJ, Godley PA, et al. Race and age disparities in receipt of sentinel lymph node biopsy for early-stage breast cancer. Breast Cancer Res Treat. 2011;128(3):863–71. doi: 10.1007/s10549-011-1398-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Black DM, Jiang J, Kuerer HM, Buchholz TA, Smith BD. Racial disparities in adoption of axillary sentinel lymph node biopsy and lymphedema risk in women with breast cancer. JAMA Surg. 2014;149(8):788–96. doi: 10.1001/jamasurg.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carpenter WR, Reeder-Hayes K, Bainbridge J, Meyer AM, Amos KD, Weiner BJ, et al. The role of organizational affiliations and research networks in the diffusion of breast cancer treatment innovation. Med Care. 2011;49(2):172–9. doi: 10.1097/MLR.0b013e3182028ff2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyer AM, Reeder-Hayes KE, Liu H, Wheeler SB, Penn D, Weiner BJ, et al. Differential receipt of sentinel lymph node biopsy within practice-based research networks. Med Care. 2013;51(9):812–8. doi: 10.1097/MLR.0b013e31829c8ca4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alderman AK, Hawley ST, Janz NK, Mujahid MS, Morrow M, Hamilton AS, et al. Racial and ethnic disparities in the use of postmastectomy breast reconstruction: results from a population-based study. J Clin Oncol. 2009;27(32):5325–30. doi: 10.1200/jco.2009.22.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freedman RA, Kouri EM, West DW, Keating NL. Racial/Ethnic Differences in Patients’ Selection of Surgeons and Hospitals for Breast Cancer Surgery. JAMA oncology. 2015;1(2):222–30. doi: 10.1001/jamaoncol.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wheeler SB, Carpenter WR, Peppercorn J, Schenck AP, Weinberger M, Biddle AK. Structural/organizational characteristics of health services partly explain racial variation in timeliness of radiation therapy among elderly breast cancer patients. Breast Cancer Res Treat. 2012;133(1):333–45. doi: 10.1007/s10549-012-1955-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keating NL, Kouri E, He Y, Weeks JC, Winer EP. Racial differences in definitive breast cancer therapy in older women: are they explained by the hospitals where patients undergo surgery? Med Care. 2009;47(7):765–73. doi: 10.1097/MLR.0b013e31819e1fe7. [DOI] [PubMed] [Google Scholar]
- 29.Parise CA, Bauer KR, Caggiano V. Disparities in receipt of adjuvant radiation therapy after breast-conserving surgery among the cancer-reporting regions of California. Cancer. 2012;118(9):2516–24. doi: 10.1002/cncr.26542. [DOI] [PubMed] [Google Scholar]
- 30.Royak-Schaler R, Pelser C, Langenberg P, Hayes J, Gardner L, Nesbitt K, et al. Characteristics associated with the initiation of radiation therapy after breast-conserving surgery among African American and white women diagnosed with early-stage breast cancer in Maryland, 2000–2006. Annals of epidemiology. 2012;22(1):28–36. doi: 10.1016/j.annepidem.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Griggs JJ, Hawley ST, Graff JJ, Hamilton AS, Jagsi R, Janz NK, et al. Factors associated with receipt of breast cancer adjuvant chemotherapy in a diverse population-based sample. J Clin Oncol. 2012;30(25):3058–64. doi: 10.1200/jco.2012.41.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chavez-MacGregor M, Clarke CA, Lichtensztajn DY, Giordano SH. Delayed Initiation of Adjuvant Chemotherapy Among Patients With Breast Cancer. JAMA oncology. 2016;2(3):322–9. doi: 10.1001/jamaoncol.2015.3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nurgalieva ZZ, Franzini L, Morgan RO, Vernon SW, Liu CC, Du XL. Impact of timing of adjuvant chemotherapy initiation and completion after surgery on racial disparities in survival among women with breast cancer. Medical oncology (Northwood, London, England) 2013;30(1):419. doi: 10.1007/s12032-012-0419-1. [DOI] [PubMed] [Google Scholar]
- 34.Griggs JJ, Sorbero ME, Stark AT, Heininger SE, Dick AW. Racial disparity in the dose and dose intensity of breast cancer adjuvant chemotherapy. Breast Cancer Res Treat. 2003;81(1):21–31. doi: 10.1023/A:1025481505537. [DOI] [PubMed] [Google Scholar]
- 35.Griggs JJ, Culakova E, Sorbero ME, Poniewierski MS, Wolff DA, Crawford J, et al. Social and racial differences in selection of breast cancer adjuvant chemotherapy regimens. J Clin Oncol. 2007;25(18):2522–7. doi: 10.1200/JCO.2006.10.2749. doi 25/18/2522 [pii] [DOI] [PubMed] [Google Scholar]
- 36.Freedman RA, Partridge AH. Breast Cancer in Special Populations: Where Are We Now and Where To Go From Here? Clin Can Res. 2017 in press;23. [Google Scholar]
- 37.Freedman RA, Vaz-Luis I, Barry WT, Lii H, Lin NU, Winer EP, et al. Patterns of chemotherapy, toxicity, and short-term outcomes for older women receiving adjuvant trastuzumab-based therapy. Breast Cancer Res Treat. 2014;145(2):491–501. doi: 10.1007/s10549-014-2968-9. [DOI] [PubMed] [Google Scholar]
- 38.Reeder-Hayes K, Peacock Hinton S, Meng K, Carey LA, Dusetzina SB. Disparities in Use of Human Epidermal Growth Hormone Receptor 2-Targeted Therapy for Early-Stage Breast Cancer. J Clin Oncol. 2016;34(17):2003–9. doi: 10.1200/jco.2015.65.8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Freedman RA, Hughes ME, Ottesen RA, Weeks JC, He Y, Wong YN, et al. Use of adjuvant trastuzumab in women with human epidermal growth factor receptor 2 (HER2)-positive breast cancer by race/ethnicity and education within the National Comprehensive Cancer Network. Cancer. 2013;119(4):839–46. doi: 10.1002/cncr.27831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reeder-Hayes K, Meyer AM, Dusetzina SB, Liu H, Wheeler SB. Racial disparities in intiation of endocrine therapy for early-stage breast cancer. J Clin Oncol. 2013;31(15_suppl):1077. [Google Scholar]
- 41.Hershman DL, Kushi LH, Shao T, Buono D, Kershenbaum A, Tsai WY, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28(27):4120–8. doi: 10.1200/jco.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21(4):602–6. doi: 10.1200/jco.2003.07.071. [DOI] [PubMed] [Google Scholar]
- 43.Roberts MC, Wheeler SB, Reeder-Hayes K. Racial/Ethnic and socioeconomic disparities in endocrine therapy adherence in breast cancer: a systematic review. American journal of public health. 2015;105(Suppl 3):e4–e15. doi: 10.2105/ajph.2014.302490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hershman DL, Tsui J, Meyer J, Glied S, Hillyer GC, Wright JD, et al. The change from brand-name to generic aromatase inhibitors and hormone therapy adherence for early-stage breast cancer. Journal of the National Cancer Institute. 2014;106(11) doi: 10.1093/jnci/dju319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neugut AI, Subar M, Wilde ET, Stratton S, Brouse CH, Hillyer GC, et al. Association between prescription co-payment amount and compliance with adjuvant hormonal therapy in women with early-stage breast cancer. J Clin Oncol. 2011;29(18):2534–42. doi: 10.1200/jco.2010.33.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neuner JM, Kamaraju S, Charlson JA, Wozniak EM, Smith EC, Biggers A, et al. The introduction of generic aromatase inhibitors and treatment adherence among Medicare D enrollees. Journal of the National Cancer Institute. 2015;107(8) doi: 10.1093/jnci/djv130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.BD S, AY S, AR N, editors. Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. Unequal Treatment: Confronting Racial and Ethnic Disaparities in Health Care. Washington DC: National Academies Press (US); 2003. [PubMed] [Google Scholar]
- 48.McGuire TG, Alegria M, Cook BL, Wells KB, Zaslavsky AM. Implementing the Institute of Medicine Definition of Disparities: An Application to Mental Health Care. Health services research. 2006;41(5):1979–2005. doi: 10.1111/j.1475-6773.2006.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cook BL, McGuire TG, Zaslavsky AM. Measuring racial/ethnic disparities in health care: methods and practical issues. Health services research. 2012;47(3 Pt 2):1232–54. doi: 10.1111/j.1475-6773.2012.01387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller JW, Hanson V, Johnson GD, Royalty JE, Richardson LC. From cancer screening to treatment: service delivery and referral in the National Breast and Cervical Cancer Early Detection Program. Cancer. 2014;120 (Suppl 16):2549–56. doi: 10.1002/cncr.28823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Howard DH, Ekwueme DU, Gardner JG, Tangka FK, Li C, Miller JW. The impact of a national program to provide free mammograms to low-income, uninsured women on breast cancer mortality rates. Cancer. 2010;116(19):4456–62. doi: 10.1002/cncr.25208. [DOI] [PubMed] [Google Scholar]
- 52.Courtemanche C, Marton J, Ukert B, Yelowitz A, Zapata D. Early Impacts of the Affordable Care Act on Health Insurance Coverage in Medicaid Expansion and Non-Expansion States. Journal of policy analysis and management : [the journal of the Association for Public Policy Analysis and Management] 2017;36(1):178–210. doi: 10.1002/pam.21961. [DOI] [PubMed] [Google Scholar]
- 53.Kehl KL, Liao KP, Krause TM, Giordano SH. Access to Accredited Cancer Hospitals Within Federal Exchange Plans Under the Affordable Care Act. J Clin Oncol. 2017;35(6):645–51. doi: 10.1200/jco.2016.69.9835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Battaglia TA, Darnell JS, Ko N, Snyder F, Paskett ED, Wells KJ, et al. The impact of patient navigation on the delivery of diagnostic breast cancer care in the National Patient Navigation Research Program: a prospective meta-analysis. Breast Cancer Res Treat. 2016;158(3):523–34. doi: 10.1007/s10549-016-3887-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rocque GB, Partridge EE, Pisu M, Martin MY, Demark-Wahnefried W, Acemgil A, et al. The Patient Care Connect Program: Transforming Health Care Through Lay Navigation. J Oncol Pract. 2016;12(6):e633–42. doi: 10.1200/jop.2015.008896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meade CD, Wells KJ, Arevalo M, Calcano ER, Rivera M, Sarmiento Y, et al. Lay navigator model for impacting cancer health disparities. Journal of cancer education : the official journal of the American Association for Cancer Education. 2014;29(3):449–57. doi: 10.1007/s13187-014-0640-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steinberg ML, Fremont A, Khan DC, Huang D, Knapp H, Karaman D, et al. Lay patient navigator program implementation for equal access to cancer care and clinical trials: essential steps and initial challenges. Cancer. 2006;107(11):2669–77. doi: 10.1002/cncr.22319. [DOI] [PubMed] [Google Scholar]
- 58.Rocque GB, Pisu M, Jackson BE, Kvale EA, Demark-Wahnefried W, Martin MY, et al. Resource Use and Medicare Costs During Lay Navigation for Geriatric Patients With Cancer. JAMA oncology. 2017 doi: 10.1001/jamaoncol.2016.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jean-Pierre P, Cheng Y, Wells KJ, Freund KM, Snyder FR, Fiscella K, et al. Satisfaction with cancer care among underserved racial-ethnic minorities and lower-income patients receiving patient navigation. Cancer. 2016;122(7):1060–7. doi: 10.1002/cncr.29902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Myers SN, Ghani KR, Dunn RL, Lane BR, Schervish EW, Gao Y, et al. Notable Outcomes and Trackable Events after Surgery: Evaluating an Uncomplicated Recovery after Radical Prostatectomy. J Urol. 2016;196(2):399–404. doi: 10.1016/j.juro.2016.02.083. [DOI] [PubMed] [Google Scholar]
- 61.Hurley P, Dhir A, Gao Y, Drabik B, Lim K, Curry J, et al. A State-wide Intervention Improves Appropriate Imaging in Localized Prostate Cancer. J Urol. 2016 doi: 10.1016/j.juro.2016.11.098. [DOI] [PubMed] [Google Scholar]
- 62.Dangi-Garimella S. QOPI, the ASCO initiative, improves compliance and promotes quality of patient care. The American journal of managed care. 2014;20(5 Spec No):E1. [PubMed] [Google Scholar]
- 63.Hoverman JR, Klein I, Harrison DW, Hayes JE, Garey JS, Harrell R, et al. Opening the black box: the impact of an oncology management program consisting of level I pathways and an outbound nurse call system. J Oncol Pract. 2014;10(1):63–7. doi: 10.1200/jop.2013.001210. [DOI] [PubMed] [Google Scholar]
- 64.McAlearney AS, Murray K, Sieck C, Lin JJ, Bellacera B, Bickell NA. The Challenge of Improving Breast Cancer Care Coordination in Safety-net Hospitals: Barriers, Facilitators, and Opportunities. Med Care. 2016;54(2):147–54. doi: 10.1097/mlr.0000000000000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.ClinicalTrials.Gov. Accountability for Cancer Care Through Undoing Racism and Equity (ACCURE) 2016 [Google Scholar]
- 66.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. 2013 Available from: http://globocan.iarc.fr, accessed on 29/10/2015. International Agency for Research on Cancer. Accessed 2015.
- 67.Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000;50(1):7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- 68.DeSantis CE, Bray F, Ferlay J, Lortet-Tieulent J, Anderson BO, Jemal A. International Variation in Female Breast Cancer Incidence and Mortality Rates. Cancer Epidemiol Biomarkers Prev. 2015;24(10):1495–506. doi: 10.1158/1055-9965.EPI-15-0535. [DOI] [PubMed] [Google Scholar]
- 69.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 70.Forouzanfar MH, Foreman KJ, Delossantos AM, Lozano R, Lopez AD, Murray CJ, et al. Breast and cervical cancer in 187 countries between 1980 and 2010: a systematic analysis. Lancet. 2011;378(9801):1461–84. doi: 10.1016/S0140-6736(11)61351-2. [DOI] [PubMed] [Google Scholar]
- 71.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 72.Kalager M, Zelen M, Langmark F, Adami HO. Effect of screening mammography on breast-cancer mortality in Norway. N Engl J Med. 2010;363(13):1203–10. doi: 10.1056/NEJMoa1000727. [DOI] [PubMed] [Google Scholar]
- 73.Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–41. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 74.Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347(16):1227–32. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 75.Early Breast Cancer Trialists’ Collaborative G. Darby S, McGale P, Correa C, Taylor C, Arriagada R, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804):1707–16. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Early Breast Cancer Trialists’ Collaborative G. McGale P, Taylor C, Correa C, Cutter D, Duane F, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383(9935):2127–35. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Early Breast Cancer Trialists’ Collaborative G. Davies C, Godwin J, Gray R, Clarke M, Cutter D, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771–84. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group [see comments] Lancet. 1998;351(9114):1451–67. [PubMed] [Google Scholar]
- 79.Early Breast Cancer Trialists’ Collaborative G. Dowsett M, Forbes JF, Bradley R, Ingle J, Aihara T, et al. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386(10001):1341–52. doi: 10.1016/S0140-6736(15)61074-1. [DOI] [PubMed] [Google Scholar]
- 80.Early Breast Cancer Trialists’ Collaborative G. Peto R, Davies C, Godwin J, Gray R, Pan HC, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379(9814):432–44. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weir HK, Thun MJ, Hankey BF, Ries LA, Howe HL, Wingo PA, et al. Annual report to the nation on the status of cancer, 1975–2000, featuring the uses of surveillance data for cancer prevention and control. J Natl Cancer Inst. 2003;95(17):1276–99. doi: 10.1093/jnci/djg040. [DOI] [PubMed] [Google Scholar]
- 82.Broeders M, Moss S, Nystrom L, Njor S, Jonsson H, Paap E, et al. The impact of mammographic screening on breast cancer mortality in Europe: a review of observational studies. J Med Screen. 2012;19(Suppl 1):14–25. doi: 10.1258/jms.2012.012078. [DOI] [PubMed] [Google Scholar]
- 83.Njor S, Nystrom L, Moss S, Paci E, Broeders M, Segnan N, et al. Breast cancer mortality in mammographic screening in Europe: a review of incidence-based mortality studies. J Med Screen. 2012;19(Suppl 1):33–41. doi: 10.1258/jms.2012.012080. [DOI] [PubMed] [Google Scholar]
- 84.Paci E, Broeders M, Hofvind S, Puliti D, Duffy SW. European breast cancer service screening outcomes: a first balance sheet of the benefits and harms. Cancer Epidemiol Biomarkers Prev. 2014;23(7):1159–63. doi: 10.1158/1055-9965.epi-13-0320. [DOI] [PubMed] [Google Scholar]
- 85.Anderson BO, Lipscomb J, Murillo RH, Thomas DB. Breast Cancer. In: Gelband H, Jha P, Sankaranarayanan R, Horton S, editors. Cancer: Disease Control Priorities. Third. Vol. 3. Washington (DC): 2015. [Google Scholar]
- 86.Smith RA, Caleffi M, Albert US, Chen TH, Duffy SW, Franceschi D, et al. Breast cancer in limited-resource countries: early detection and access to care. Breast J. 2006;12(Suppl 1):S16–26. doi: 10.1111/j.1075-122X.2006.00200.x. [DOI] [PubMed] [Google Scholar]
- 87.McPhail S, Johnson S, Greenberg D, Peake M, Rous B. Stage at diagnosis and early mortality from cancer in England. Br J Cancer. 2015;112(Suppl 1):S108–15. doi: 10.1038/bjc.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Elkin EB, Hudis C, Begg CB, Schrag D. The effect of changes in tumor size on breast carcinoma survival in the U.S.: 1975–1999. Cancer. 2005;104(6):1149–57. doi: 10.1002/cncr.21285. [DOI] [PubMed] [Google Scholar]
- 89.Independent UKPoBCS. The benefits and harms of breast cancer screening: an independent review. Lancet. 2012;380(9855):1778–86. doi: 10.1016/S0140-6736(12)61611-0. [DOI] [PubMed] [Google Scholar]
- 90.Lauby-Secretan B, Scoccianti C, Loomis D, Benbrahim-Tallaa L, Bouvard V, Bianchini F, et al. Breast-cancer screening–viewpoint of the IARC Working Group. N Engl J Med. 2015;372(24):2353–8. doi: 10.1056/NEJMsr1504363. [DOI] [PubMed] [Google Scholar]
- 91.Mittra I, Mishra GA, Singh S, Aranke S, Notani P, Badwe R, et al. A cluster randomized, controlled trial of breast and cervix cancer screening in Mumbai, India: methodology and interim results after three rounds of screening. Int J Cancer. 2010;126(4):976–84. doi: 10.1002/ijc.24840. [DOI] [PubMed] [Google Scholar]
- 92.Sankaranarayanan R, Ramadas K, Thara S, Muwonge R, Prabhakar J, Augustine P, et al. Clinical breast examination: preliminary results from a cluster randomized controlled trial in India. J Natl Cancer Inst. 2011;103(19):1476–80. doi: 10.1093/jnci/djr304. [DOI] [PubMed] [Google Scholar]
- 93.Miller AB, Wall C, Baines CJ, Sun P, To T, Narod SA. Twenty five year follow-up for breast cancer incidence and mortality of the Canadian National Breast Screening Study: randomised screening trial. Bmj. 2014;348:g366. doi: 10.1136/bmj.g366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rajaraman P, Anderson BO, Basu P, Belinson JL, Cruz AD, Dhillon PK, et al. Recommendations for screening and early detection of common cancers in India. Lancet Oncol. 2015;16(7):e352–61. doi: 10.1016/S1470-2045(15)00078-9. [DOI] [PubMed] [Google Scholar]
- 95.WHO Cancer Control Programme DoCDaHP. Early detection of cancer. 2015 7/24/2015. Available at: http://www.who.int/cancer/detection/en/. In Programmes: Cancer. WHO. 7/24/2015.
- 96.Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353(17):1784–92. doi: 10.1056/NEJMoa050518. doi 353/17/1784 [pii] [DOI] [PubMed] [Google Scholar]
- 97.Slamon DJ, E W, Robert NJ, Giermek J, Martin M, Jasiowka M, Mackey JR, Chan A, Liu M-C, Pinter T, Valero V, Falkson C, Fornander T, Shiftan TA, Bensfia S, Hitier S, Xu N, Bée-Munteanu V, Drevot P, Press MF, Crown J, On Behalf of the BCIRG-006 Investigators Ten year follow-up of BCIRG-006 comparing doxorubicin plus cyclophosphamide followed by docetaxel (AC→T) with doxorubicin plus cyclophosphamide followed by docetaxel and trastuzumab (AC→TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2+ early breast cancer San Antonio Breast Cancer Symposium. 2015 [Google Scholar]
- 98.Coleman MP, Quaresma M, Berrino F, Lutz JM, De Angelis R, Capocaccia R, et al. Cancer survival in five continents: a worldwide population-based study (CONCORD) Lancet Oncol. 2008;9(8):730–56. doi: 10.1016/S1470-2045(08)70179-7. doi S1470-2045(08)70179-7 [pii] [DOI] [PubMed] [Google Scholar]
- 99.The World Bank. Health expenditure per capita (current US$) 2015 2/10/2015. Available at http://data.worldbank.org/. 2/10/2015.
- 100.Farmer P, Frenk J, Knaul FM, Shulman LN, Alleyne G, Armstrong L, et al. Expansion of cancer care and control in countries of low and middle income: a call to action. Lancet. 2010;376(9747):1186–93. doi: 10.1016/S0140-6736(10)61152-X. [DOI] [PubMed] [Google Scholar]
- 101.United Nations General Assembly. Political Declaration of the High-level Meeting of the General Assembly on the Prevention and Control of Non-communicable Diseases (A/RES/66/2) New York, NY: Sep 19, 2011. [Google Scholar]
- 102.Bazargani YT, de Boer A, Schellens JH, Leufkens HG, Mantel-Teeuwisse AK. Essential medicines for breast cancer in low and middle income countries. BMC Cancer. 2015;15(1):591. doi: 10.1186/s12885-015-1583-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Price AJ, Ndom P, Atenguena E, Mambou Nouemssi JP, Ryder RW. Cancer care challenges in developing countries. Cancer. 2012;118(14):3627–35. doi: 10.1002/cncr.26681. [DOI] [PubMed] [Google Scholar]
- 104.Anderson BO, Ilbawi AM, El Saghir NS. Breast cancer in low and middle income countries (LMICs): a shifting tide in global health. Breast J. 2015;21(1):111–8. doi: 10.1111/tbj.12357. [DOI] [PubMed] [Google Scholar]
- 105.Ilbawi AM, Anderson BO. Global cancer consortiums: moving from consensus to practice. Ann Surg Oncol. 2015;22(3):719–27. doi: 10.1245/s10434-014-4346-6. [DOI] [PubMed] [Google Scholar]
- 106.Anderson BO, Duggan C. Resource-Stratified Guidelines for Cancer Management: Correction and Commentary. Journal of Global Oncology. 2016;0(0):JGO006213. doi: 10.1200/JGO.2016.006213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Anderson BO, Lipscomb J, Murillo R, Thomas DB. Disease Control Priorities. Washington, DC: World Bank Publications; 2015. Breast Cancer (3) [Google Scholar]


