Abstract
Background
Although physical activity (PA) interventions have been effective for improving health outcomes in breast cancer survivors, little is known relative to their potential for translation into practice.
Purpose
This review was designed to provide a quantitative estimate of the reporting of both internal and external validity in recent studies of PA in breast cancer survivors (BCS).
Methods
The Reach, Efficacy/Effectiveness, Adoption, Implementation, and Maintenance (RE-AIM) framework was utilized to assess the reporting of internal and external validity in 25 randomized controlled trials (RCTs) of PA and BCS published between 1998 and 2008. Each trial was evaluated relative to the degree it met criteria for each of the above dimensions.
Results
The majority of studies in this review reported dimensions reflecting internal validity. The overall level of detail relative to external validity of PA interventions was rarely reported, limiting the generalizability of study findings.
Conclusions
As with many RCTs of health behavior change, detail relative to contextual elements of published PA interventions in BCS is limited. It is recommended that future physical activity interventions in BCS be designed to facilitate scalable and sustainable interventions for improving health outcomes in this population.
Keywords: External validity, Breast cancer, Physical activity interventions
Introduction
There are more than 2.4 million breast cancer survivors in the US accounting for 22% of all cancer survivors [1]. The 5-year survival rate of 88.6% [1], coupled with a one in eight lifetime chance of diagnosis [1], portends a continued increase in the number of breast cancer survivors. The combined high incidence and prevalence rates have created a relatively large population with distinct public health needs [2]. Many of these special needs stem from the adverse side effects associated with common treatment options including surgery, chemotherapy, radiation therapy, hormone therapy, and biologic therapy. These side effects vary in severity and may persist, or even increase, several years postdiagnosis [3]. In addition, breast cancer survivors are at an increased risk of cancer recurrence, comorbidities, and premature death [4, 5].
It has been well established that the side effects associated with breast cancer are not entirely unmanageable [6–8]. Behavioral and lifestyle programs continue to be developed to address issues relative to increased survivor-ship and prolonged treatment side effects. These approaches seek to maximize disease-free survivorship and quality of life (QOL) while, at the same time, minimize the risk of recurrence and comorbidities in breast cancer survivors. Physical activity has been one lifestyle factor identified to have excellent potential for ameliorating the aversive side effects of treatment and positively influencing disease-related outcomes [5–7, 9]. The existing evidence, however, has focused almost exclusively on the efficacy of physical activity with little attention as to how these physical activity interventions might be translated into practice.
To date, physical activity interventions intended for breast cancer survivors have been tested using methodologies designed to ensure high internal validity, while issues related to external validity have received less attention [10]. Consequently, although physical activity interventions appear to be beneficial following a breast cancer diagnosis, the extent to which successful interventions can be effectively disseminated to create sustainable physical activity programs for this population is unclear. One approach to determining the external validity of physical activity interventions in breast cancer survivors is to utilize the Reach, Efficacy/Effectiveness, Adoption, Implementation, and Maintenance (RE-AIM) framework [11]. This framework has been successfully applied to a number of health behaviors and evaluates the dimensions of reach, efficacy/effectiveness, adoption, implementation, and maintenance to determine the public health impact of an intervention [12–15]. Briefly, reach is characterized by the absolute number, proportion, and representativeness of individuals willing to participate in a given program. Efficacy or effectiveness (depending on study design) assesses the impact of an intervention on important outcomes, including potential negative effects, and cost. Adoption reflects the absolute number, proportion, and representativeness of settings and intervention agents who are willing to initiate the program in question. Implementation is concerned with the extent to which the intervention agents and participants have adhered to the intervention protocol, whether the intervention was delivered or completed appropriately, and the cost and time taken to deliver the intervention. Maintenance is determined by assessing the extent to which a program or policy has been institutionalized or become part of organizational practices and policies. Maintenance can also be operationalized as the degree to which initial changes in participant behavior are sustained 6 months post-intervention. Reach, efficacy/effectiveness, and behavioral maintenance are generally considered to be measures of individual-level impact whereas adoption, implementation, and organizational maintenance are considered to be indices of the setting-level impact of an intervention [16].
Given the number of existing physical activity trials in breast cancer survivors and the existing narrative and meta-analytic reviews supporting the efficacy of physical activity to improve psychological and physical health outcomes, we adopted the RE-AIM framework to achieve two objectives: (1) to provide a quantitative estimate of the extent to which recent studies have addressed issues related to both internal and external validity and (2) to offer suggestions on methods to improve the design and reporting of future interventions to enhance their dissemination potential.
Methods
Selection of Studies for Review
Studies selected for inclusion in this review were identified through literature searches conducted in 2008 using the PubMed, GoogleScholar, and Scirus search engines and a combination of the following terms: breast cancer survivors, breast cancer patients, physical activity, exercise, physical functioning, fatigue, weight management, randomized controlled trial, and fitness. Studies which met the following criteria were included in the review: (1) designed as a controlled physical activity trial for breast cancer survivors with participants randomized to a physical activity or control condition; (2) targeted primary intervention outcomes other than physical activity behavior change; (3) published the results of the primary outcomes; and (4) were published or in press in English between 1998 and 2008. If studies met all inclusion criteria, they were included in the review.
RE-AIM Criteria
In order to effectively evaluate the extent to which studies included each of the RE-AIM components, we assessed multiple components of each. Thus, for reach, we identified four indicators: (1) methods used to identify the target population; (2) identification of inclusion and exclusion criteria; (3) whether the sample size and participation rate (number participating/number eligible) were reported or could be calculated from the information provided; and (4) whether the characteristics of both participants and non-participants were reported. Efficacy/effectiveness was assessed relative to whether studies reported: (1) measures and results for at least one follow-up period (i.e., post-intervention); (2) the use of intention-to-treat analyses; (3) a quality of life outcome; and (4) the degree of participant attrition from the trial.
Adoption criteria included: (1) description of the intervention location; (2) description of the staff who delivered intervention; (3) method to identify target delivery agent; (4) level of expertise of the delivering agent; (5) inclusion/exclusion criteria for settings; (6) adoption rate; and (7) the characteristics of adoption/nonadoption. The implementation aspect can be assessed at both the individual and setting levels and was evaluated relative to studies reporting: (1) the intervention type and intensity level of the activity; (2) the extent to which the protocol was delivered as intended; and (3) measures of the cost of intervention implementation. Finally, assessment of the maintenance dimension was based upon whether studies included: (1) an assessment of individual behavior at least 6 months following completion of the intervention; (2) the current status of the program; and (3) measures of the cost of maintaining the intervention.
The coding procedures used in this study were consistent with previous RE-AIM reviews. Additional details on adoption at the “delivery agent” level, as well as cost indicators, were also included. These areas were not explicitly reviewed in previous manuscripts but are consistent with the definitions and recommendations for reporting based on the RE-AIM framework (see Glasgow et al. [10]). Equal weight was given to each of the components within a particular RE-AIM indicator.
Coding Protocol and Scoring
All included studies were coded along each of the RE-AIM dimensions in a two-step process. First, two of the coauthors (SW, EM) independently coded each of the articles utilizing a tabular checklist based on the operational definitions of RE-AIM dimensions (see www.re-aim.org). The checklist required the coder to indicate “Yes” if the information was reported and “No” if it was not. In order to be classified as having met a specific criterion, all conditions had to be satisfied. For example, a study was given a “No” for sample size and participation rate if only the sample size was reported and vice versa. Data reflecting sample size, participation and adherence rates, and attrition were also assessed. Initial coding resulted in high concordance with >90% agreement on each of the dimensions. This evaluation was then validated independently by the other two coauthors (PE, KC). The only disagreement on the initial evaluation was based on a different operational definition of the denominator to be used for the calculation of participation rate. Specifically, in one case, one reviewer used the total number of patients within the target population as the denominator while another reviewer used the total number of patients that were invited to participate as the denominator. The latter denominator was the agreed upon operational definition used for all calculations of participation rate and further deliberations resulted in the resolution of any discrepancies resulting in 100% agreement.
After full agreement of all authors, studies were scored based on the percentage of the criteria for each dimension reported in the article. For example, if a study reported on four of the five elements of reach, it was given a score of 80% (4/5×100). The mean reach score across all studies was then calculated to provide the overall availability of reach data for all physical activity interventions. A similar procedure was implemented for the other RE-AIM dimensions.
Results
Intervention Characteristics
Twenty-five studies met the inclusion criteria for review. Characteristics of these studies are provided in Table 1. All studies were randomized controlled exercise trials with a standard care control condition. The median number of participants across all 25 studies was 41. Trials were conducted either during or postadjuvant therapies with the majority (60%) being conducted on women who had completed all adjuvant breast cancer therapies with the exception of hormone therapy. Intervention duration ranged from 6 to 24 weeks. Over two thirds of the studies (68%) included at least some supervised physical activity component, and the remaining studies consisted of home-based programs. The primary physical activity modality in most interventions was aerobic activity (60%) or a combination of aerobic and strength training (28%). Two studies included an isolated resistance training arm and three additional studies included a yoga/tai chi arm. Most studies (72%) reported some measure of aerobic fitness with just under half of the studies (44%) utilizing a submaximal exercise test.
Table 1.
Intervention characteristics of studies reviewed
| Study | Number | Int. type | Int. length (weeks) | Physical activity modality | PI follow-up | LT follow-upa |
|---|---|---|---|---|---|---|
| Basen-Engquist et al. [20] | 60 | II | 24 | Aerobic | ✓ | |
| Battaglini et al. [42] | 20 | I | 15 | Combination | ✓ | |
| Campbell et al. [43] | 22 | I | 12 | Combination | ✓ | |
| Courneya et al. [44] | 53 | I | 15 | Aerobic | ✓ | |
| Courneya et al. [17] | 242 | I | ~17b | Aerobic; strength | ✓ | ✓ |
| Culos-Reed et al. [45] | 38 | I | 7 | Yoga | ✓ | |
| Daley et al. [18] | 108 | I | 8 | Aerobic; flexibility | ✓ | ✓ |
| Drouin et al. [46] | 23 | II | 7 | Aerobic | ✓ | |
| Galantino et al. [47] | 11 | II | 6 | Aerobic; tai chi | ✓ | |
| Headley et al. [48] | 32 | II | ~17b | Aerobic | ✓ | |
| Herrero et al. [49] | 20 | I | 8 | Combination | ✓ | |
| Kim et al. [50] | 41 | III | 8 | Aerobic | ✓ | |
| Ligibel et al. [51] | 101 | III | 16 | Combination | ✓ | |
| McKenzie et al. [21] | 14 | I | 8 | Combination | ✓ | |
| Milne et al. [52] | 58 | I | 12d | Combination | ✓ | |
| Mock et al. [24] | 50 | II | 6 or 16–24c | Aerobic | ✓ | |
| Mock et al. [23] | 119 | II | 6 or 16–24c | Aerobic | ✓ | |
| Mustian et al. [53] | 31 | I | 12 | Tai Chi | ✓ | |
| Mutrie et al. [54] | 203 | I | 12 | Aerobic | ✓ | ✓ |
| Pinto et al. [55] | 24 | I | 12 | Combination | ✓ | |
| Pinto et al. [56] | 86 | II | 12 | Aerobic | ✓ | |
| Sandel et al. [22] | 38 | I | 12d | Aerobic | ✓ | |
| Schmitz et al. [57] | 86 | III | 26d | Strength | ✓ | ✓ |
| Segal et al. [58] | 123 | IV | 26 | Aerobic | ✓ | |
| Segar et al. [59] | 30 | II | 10d | Aerobic | ✓ |
Int. intervention, PI post-intervention, LT long-term, I supervised, II home-based, III intervention combined supervised and home-based components at the same time, IV intervention had a supervised and home-based arm collapsed for analysis, ✓ included
At least 6 months post-intervention
Conducted during treatment with value reflecting average treatment duration
Six weeks for those receiving radiotherapy and 16–24 weeks for those receiving chemotherapy
Incorporated crossover period of same time length
Assessing RE-AIM Characteristics
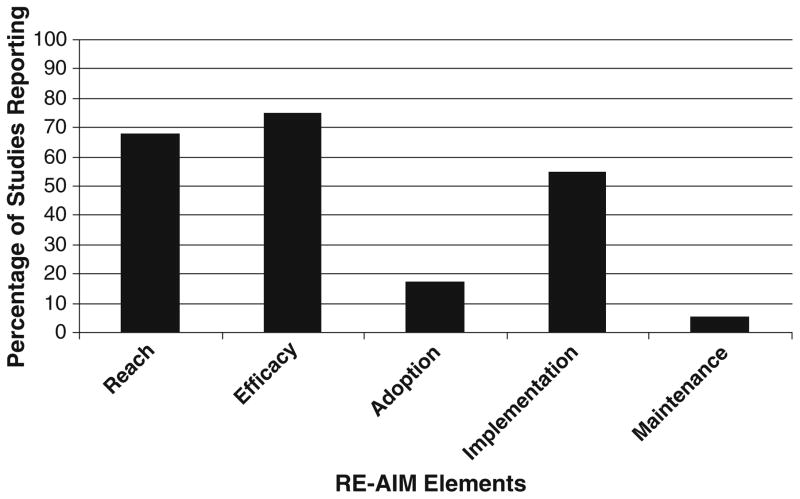
Table 2 and Fig. 1 provide a summary of the overall percentage of studies reporting on RE-AIM dimensions. In general, information relative to generalizability and dissemination were not reported. Additionally, as most of the studies were single-site interventions, data relative to setting-level indices (i.e., adoption, implementation, and maintenance) were scarce.
Table 2.
Proportion of studies reviewed reporting RE-AIM dimensions and components
| Reach | 68.0 |
| Method to identify target population | 92.0 |
| Inclusion criteria | 100.0 |
| Exclusion criteria | 96.0 |
| Sample size and participation rate | 52.0 |
| Characteristics of nonparticipants | 0.0 |
| Efficacy/effectiveness | 75.0 |
| Measures/results for at least one follow-up | 100.0 |
| Intent to treat analysis utilized | 52.0 |
| Quality-of-life measure | 52.0 |
| Percent attrition | 96.0 |
| Adoption | 17.1 |
| Description of intervention location | 48.0 |
| Description of staff who delivered intervention | 60.0 |
| Method to identify target delivery agent | 0.0 |
| Level of expertise of delivery agent | 12.0 |
| Inclusion/exclusion criteria | 0.0 |
| Adoption rate | 0.0 |
| Characteristics of adoption/nonadoption | 0.0 |
| Implementation | 54.7 |
| Intervention type and intensity | 100.0 |
| Extent protocol delivered as intended (%) | 64.0 |
| Measures of cost of implementation | 0.0 |
| Maintenance | 5.3 |
| Was individual behavior assessed at least 6 months following the completion of the intervention? | 16.0 |
| Is the program still in place? | 0.0 |
| Measures of cost of maintenance | 0.0 |
Mean level reporting across all components of each dimension is shown to the immediate right of the RE-AIM dimension
Fig. 1.
Overall reporting of RE-AIM elements across studies
Reach
All studies (100%) reported inclusion criteria, and the majority (96%) reported exclusion criteria for study participation. A majority of the studies (92%) reported the methods utilized to identify and recruit participants. The sample size and participation rate were reported in just over half (52%) of the studies with 53.8% of those studies indicating that less than half of eligible individuals participated in the trials. No studies reported on any aspect of the representativeness of the recruited samples compared to non-participants or the general target population. All of these factors were combined to achieve a mean reach score across studies of 68%.
Efficacy/Effectiveness
This was the most consistently reported RE-AIM element across all studies (75%). All studies reported at least one follow-up of the primary study outcomes, typically immediately post-intervention. Just over half of the studies (52%) reported using intent-to-treat methods to analyze study data. The remaining studies either did not specify analytical methods or conducted complete case analyses. Quality of life measures were utilized in about half of the studies (52%). Most studies (96%) reported trial attrition rates (M=14.2%).
The most frequently reported outcome categories in the 25 studies reviewed were cardiovascular fitness, muscular strength, body composition, overall QOL, fatigue, physical functioning, other psychosocial variables, biological markers, and lymphedema-related measures. The most common psychosocial variables reported were depression, mood, self-esteem, and anxiety. Biological markers assessed included: insulin, insulin resistance, glucose, and insulin-like growth factor axis measures. Lymphedema-related outcomes included measurement of arm circumference, shoulder range of motion, and water displacement. For those studies involving more than one intervention arm [17, 18], each group was analyzed separately in comparison to the usual care group. Aerobic fitness was measured in 18 of the intervention arms with 72.2% of those studies reporting a statistically significant improvement due to the intervention. Strength was assessed and analyzed in six intervention arms with 66.7% reporting a significant positive intervention effect. Body composition effects were adequately documented in ten intervention arms with 44.4% reporting a significant improvement due to the physical activity intervention. Valid QOL measures were utilized and reported in 14 study arms. Interestingly, the SF-36 [19], an accepted measure of health-related QOL, was used as either a QOL measure or a measure of physical function. In the case of QOL, three studies [20–22] used the SF-36. Of the 14 study arms, 12 reported an overall QOL of life score and 66.7% exhibited improvements as a function of physical activity participation.
Measures of physical functioning, by self-report or functional performance tests, were utilized and analyzed in six of the intervention arms with 66.7% of these studies showing significant improvements. Two of these studies [23, 24] used the physical function subscale of the SF-36 as their outcome measure. Of the 11 studies reporting intervention effects on fatigue, 54.5% had significant reductions in fatigue symptoms. Sixteen studies examined physical activity effects on psychosocial function with significant improvements in at least one aspect of psychosocial function being reported in 62.5% of these studies. Intervention effects on biological markers were reported in two studies with significant reductions demonstrated on at least one marker in both (100%) of the studies. Lymphedema-related outcomes were assessed in seven intervention arms. No studies reported increases in lymphedema during exercise training and two (28.6%) reported a positive effect of exercise on shoulder mobility measures. Those studies that did not find significant effects for the primary and secondary outcomes exhibited a trend towards a positive effect for the intervention groups. Finally, details relative to the occurrence of any adverse events across the trial period were reported in eight (32%) of the 25 studies.
Adoption
The mean level of reporting for the adoption elements (Table 2) across all studies was 17.1%. The most commonly reported adoption element was the description of intervention staff (60%) and intervention location (48%). The level of expertise of the delivery agents was reported in only 12% of the studies reviewed and no studies reported methods to identify the target delivery agent, delivery agent inclusion/exclusion criteria, adoption rate, or representativeness of study settings to the broader population of clinical settings that would ultimately deliver such an intervention. As all the studies reviewed herein were efficacy trials and were not implemented across settings, the adoption of these types of interventions at an organizational level cannot be adequately assessed [25].
Implementation
The mean level of reporting for individual-level implementation was 54.7% (see Table 2). All studies (100%) reported elements comprising the implementation dimension at the individual level in that they documented the intervention type and included a description of the physical activity program. The extent to which the physical activity program was delivered as intended was reported by 64% of the studies and was most commonly conceptualized as participants adherence to the physical activity intervention. The average adherence level across all studies reporting on this element was 78.5%. No studies reported details of setting-level implementation or any information relative to the cost associated with implementing the intervention.
Maintenance
Elements of the maintenance dimension were reported in only a few trials (5.3%). Specifically, assessment of individual behavior at least 6 months following the completion of the intervention was reported in 16% of the studies. No studies reported on the current status of the physical activity interventions, cost of maintenance, or on the potential for these interventions to be sustained in typical practice.
Discussion
Increased attention has been focused on the importance of demonstrating the external validity of health behavior change interventions [26, 27]. A number of recent reports have used the RE-AIM framework to evaluate the public health impact of interventions across studies and have further underscored the importance of detailing external validity criteria [28, 29]. In the context of breast cancer survivors, an increasing number of studies have concluded that physical activity is more effective than usual care for improving health outcomes in breast cancer survivors [6, 7]. The extent to which this literature can be viewed as generalizable, however, is not clear. In the present study, we utilized the RE-AIM framework to examine the external validity of those published randomized controlled physical activity trials for breast cancer survivors.
That we identified 25 studies using randomized controlled designs to examine physical activity in breast cancer survivors is encouraging. However, the extent to which the content of these programs is capable of being translated into breast cancer survivor care is unclear due to the lack of detail relative to issues of generalizability. In the absence of such detail, it is not yet possible to address the potential for physical activity to be widely disseminated in an increasingly growing population of breast cancer survivors. Our findings echo those of Klesges and others [14, 29, 30] who conclude in their reviews that there is a dearth of detail relative to contextual elements of randomized controlled trials of health behavior change. Without this information, the relevance of study outcomes and their practical utility are difficult to determine. As also noted by Klesges et al. [29] and Victora et al. [31], this state of affairs, in many ways, is to be expected given that internal validity concerns dominate most study designs.
Our review further suggests that physical activity interventions designed for breast cancer survivors are more likely to report individual-level components of the RE-AIM framework than setting- or organizational-level indices. Additionally, the external validity and ability of these interventions to be adopted, implemented, and maintained at a community level appear to be low. The majority of the interventions targeted convenience samples leading to the recruitment of populations that may not be generalizable across age, race, socioeconomic status, disease stage, time from diagnosis, marital status, or employment status suggesting limited overall reach.
To determine the public health impact of any intervention, it is critical to detail the reach and potential for adoption of an intervention relative to the targeted population or setting, as this is a major concern in efficacy and effectiveness intervention trials [10]. The strict criteria for participation often reported in these interventions suggest a well-defined population. In reality, however, this strategy typically results in participation by groups of highly motivated individuals or organizations, further exacerbating the issue of generalizability [6, 10]. Representativeness of samples in physical activity interventions, in general, and specifically for breast cancer survivors, is rarely reported [6, 10]; this shortcoming is further highlighted in this review with no studies providing any detail relative to the characteristics of the external population from which the samples were drawn (non-participants). Some inference relative to the limited representativeness of the study populations can be made from the demographic characteristics reported, as the participants were primarily white, well educated, partnered, middle class, and early-stage breast cancer survivors. Individuals not matching this profile may respond differently to exercise interventions.
One approach to alleviating this concern may be to consult local or national cancer registries in future studies to: (a) determine the representativeness of study populations and (b) serve as a potential resource for the recruitment of samples reflecting the entire spectrum of breast cancer survivors. It is acknowledged that low response rates from registry recruitment may well reflect a selection bias of a different nature from recruitment in a clinical setting. Nevertheless, without documenting the representativeness of those recruited, the degree to which the selection bias is problematic cannot be determined. At the very least, we recommend that the characteristics of those individuals electing not to participate should be measured and reported. If such information is unavailable, an assessment of basic demographic or motivational characteristics of those declining participation can be used to determine if systematic differences exist between participants and non-participants.
Although the reach and efficacy of interventions can provide initial guidance to organizations considering the adoption of a specific intervention, other factors such as the extent to which the interventions are demanding, are costly, necessitate high levels of expertise, or require considerable time to deliver can also be considered. The reporting of these implementation indicators of physical activity interventions at the organizational level is lacking in both the general literature [12] and the physical activity and breast cancer literature [6]. Interventions requiring fewer resources, demanding less staff expertise, and allowing for greater flexibility are more likely to be amenable to higher levels of adoption [25].
Detail regarding cost is necessary for any intervention or program with dissemination potential. No studies in the present review reported any measure of cost. However, the majority of the interventions in this review had features characteristic of relatively intense and costly interventions requiring considerable personnel resources in terms of staff training and time commitment. Additionally, they typically required participants to travel to a physical location. Even the unsupervised interventions reviewed required some degree of external staff involvement, as participants were generally contacted on a weekly or bi-weekly basis, a potentially expensive and time-consuming resource when large samples are involved. One approach to improving the adoption of physical activity interventions in breast cancer survivors might be to design low-cost detailed intervention and training materials. Such materials should be easily interpreted by the general public and applicable to existing community agencies and settings including cancer centers, health centers, outpatient clinics, and community organizations [32].
To successfully implement physical activity interventions designed for breast cancer survivors, some form of process evaluation at the setting level is necessary and might include observation of exercise classes within organizational settings and monitoring information delivered to participants, staff phone calls, mailings, and participant exercise logs. A number of studies have assessed implementation at the individual level by documenting physical activity behavior via calendars [33]. Incorporating such “built-in” process evaluations not only permits assessment of program implementation but also provides information necessary to modify programs to maximize effectiveness and cost feasibility prior to dissemination [32].
Maintenance of physical activity post-intervention is rarely measured in efficacy studies [34] and this review of the breast cancer literature is reflective of a general lack of reporting on the maintenance of behavior changes after the intervention is complete. Assessing the maintenance of physical activity at the individual level is important in determining the sustainability of an intervention’s impact on individual behavior, thereby providing an indicator of program utility [12]. Moreover, little is known about the optimal dose of physical activity necessary to achieve and maintain beneficial outcomes in breast cancer survivors. In addition, there is a need to determine the extent to which the following are implicated in long-term physical activity change: continued contact with participants; increased social support for physical activity; and programs tailored specifically to overcoming barriers to physical activity for breast cancer survivors [32]. Identifying factors which contribute to the maintenance of physical activity in breast cancer survivors (e.g., Courneya et al. [35]) not only have implications for translation but also for reductions in the health care costs associated with more costly palliative treatments and cancer recurrence.
One important element of any intervention is the reporting of adverse events attributable to the intervention. The reporting of adverse, negative, or unexpected outcomes serves as a protection to the population at large should the intervention be chosen for dissemination [11]. Physical activity intervention benefits for breast cancer survivors must outweigh the potential harm, and risks must be determined before intervening at the population level. CONSORT diagrams [36, 37] for the reporting of participant flow through randomized controlled trials are now required by most journals. We are hopeful that incorporating additional elements related to external validity and reporting of any adverse and negative events associated with the implementation of physical activity will be incorporated into future standards for reporting.
Although our review suggests a general lack of reporting of external validity criteria in the physical activity and BCS literature, we believe it important to highlight two important issues for consideration in evaluating this literature. First, the earliest studies reviewed were conducted in the late 1990s; the goals of many of the studies reviewed were to determine efficacy within a climate of skepticism from health care providers as to whether physical activity interventions were safe for their patients. Thus, the importance of these early trials cannot be underestimated. Our intent is not to suggest that early studies with strong internal validity were not valuable to the end of providing information related to efficacy and safety nor is it to retrospectively apply RE-AIM standards to these trials. Rather, it is our intent to suggest that even these early studies could have benefited from attention to external validity issues and that future studies should build substantially on this base by including methods to address RE-AIM dimensions. The concerns of safety and efficacy would be further served by providing information on the potential reach of a prospective intervention (i.e., what proportion of the patient population would engage in the intervention), the representativeness of the sample (i.e., for what types of patients do the results apply), and conditions under which safety and efficacy were determined.
The second consideration is that our review included a number of efficacy studies with a primary outcome that was not physical activity behavior (e.g., fitness, quality of life, fatigue, physical functioning, etc). In a traditional view, efficacy studies, by their very nature, involve select populations and expensive interventions, and one could question whether RE-AIM is an appropriate framework to apply here. Further, it could be argued that these researchers do not see their facility-based exercise programs with highly trained staff and intensive follow-up to increase retention as a disseminable model for changing physical activity behavior in cancer survivors. These are reasonable perspectives. However, it is possible for even large-scale efficacy trials to incorporate external validity criteria. A good case in point is the Diabetes Prevention Program (DPP; [38–41]), an efficacy trial to determine whether lifestyle changes delay diabetes onset. Although the DPP was a tightly controlled randomized study, considerable detail was collected on the reach of the study, cost of different recruitment strategies, the characteristics of the settings and interventionists, the degree to which the intervention was delivered as intended, and the maintenance of individual effects. This type of information is invaluable for use in the dissemination and adaptation of programs in the public health domain. Thus, the inclusion of external validity metrics can add value to even tightly controlled outcomes-based efficacy trials.
As a lack of information on external validity hinders translation of research into practice, reviewers of other health behaviors [29] and editors [27] have attempted to facilitate this practice by calling for researchers and journal editors to determine how best to include external validity evidence in published reports without unduly increasing manuscript length. Furthermore, funding agencies could facilitate the process of translating research to practice by, at the very least, requiring grant applications to detail how proposed interventions will satisfy some minimal standard of external validity criteria. We acknowledge that journal space limitations sometimes make it difficult to include RE-AIM metrics. There are at least two potential solutions to this issue. One is to simply publish a separate manuscript detailing the external validity of any given intervention. The second is to consider changing reporting procedures to facilitate understanding the generalizability of findings without substantially increasing manuscript length. Detailed below are some recommendations to improve the potential for translation from research to practice and examples of expressing the information in manuscript text:
Assess and report the number, percent of target audience, and representativeness of those who participate. Representativeness can be determined through comparison to the entire population (e.g., cancer registries) or to the characteristics of those who declined to participate. Example: Sixty-five percent (n=100) of breast cancer survivors invited to participate were randomized; those declining were more likely to be Latino, of lower SES, and diagnosed with later-stage cancer compared to those who agreed to participate.
Document anticipated effects, use quality of life as a common metric across conditions and interventions to allow for a patient-centered approach, and assess and report any potential unintended negative consequences. The minimally important difference should be reported for all of these factors. Example: The exercise intervention increased quality of life in breast cancer survivors by the minimally important difference with no adverse effects or interference with treatment adherence.
Provide clear information on the number of settings that participated, the level of expertise of intervention delivery staff, and the type, and, if appropriate, the representativeness, of program space. Example: The intervention was delivered at a single community health center by four research assistants completing graduate work in Kinesiology. The community health center was typical of other settings in the region in terms of size, health mission, and populations served.
Increase the focus on the extent to which an intervention was delivered consistently by study staff and the time and costs of the program. Example: Research assistants completed 95% of intervention activities across sessions although this rate varied between 80% and 100%. Components that were delivered less often included self-monitoring support and flexibility activities. The program leader dedicated approximately 30 min per participant per week of the study.
Monitor and report whether initial effects were sustained 6 months post-intervention and, when appropriate, report on data related to organizational sustainability. Example: Initial physical activity increases and quality of life benefits were sustained by those who completed the 6-month post-intervention assessment. Because the trial focused on demonstrating the efficacy of this approach, organizational indicators of program sustainability were not assessed.
Glasgow and associates [10] completed a review of 119 behavioral intervention studies, with primarily healthy adults using RE-AIM as a template. The review included 34 studies that targeted physical activity behavior change. In comparing our findings to those physical activity studies reviewed by Glasgow [10], it would appear that physical activity intervention research with breast cancer survivors is less consistent in reporting participation rate (52% vs. 76%), representativeness (0% vs. 14%), and maintenance of effects (16% vs. 36%). However, the BCS literature was more consistent in reporting the extent to which treatment was delivered as intended (64% vs. 46%) and participant attrition (96% v. 79%). Neither body of literature reported cost data to a significant degree. Thus, the low level of reporting on some components of RE-AIM is not unique to the physical activity and breast cancer literature but is also evident in the general physical activity literature.
The population of breast cancer survivors will continue to increase in the foreseeable future. If physical activity is to be a sufficiently scalable and sustainable intervention for improving health outcomes in breast cancer survivors, we must design multilevel studies which will ensure that interventions are suitably representative of breast cancer survivors and intervention effects are consistent and replicable across settings, staff, and conditions. Although this is a formidable challenge, the public health significance is undeniable in terms of potential reductions in the health care costs associated with physical inactivity and enhancement of the overall well-being of breast cancer survivors.
Acknowledgments
Edward McAuley is supported in part by a Shahid and Ann Carlston Khan Professorship in Applied Health Sciences and a grant from the National Institute on Aging (RO1 AG025667). Kerry S. Courneya is supported by the Canada Research Chairs Program and a Research Team Grant from the National Cancer Institute of Canada (NCIC), with funds from the Canadian Cancer Society (CCS) and the NCIC/CCS Sociobehavioral Cancer Research Network. Paul Estabrooks is supported in part by grants from the National Institute of Diabetes, Digestive, and Kidney Diseases (R01 DK070553; R01 DK071664). We are indebted to two anonymous reviewers and the Editor for their insightful comments on an earlier draft on this manuscript.
Footnotes
The authors have no disclosures.
Contributor Information
Siobhan M. White, Department of Kinesiology and Community Health, University of Illinois, 336 Freer Hall, 906 S. Goodwin Ave, Urbana, IL 61801, USA
Edward McAuley, Department of Kinesiology and Community Health, University of Illinois, 336 Freer Hall, 906 S. Goodwin Ave, Urbana, IL 61801, USA
Paul A. Estabrooks, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA
Kerry S. Courneya, University of Alberta, Edmonton, Canada
References
- 1.Ries LAG, Melbert D, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975–2004. Bethesda, MD: National Cancer Institute; 2006. [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Ganz PA, Desmond KA, Leedham B, Rowland JH, Meyerowitz BE, Belin TR. Quality of life in long term, disease-free survivors of breast cancer: A follow-up study. J Natl Cancer Inst. 2002;94:39–49. doi: 10.1093/jnci/94.1.39. [DOI] [PubMed] [Google Scholar]
- 4.Hewitt M, Rowland JH, Yancik R. Cancer survivors in the United States: Age, health, and disability. J Gerontol A Biol Sci Med Sci. 2003;58:82–91. doi: 10.1093/gerona/58.1.m82. [DOI] [PubMed] [Google Scholar]
- 5.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 6.Kirshbaum MN. A review of the benefits of whole body exercise during and after treatment for breast cancer. J Clin Nurs. 2007;16:104–121. doi: 10.1111/j.1365-2702.2006.01638.x. [DOI] [PubMed] [Google Scholar]
- 7.McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: A systematic review and meta-analysis. Can Med Assoc J. 2006;175:34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Redd WH, Montgomery GH, DuHamel KN. Behavioral intervention for cancer treatment side effects. J Natl Cancer Inst. 2001;93:810–823. doi: 10.1093/jnci/93.11.810. [DOI] [PubMed] [Google Scholar]
- 9.Holick CN, Newcomb PA, Trentham-Dietz A, et al. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:379–386. doi: 10.1158/1055-9965.EPI-07-0771. [DOI] [PubMed] [Google Scholar]
- 10.Glasgow RE, Klesges LM, Dzewaltowski DA, Bull SS, Estabrooks P. The future of behavior change research: What is needed to improve translation of research into health promotion practice. Ann Behav Med. 2004;27:3–12. doi: 10.1207/s15324796abm2701_2. [DOI] [PubMed] [Google Scholar]
- 11.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: The RE-AIM framework. Am J Public Health. 1999;89:1322–1327. doi: 10.2105/ajph.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Estabrooks PA, Gyurcsik NC. Evaluating the impact of behavioral interventions that target physical activity: Issues of generalizability and public health. Psychol Sport Exerc. 2003;4:41–55. [Google Scholar]
- 13.Glasgow RE. Chapter 23: Evaluation of theory-based interventions: The RE-AIM model. In: Glanz K, Rimer BK, Lewis FM, editors. Health Behavior and Health Education. San Francisco: Wiley; 2002. [Google Scholar]
- 14.Glasgow RE, Nelson CC, Strycker LA, King DK. Using RE-AIM metrics to evaluate diabetes self-management support interventions. Am J Prev Med. 2006;30:67–73. doi: 10.1016/j.amepre.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 15.Glasgow RE, McKay HG, Piette JD, Reynolds KD. The RE-AIM framework for evaluating interventions: What can it tell us about approaches to chronic illness management. Patient Educ Couns. 2001;44:119–127. doi: 10.1016/s0738-3991(00)00186-5. [DOI] [PubMed] [Google Scholar]
- 16.Glasgow RE, Klesges LM, Dzewaltowski DA, Estabrooks PA, Vogt TM. Evaluating the impact of health promotion programs: Using the RE-AIM framework to form summary measures for decision making involving complex issues. Health Educ Res. 2006;21:688–694. doi: 10.1093/her/cyl081. [DOI] [PubMed] [Google Scholar]
- 17.Courneya KS, Segal RJ, Mackey JR, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: A multicenter randomized controlled trial. J Clin Oncol. 2007;25:4396–4404. doi: 10.1200/JCO.2006.08.2024. [DOI] [PubMed] [Google Scholar]
- 18.Daley AJ, Crank H, Saxton JM, Mutrie N, Coleman R, Roalfe A. Randomized trial of exercise therapy in women treated for breast cancer. J Clin Oncol. 2007;25:1713–1721. doi: 10.1200/JCO.2006.09.5083. [DOI] [PubMed] [Google Scholar]
- 19.Ware J, Sherbourne C. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 20.Basen-Enquist K, Carmack Taylor CL, Rosenblum C, et al. Randomized pilot test of a lifestyle physical activity intervention for breast cancer survivors. Patient Educ Couns. 2006;64:225–234. doi: 10.1016/j.pec.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 21.McKenzie DC, Kalda AL. Effect of upper extremity exercise on secondary lymphedema in breast cancer patients: A pilot study. J Clin Oncol. 2003;21:463–466. doi: 10.1200/JCO.2003.04.069. [DOI] [PubMed] [Google Scholar]
- 22.Sandel SL, Judge JO, Landry N, Faria L, Ouellette R, Majczak M. Dance and movement program improves quality-of-life measures in breast cancer survivors. Cancer Nurs. 2005;28:301–309. doi: 10.1097/00002820-200507000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Mock V, Frangakis C, Davidson NE, et al. Exercise manages fatigue during breast cancer treatment: A randomized controlled trial. Psychooncology. 2005;14:464–477. doi: 10.1002/pon.863. [DOI] [PubMed] [Google Scholar]
- 24.Mock V, Pickett M, Ropka ME, et al. Fatigue and quality of life outcomes of exercise during cancer treatment. Cancer Pract. 2001;9:119–127. doi: 10.1046/j.1523-5394.2001.009003119.x. [DOI] [PubMed] [Google Scholar]
- 25.Dzewaltowski DA, Estabrooks PA, Glasgow RE. The future of physical activity behavior change research: What is needed to improve translation of research into health promotion practice. Exerc Sport Sci Rev. 2004;32:57–63. doi: 10.1097/00003677-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Patrick K, Scutchfield FD, Woolf SH. External validity reporting in prevention research. Am J Prev Med. 2008;34:260–262. doi: 10.1016/j.amepre.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 27.Steckler A, McLeroy KR. The importance of external validity. Am J Public Health. 2008;98:9–10. doi: 10.2105/AJPH.2007.126847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Estabrooks P, Dzewaltowski DA, Glasgow RE, Klesges LM. Reporting of validity from school health promotion studies published in 12 leading journals, 1996–2000. J Sch Health. 2003;73:21–28. doi: 10.1111/j.1746-1561.2003.tb06554.x. [DOI] [PubMed] [Google Scholar]
- 29.Klesges LM, Dzewaltowski DA, Glasgow RE. Review of external validity reporting in childhood obesity prevention research. Am J Prev Med. 2008;34:216–223. doi: 10.1016/j.amepre.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 30.Dzewaltowski DA, Estabrooks PA, Klesges LM, Bull S, Glasgow RE. Behavior change intervention research in community settings: How generalizable are the results. Health Promot Int. 2004;19:235–245. doi: 10.1093/heapro/dah211. [DOI] [PubMed] [Google Scholar]
- 31.Victora CG, Habicht JP, Bryce J. Evidence-based public health: Moving beyond randomized trials. Am J Public Health. 2004;94:400–405. doi: 10.2105/ajph.94.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klesges LM, Estabrooks PA, Dzewaltowski DA, Bull SS, Glasgow RE. Beginning with the application in mind: Designing and planning health behavior change interventions to enhance dissemination. Ann Behav Med. 2005;29:66–75. doi: 10.1207/s15324796abm2902s_10. [DOI] [PubMed] [Google Scholar]
- 33.Vallance JKH, Courneya KS, Plotnikoff RC, Yasui Y, Mackey JR. Effects of print materials and step pedometers on physical activity and quality of life in breast cancer survivors: A randomized controlled trial. J Clin Oncol. 2007;25:2352–2359. doi: 10.1200/JCO.2006.07.9988. [DOI] [PubMed] [Google Scholar]
- 34.Marcus BH, Dubbert PM, Forsyth LH, et al. Physical activity behavior change: Issues in adoption and maintenance. Health Psychol. 2000;19:32–41. doi: 10.1037/0278-6133.19.suppl1.32. [DOI] [PubMed] [Google Scholar]
- 35.Courneya KS, Friedenreich CM, Reid RD, et al. Predictors of follow-up exercise behavior 6 months after a randomized trial of exercise training during breast cancer chemotherapy. Breast Cancer Res Treat. 2009;114:179–187. doi: 10.1007/s10549-008-9987-3. [DOI] [PubMed] [Google Scholar]
- 36.Altman DG, Schulz KF, Moher D, et al. The revised CONSORT statement for reporting randomized trials: Explanation and elaboration. Ann Intern Med. 2001;134:663–694. doi: 10.7326/0003-4819-134-8-200104170-00012. [DOI] [PubMed] [Google Scholar]
- 37.Moher D, Schulz KF, Altman DG. The CONSORT statement: Revised recommendations for improving the quality of reports of parallel-group randomized trials. J Am Podiatr Med Assoc. 2001;91:437–442. doi: 10.7547/87507315-91-8-437. [DOI] [PubMed] [Google Scholar]
- 38.The Diabetes Prevention Program Research Group. The Diabetes Prevention Program (DPP): Description of lifestyle intervention. Diabetes Care. 2002;25:2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.The Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The Diabetes Prevention Program Research Group. Impact of intensive lifestyle and metformin therapy on cardiovascular disease risk factors in the Diabetes Prevention Program. Diabetes Care. 2005;28:888–894. doi: 10.2337/diacare.28.4.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.The Diabetes Prevention Program Research Group. Within-trial cost-effectiveness of lifestyle intervention or metformin for the primary prevention of type 2 diabetes. Diabetes Care. 2003;26:2518–2523. doi: 10.2337/diacare.26.9.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Battaglini C, Bottaro M, Dennehy C, et al. The effects of an individualized exercise intervention on body composition in breast cancer patients undergoing treatment. Sao Paulo Med J. 2007;125:22–28. doi: 10.1590/S1516-31802007000100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Campbell A, Mutrie N, White F, McGuire F, Kearney N. A pilot study of a supervised group exercise program as a rehabilitation treatment for women with breast cancer receiving adjuvant treatment. Eur J Oncol Nurs. 2005;9:56–63. doi: 10.1016/j.ejon.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 44.Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmen-opausal breast cancer survivors: Cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21:1600–1668. doi: 10.1200/JCO.2003.04.093. [DOI] [PubMed] [Google Scholar]
- 45.Culos-Reed SN, Carlson LE, Daroux LM, Hately-Aldous S. A pilot study of yoga for breast cancer survivors: Physical and psychological benefits. Psychooncology. 2006;15:891–897. doi: 10.1002/pon.1021. [DOI] [PubMed] [Google Scholar]
- 46.Drouin JS, Armstrong H, Krause S, Orr J. Effects of aerobic exercise training on peak aerobic capacity, fatigue, and psychological factors during radiation for breast cancer. Rehabilitation Oncology. 2005;23:11–17. [Google Scholar]
- 47.Galantino ML, Capito L, Kane RJ, Ottey N, Switzer S, Packel L. The effects of tai chi and walking on fatigue and body mass index in women living with breast cancer: A pilot study. Rehabilitation Oncology. 2003;21:17–22. [Google Scholar]
- 48.Headley JA, Ownby KK, John LD. The effect of seated exercise on fatigue and quality of life in women with advanced breast cancer. Oncol Nurs Forum. 2004;31:977–983. doi: 10.1188/04.ONF.977-983. [DOI] [PubMed] [Google Scholar]
- 49.Herrero F, San Juan AF, Fleck SJ, et al. Combined aerobic and resistance training in breast cancer survivors: A randomized, controlled pilot trial. Int J Sports Med. 2006;27:573–580. doi: 10.1055/s-2005-865848. [DOI] [PubMed] [Google Scholar]
- 50.Kim CJ, Kang DH, Smith BA, Landers KA. Cardiopulmonary responses and adherence to exercise in women newly diagnosed with breast cancer undergoing adjuvant therapy. Cancer Nurs. 2006;29:156–165. doi: 10.1097/00002820-200603000-00013. [DOI] [PubMed] [Google Scholar]
- 51.Ligibel JA, Campbell N, Partridge A, et al. Impact of a mixed strength and endurance exercise intervention on insulin levels in breast cancer survivors. J Clin Oncol. 2008;26:907–912. doi: 10.1200/JCO.2007.12.7357. [DOI] [PubMed] [Google Scholar]
- 52.Milne HM, Wallman KE, Gordon S, Courneya KS. Effects of a combined aerobic and resistance exercise program in breast cancer survivors: A randomized controlled trial. Breast Cancer Res Treat. 2008;108:279–288. doi: 10.1007/s10549-007-9602-z. [DOI] [PubMed] [Google Scholar]
- 53.Mustian KM, Katula JA, Gill DL, Roscoe JA, Lang D, Murphy K. Tai Chi Chuan, health-related quality of life and self-esteem: A randomized trial with breast cancer survivors. Support Care Cancer. 2004;12:871–876. doi: 10.1007/s00520-004-0682-6. [DOI] [PubMed] [Google Scholar]
- 54.Mutrie N, Campbell AM, Whyte F, et al. Benefits of supervised group exercise programme for women being treated for early stage breast cancer: Pragmatic randomised controlled trial. BMJ. 2007;334:517–523. doi: 10.1136/bmj.39094.648553.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pinto BM, Clark MM, Maruyama NC, Feder SI. Psychological and fitness changes associated with exercise participation among women with breast cancer. Psychooncology. 2003;12:118–126. doi: 10.1002/pon.618. [DOI] [PubMed] [Google Scholar]
- 56.Pinto BM, Frierson GM, Rabin C, Trunzo JJ, Marcus BH. Home-based physical activity intervention for breast cancer patients. J Clin Oncol. 2005;20:3577–3587. doi: 10.1200/JCO.2005.03.080. [DOI] [PubMed] [Google Scholar]
- 57.Schmitz KH, Ahmed RL, Hannan PJ, Yee D. Safety and efficacy of weight training in recent breast cancer survivors to alter body composition, insulin, and insulin-like growth factor axis proteins. Cancer Epidemiol Biomarkers Prev. 2005;14:1672–1680. doi: 10.1158/1055-9965.EPI-04-0736. [DOI] [PubMed] [Google Scholar]
- 58.Segal R, Evans W, Johnson D, et al. Structured exercise improves physical functioning in women with stages I and II breast cancer: Results of a randomized controlled trial. J Clin Oncol. 2001;19:657–665. doi: 10.1200/JCO.2001.19.3.657. [DOI] [PubMed] [Google Scholar]
- 59.Segar ML, Katch VL, Roth RS, et al. The effect of aerobic exercise on self-esteem and depressive and anxiety symptoms among breast cancer survivors. Oncol Nurs Forum. 1998;25:107–113. [PubMed] [Google Scholar]