Abstract
Smokers are at high risk for oral disease. As a result, they represent an important target group for population- level, public oral health promotion efforts. While dental health professionals often address smoking with their patients, no systematic efforts have been made to offer smokers an intervention to improve their use of oral health care. This paper details the rationale, design, and methods of a large, semi-pragmatic, randomized clinical trial designed to address this gap. Participants are recruited via the Oregon, Nebraska and Louisiana state-sponsored tobacco quitlines and randomized to receive standard quitline care versus standard care plus a multi- modal oral health promotion program (Oral Health 4 Life) integrated within the quitline services. All participants are followed for 6 months to assess the impact of the intervention on smoking abstinence and utilization of professional dental care. In addition, the study will assess the cost of the intervention and provide practical guidance to states on whether the intervention is financially feasible to implement, should the intervention be effective. This study protocol may be useful to others interested in promoting oral health among smokers, those interested in partnering with tobacco quitlines to extend standard services to address other high risk health behaviors among smokers, or those interested in semi-pragmatic trial design.
Keywords: oral health, dental care, smoking cessation, behavior change, quitline, pragmatic trial
1. Introduction
Oral disease affects millions of people in the U.S., resulting in unnecessary pain, potential disfigurement, emotional suffering, and greater risk for morbidity and mortality.[1] Treating acute oral disease and preventing future oral disease are important public health goals. To achieve these goals, the Office of the Surgeon General [2] and the Centers for Disease Control and Prevention [1] have called for greater partnerships between the public and private sectors.
Smokers are at particularly high risk for oral disease due to their tobacco use and other lifestyle choices (e.g., low dental care utilization [3, 4], poor oral hygiene [5], poor diet [6], and alcohol use [7–10]). As a result, they are a priority audience for population-level oral health promotion efforts. While prior efforts have focused on empowering dental providers to promote tobacco abstinence, dental professionals often have limited time, resources and training to provide behavioral counseling, and their efforts fail to reach individuals who do not visit a dentist. Moreover, many dental professionals are not trained in behavioral counseling and simply providing oral health education alone does not result in lasting behavior change.[11–16]
A complementary strategy for reaching smokers, particularly those not routinely seeking dental care, is to integrate oral health promotion into tobacco quitline programs. Quitlines provide behavioral counseling for tobacco cessation, primarily through proactive calls (i.e., calls initiated by the quitline on a pre-determined schedule) with supplemental outreach via online materials, mail, and/or text messaging. Quitlines are available in all U.S. states and are an effective public health intervention for smoking cessation.[17–20] In 2015, an estimated 1% of the 40 million U.S. smokers received care through a state-sponsored quitline.[21] This equates to approximately 400,000 smokers, the majority of whom are female (57%) and have less than a college degree (57%). A significant portion of these callers receive Medicaid (38%) or are uninsured (22%).[21] Based on the reach of the quitlines, we hypothesized that partnering with them may be an effective strategy to reach smokers and promote better oral health care. In preliminary research, we found many quitline callers do not seek routine dental care [5], so there is a need for intervention. We also found that quitline callers and the key stakeholders responsible for funding and providing these services were supportive of this intervention concept [5, 22, 23].
Thus, we developed a theoretically-grounded, comprehensive, multi- modal behavioral program (Oral Health 4 Life, OH4L) to promote better oral health care in conjunction with standard tobacco quitline counseling. The program consists of behavioral counseling, supportive outreach via text messaging, and other health education materials and resources delivered in print and online—all designed to fit within the quitline infrastructure. By partnering with state quitlines, the program can reach a high-risk, high-need, lower-socioeconomic status audience.
This paper describes the rationale, design, and methods of the OH4L study. At the time of this writing (October, 2016), recruitment has ended, but intervention delivery and data collection are ongoing and expected to be complete in March 2017. Study findings are not presented in this paper, but will inform the cost and effectiveness of the OH4L intervention. This study could serve as a model for leveraging the tobacco quitline infrastructure to address other high-risk health behaviors among smokers nationwide.
2. Methods
2.1 Collaborating Sites and Oversight
This study is a collaboration between researchers at the Kaiser Permanente Washington Health Research Institute (KPWHRI), University of California at Davis (UCD), and Alere Wellbeing (AW). At the time the study was initiated and data collected, KPWHRI was known as the Group Health Research Institute. The intervention was developed by KPWHRI and designed to be integrated into standard quitline services provided by AW, the leading provider of tobacco quitline services in the United States. AW treats approximately 350,000 thousand smokers each year across 25 state quitline contracts. All phone-based counseling in the study is provided by AW. Mail and text message-based intervention content are provided by KPWHRI, and all follow-up data collection is conducted by KPWHRI’s Survey Research Program. Treatment fidelity monitoring is conducted by KPWHRI, under the supervision of staff at UCD.
The OH4L trial is funded by the National Institute for Dental and Craniofacial Research (NIDCR) and is registered with ClinicalTrials.gov (NCT02347124). All research activities were reviewed and approved by the KP Washington Institutional Review Board. Recruitment and treatment activities at AW were also approved by the Washington Institutional Review Board and study participation was approved by authorities in the Oregon, Louisiana, and Nebraska state departments of health who contract quitline services with AW. The project is overseen by a Data and Safety Monitoring Board (DSMB) convened by NIDCR and an NIDCR medical monitor.
2.2 Study Objectives
The primary objective of this study is to assess the effects of the OH4L program on tobacco abstinence and utilization of professional dental services. Both outcomes are considered primary because quitline stakeholders told us the intervention would only be viable to implement if improving oral health care did not deter from cessation.[23]
Secondary objectives include: a) assessing the impact of the OH4L program on key secondary behavioral outcomes and select intermediate outcomes/process measures that could mediate treatment effects, and if warranted based on the results, b) calculating the incremental cost of the OH4L program, and c) providing these data to key stakeholders to inform decisions about whether the OH4L program warrants dissemination in its current form or further refinement and evaluation. Relevant details to these objectives are discussed in the following sections.
2.3 Pragmatic Design
On the continuum of explanatory to pragmatic trials, this study uses a semi-pragmatic trial design. Explanatory studies are randomized trials conducted under idealized conditions with tight experimental control. In contrast, pragmatic trials evaluate interventions using usual care systems and real world conditions.[24] While explanatory studies seek to inform if an intervention can be effective when conditions are tightly controlled, pragmatic trials seek to inform intervention effectiveness in the real world.[25] The latter is more useful when informing clinical and policy decisions.
Loudon et al. [24] have offered a Pragmatic-Explanatory Continuum Indicator Summary (PRECIS-2) to help researchers make study design decisions that align with the goals of their trial. The tool describes a continuum between explanatory and pragmatic study designs using nine domains: (1) the extent to which participants are similar to usual care recipients, (2) the amount of extra effort required for recruitment beyond usual care activities, (3) how closely the setting matches usual care, (4) how well the organizational resources for intervention delivery match usual care, (5) flexibility of the intervention delivery compared to usual care, (6) flexibility in how participants are monitored and the intervention adhered to compared to usual care, (7) how the intensity of follow-up data collection compares to usual care; (8) relevance of the primary outcomes to participants, and (9) extent to which all data are included in the primary outcome analyses. Each domain is scored on a five point scale from 1 (“very explanatory”) to 5 (“very pragmatic”). Using this framework, the current project scores 36 out of 45 points, reflecting a more pragmatic trial. This score was derived based on the following characteristics and their associated domain scores: (1) participants are actual smokers seeking usual care, although some callers are screened out (score = 4); (2) no extra effort is required to identify and recruit quitline callers (score = 5); (3) the setting is a real-world tobacco quitline (score = 5); (4) the organizational resources for the intervention were identical to usual care resources (score = 5); (5) the standard care quitline intervention was not changed, but experimental participants received additional oral health services (score = 3); (6) a higher level of fidelity monitoring was included in the study design, but real world practices were used to provide feedback to quitline counselors (score = 3); (7) usual care includes phone-based follow-up assessments of smoking cessation, but with less intensive effort to retain individuals than in this study (score = 2); (8) smoking cessation and oral health are relevant outcomes for usual care quitline callers, although most are only seeking assistance quitting smoking when they enroll in services (score = 4); and (9) all participants and data collected will be included in main outcome analyses (score = 5). Thus, the resulting semi-pragmatic trial blends the best aspects of both explanatory and pragmatic trial designs.
2.4 Recruitment and Eligibility Criteria
Participants were recruited from the Oregon, Nebraska, and Louisiana State Quitlines. Each state was chosen because they contract services with AW, offer a multi-call standard tobacco counseling program, and have affordable dental care services to which participants could be referred for care.
Recruitment occurred from June 2015 through July 2016. Callers to each participating quitline were first pre-screened in real-time using standard intake data obtained during their quitline registration call. Callers were deemed potentially eligible if they smoked at least 5 cigarettes a day, were age 18 or older, were ready to quit smoking in the next month, could read and speak in English, and were eligible for their state’s multi-call treatment program. Persons who did not meet all of these criteria were excluded (n = 1,072; Figure 1). The remaining callers were potentially eligible and invited to participate in a screening interview and learn more about the study. With their agreement, these callers were transferred from the registration staff to an AW research counselor trained in the study protocol. Callers were deemed eligible for the study if they had not had a dental checkup in the prior 6 months, did not have a dental checkup scheduled for the next 6 months, had some or all of their natural teeth, had a cell phone capable of receiving text messages, and reported internet access. Individuals were excluded if they were incarcerated, enrolled in an inpatient substance abuse treatment facility, had a significant cognitive impairment at the time of the call, self-reported dementia or serious mental illness (bipolar disorder, mania, or schizophrenia), or were not able to read small text. Individuals were also excluded if they reported plans to move in the next 6 months or had a household member already enrolled in the study. The latter was based on self-report and subsequent review of participants mailing addresses.
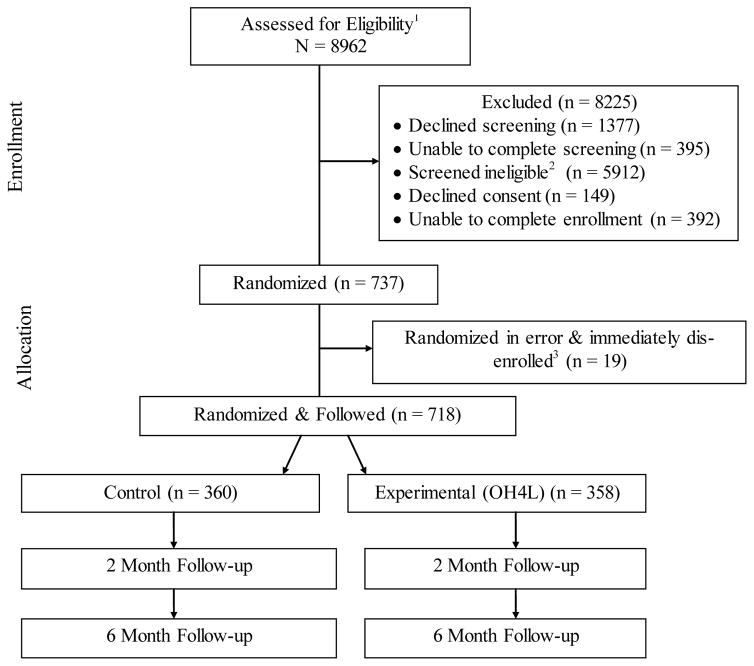
Figure 1. CONSORT Flow Diagram.
1Excludes people known to be ineligible per Quitline intake data (n = 1072)
2Primary reasons were: No internet access (n = 2283), seen dentist in prior 6 months (n = 1051), lost all natural teeth (n = 693), prior diagnosis with psychosis (n = 373), no text messaging capacity (n = 351), unwilling to discuss oral health (n = 303), dental appointment already scheduled (n = 233), and planning to move in next 6 months (n = 170).
3Individuals mistakenly randomized due to systems issues. Immediately removed from sample, not offered treatment, and not followed for data collection.
2.5 Consent, Baseline, Randomization and Enrollment
Following the eligibility screening, eligible callers provided oral consent to participate and then completed a baseline phone interview. Following completion of the interview, they were enrolled in the study and randomized to treatment using an automated algorithm built into the AW systems software. Randomization was stratified based on whether participants had dental insurance coverage (yes/no) and which tobacco quitline they were enrolled in (Louisiana, Nebraska or Oregon), to ensure balance across each treatment group. Participants were randomly assigned to either the standard care tobacco quitline program or the experimental program (standard care tobacco quitline program + multi- modal OH4L intervention).
Consent, baseline assessment, randomization and enrollment were typically completed during the initial quitline intake call by trained AW research counselors. If the enrollment process could not be completed during this call, counselors called individuals back to complete enrollment at a later date, but prior to delivery of any intervention content. If the enrollment was completed in one call, counselors then either initiated the first counseling call content (experimental group) or transferred calls to a usual care quitline counselor (control group) to initiate counseling. This process minimized the risk of contamination across treatment groups.
A total of 737 people were randomized; however, 19 of these were not eligible and should not have been randomized (9 control, 10 experimental). In each case, individuals were recognized as ineligible either during the initial contact or upon routine file review within 24 hours afterwards. As such, they were immediately notified that they were ineligible, were removed from the study, and were transferred to usual care services provided through their appropriate state quitline program. No treatment utilization or follow-up assessment data is being collected on these 19 individuals. Of this group, 12 individuals were deem ineligible because their state changed the qualifying criteria for the multi-call quitline program and these individuals were screened as eligible before the quitline’s automated systems could be updated to exclude them. Four people initially stated they were smoking, but subsequently during the first contact indicated they had actually already quit smoking. One person was already enrolled in a separate study through their quitline and mistakenly referred to this study for screening. Two people reported during the screening they had a cell phone with text messaging, but then revealed during the initial contact that they did not. One person shared a household with an enrolled participant. These exclusions were not mutually exclusive, one of the 19 people was ineligible for two reasons. We considered whether each person should be retained per our intent to treat (ITT) protocol, but ultimately determined this would be inappropriate since participants were clearly not eligible for the study and this was known at the outset. Furthermore, for the 12 individuals who were not eligible for their state’s multi-call quitline program, we did not have an option of retaining them and providing this service..
2.6 Blinding
Due to the nature of this trial, neither participants nor AW counselors in the experimental group were blinded to treatment group. Counselors delivering the control intervention were aware that participants were enrolled in a research study, but they were not trained in or provided access to the oral health intervention materials. KPWHRI staff responsible for follow-up assessment were blinded to treatment assignment. To retain this blinding during the follow-up data collection, qualitative satisfaction items (which could unblind a participant’s treatment group) were not assessed until the end of the six month survey, after primary and secondary outcome data had been assessed and saved in the electronic data systems.
2.7 Intervention
2.7.1. Control Intervention
The control intervention included participants’ standard state-sponsored tobacco quitline services (a 4 or 5 call program, depending on the state), mailed written materials, and access to an online treatment program (see overview of contact and timing in Figure 2). The protocol for the standard tobacco quitline program is proprietary and is not described in detail here, but all treatment focuses on tobacco cessation; it does not include oral health advice. The intervention is grounded in social cognitive theory and cognitive behavioral therapy (CBT), and includes a focus on the standard counseling components demonstrated effective for CBT-based tobacco cessation treatment: problem-solving, coping skills training, and support.[26] Callers are encouraged to set a target quit date (QD) and then assisted in developing and remaining adherent to their quit plan. Once a participant has quit, the focus turns to relapse prevention.
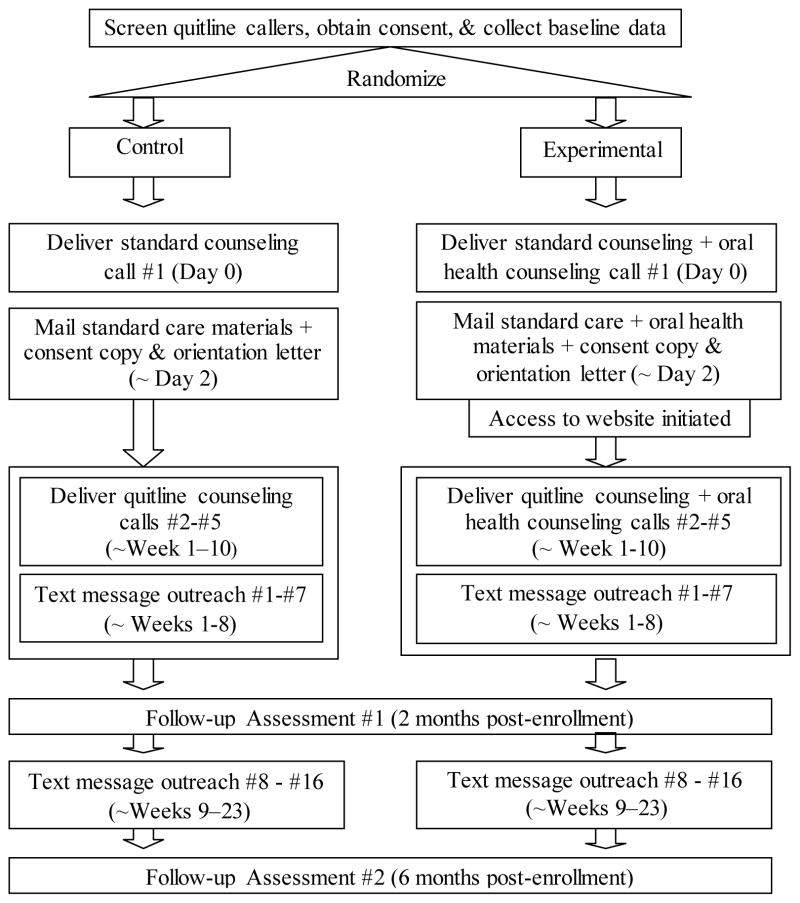
Figure 2.
Overview Study Contacts
The content and timing of the counseling is tailored to each person based on their availability to receive calls, planned QD, and continued program engagement. So, call timing can vary across people, but is designed to follow the general timeline below (see also Figure 2).
Call 1: Enrollment call/treatment session 1 (pre-target QD)
Call 2: QD or QD +1 day (~week 1–2 after Call 1)
Call 3: QD + 1–2 weeks (~week 2–4 after Call 1)
Call 4: Call 3 + 3 weeks (~week 4–6 after Call 1)
Call 5: Call 4 + 3 weeks (~week 6–10 after Call 1)
The first call is initiated by participants to the quitline, but subsequent calls are initiated by quitline staff to participants. In addition, participants can call the quitline for ad-hoc counseling, as wanted.
In addition to standard care services, control participants receive an attention- matched text messaging program focused on general health behavior recommendations (e.g., diet, physical activity). Messages do not address smoking cessation or oral health. All messages are delivered on a standardized schedule, for a total of 16 messages over 23 weeks:
| Text message 1: | One day prior to QD |
| Text messages 2–8: | Once a week |
| Text messages 9–16: | Every other week |
Finally, each state offers an initial 2 or 4 week starter kit of nicotine replacement therapy (NRT) to some participants as part of their standard care, based on the availability of state resources to provide this adjunct treatment at any given time and the medical appropriateness of this treatment for each person. The proportion of people receiving this adjunct therapy can be highly variable over time, ranging from no one to the majority of callers at any given time. Given this variability, provision and use of NRT is monitored for each study participant. All intervention contact with the quitline (number of calls, duration of calls, etc.) is also monitored using automated data.
2.7.2. Experimental Intervention
Experimental participants received the same standard tobacco quitline services described above with two exceptions. Participants in this group also receive a multi-modal oral health promotion program (OH4L) and instead of generic health promotion text messages, all text messages are focused on oral health.
Like the standard tobacco cessation program, the oral health intervention is grounded in social cognitive theory [27] and cognitive behavioral therapy, but also draws upon motivational interviewing [28, 29] and prospect theory.[30]
The multi-modal OH4L program consists of: scripted oral health counseling integrated into each quitline counseling call, a mailed oral health promotion brochure, access to an oral health promotion website, a toothbrush, floss, sugar-free gum, and a series of 16 oral health text messages. The text messages are delivered on the same schedule as in the control group. The mailed materials (brochure, toothbrush, floss, and gum) are mailed 2–3 business days following enrollment.
Similar oral health content is provided via the scripted counseling, mailed written brochure, and website. Smokers are informed about the benefits of routine oral hygiene, recommendations how to manage tobacco cravings by engaging in oral health behaviors (such as brushing teeth, flossing, and chewing sugar-free gum), information about the oral health risks of using alcohol and tobacco together, and a referral to low cost dental resources in their local area. These topics are intended to promote positive outcome expectations, build self-efficacy for change, and enhance participants’ motivation for change.
To ensure participants receive as much of this information as possible, call content is weighted to include more discussion in the first call and review “booster” content in calls 2– 5. The scripted counseling and text messages are also designed to point people to the written and online materials for additional information. Although the standard quitline intervention is not scripted, we opted to use a counseling script for the oral health counseling. Doing so helps ensure key talking points are delivered in each call. Additionally, this helps ensure that messaging is consistently presented in a gain-framed, as opposed to loss-framed context. That is, counselors discuss the benefits of better oral health care, as opposed to the risks of poor oral health care. According to prospect theory [30], potential gains are more motivating than potential losses for low risk, preventive behaviors. While the importance of gain-framing has been called into question, the strongest support for this strategy comes from its application to oral hygiene behaviors.[31]
At the request of funding officials, participants are required to enter a password to access the online intervention materials, thereby allowing treatment exposure to be monitored on an individual level. In order to minimize the burden of this requirement, participants are instructed to use the phone number they provided when they enrolled in the study as their password. Instructions for logging in are included in the counseling script, the written brochure, and on a sticker placed on the outside of the gum package mailed to participants. Additionally, instructions for contacting study staff to get help logging in are posted on the website’s homepage next to the log-in box. Access to the online materials is set-up within 2 days following enrollment.
2.8 Fidelity Monitoring
AW records counseling calls for routine quality assurance testing. To monitor fidelity to the treatment protocol, we are randomly selecting a minimum of 10% of control calls and 30% of experimental calls for review and fidelity coding. Coders use a standardized checklist of key treatment components. The goal in the control group is to confirm calls contain basic smoking cessation content and no discussion of oral health. Intervention calls are monitored to ensure they contain the same basic smoking cessation content and specific scripted oral health talking points, such as a recommendation to schedule a dental appointment, referral information for low-cost dental providers, and the use of oral health tips (e.g., brushing, flossing, chewing sugar-free gum) to manage cravings to smoke. Deviations from the protocol are shared with AW supervisors, so that protocol drift can be addressed with individual counselors. Summary reports of treatment fidelity metrics are reviewed routinely by the study team.
2.9 Assessment Methods
2.9.1 Assessment Contacts
Participants are surveyed at baseline following consent to enroll and prior to receiving any counseling. Follow-up assessments occur at 2 and 6 months post-enrollment. Follow-up assessment is conducted by the KPWHRI Survey Research Program using phone assessment with a back-up written survey by mail for non-responders. Each assessment is designed to take between 15 and 20 minutes.
2.9.3 Measures
An overview of key assessment measures and their timing is presented in Table 1. Primary outcome measures are smoking abstinence at 6 month follow-up and receipt of professional dental care following study enrollment. Smoking abstinence is assessed per convention as a self-report of no smoking, even a puff, in the past 7 days (i.e., 7-day point prevalent abstinence [PPA]). Among its advantages, self-reported PPA is easy to assess by phone and allows a direct comparison of study outcomes with other published literature. Biochemical confirmation of smoking status is not being used because it is not recommended in large cessation trials with no face to face contact or when participants are geographically dispersed and specimen collection is prohibitive. Under these circumstances, requiring collection of biological specimens to confirm abstinence can introduce a response bias unrelated to smoking status. [32]
Table 1.
Key assessment constructs and timing
| Baseline | 2-month | 6-month | |
|---|---|---|---|
| Demographics2 | x | ||
| Depression history2 | x | ||
| Oral health status2 | x | ||
| Dental anxiety12 | x | ||
| Tobacco use/abstinence 2 | x | x | x |
| Dental care utilization2 | x | x | x |
| Future dental appointment 2 | x | x | x |
| Smoking quit attempts2 | x | x | x |
| Tooth brushing2 | x | x | x |
| Flossing2 | x | x | x |
| Oral health knowledge12 | x | x | x |
| Self-efficacy12 | x | x | x |
| Motivation12 | x | x | x |
| Perceived barriers to oral health care12 | x | x | x |
| Stop-smoking pharmacotherapy23 | x | x | x |
| Treatment utilization care23 | x | ||
| Use of OH4L website4 | x | ||
| Treatment satisfaction – smoking intervention2 | x | ||
| Treatment satisfaction – oral health2 | x |
Likert scale items.
Source is self-report via phone survey.
Source is automated quitline treatment records.
Source is web analytic data.
Receipt of professional dental care is assessed using a self-report item from the 2012 National Health Interview Survey which assesses time since last seeing a dentist (including orthodontists, oral surgeons, and dental hygienists). Time since last appointment is characterized as never, less than 6 months, 6 months to one year, 1–2 years, 2–5 years, greater than 5 years, and never.[33] Since the standardized wording does not directly assess treatment use since study enrollment, we rely on a report of use in the last 6 months at the 2 or 6 month assessment to define our primary outcome. As a condition of eligibility, participants cannot have seen a dental care provider in the prior 6 months at baseline or have a dental appointment scheduled, so any new reports of dental care utilization in the past 6 months at follow-up will indicate receipt of dental care post-enrollment. The primary outcome will be defined as a self-report of a dental visit in the past 6 months reported at either the 2-month or 6-month study assessment. To discourage misreporting of dental services outcomes, a variation of the ‘bogus pipeline’ methodology [34] is used. Participants are told at the outset of the follow- up survey that they may be asked for permission to contact their dental care provider in order to confirm their reported use of services and instructed to locate their provider’s contact information prior to start of the survey. However, this information is not actually collected. Since participants in both groups receive the same smoking cessation intervention, other than the oral health content, and there is not a differential pressure to mis-report smoking status, a similar bogus pipeline is not necessary for assessing smoking outcomes. Moreover, it might be difficult to convince participants of our ability to remotely collect breath or other biological samples in order to confirm smoking status given that study staff were not located in the same states as participants.
Smoking abstinence and dental care utilization will also be examined at the 2 month follow-up as secondary outcomes. Other key secondary and process measures include: scheduling of a future dental appointment (yes/no), oral health knowledge, dental anxiety, oral health self-efficacy, and motivation for change. Oral health knowledge is assessed via a scale from Brennan et al., [35] which we modified to include five additional oral health behaviors (e.g., stopping smoking, avoiding or limiting alcohol, eating plenty of fruits and vegetables, and chewing sugar free gum). Participants rate the importance of each item on a 5-point Likert scale ranging from “definitely not important” to “definitely important.” Dental anxiety is assessed with the Modified Dental Anxiety Scale[36], a 5 item Likert-scale measure of anxiety associated with receiving dental care. Motivation and self-efficacy are assessed using 5-point Likert scales ranging from “not at all” to “very”. Participants are also asked to provide feedback on the helpfulness of, and their satisfaction with, the different components of the tobacco cessation intervention (both groups) and the OH4L intervention (experimental group only). Additional assessment measures include standard demographics, self-reported oral health status, depression history, use of pharmacotherapy for smoking cessation, quit attempts, brushing, flossing, and perceived barriers to dental care. Automated quitline records will be used to further assess treatment utilization (number of calls, duration of counseling contact) and receipt of NRT. Use of the oral health website is monitored using web analytics.
2.9.4 Retention Strategies
Participants receive $30 cash for completing each of the three assessments (baseline, 2-month follow-up, and 6-month follow- up). Reminder letters and text messages are sent one week prior to each follow-up assessment, and participants who do not complete the 6-month assessment receive additional text message reminders. Participants who are not reached within 2–3 weeks of their scheduled assessment date are mailed a written survey. To boost retention rates, mid-study we implemented an additional retention strategy. Participants are informed (by text and letter) that they can receive an additional $15 bonus ($45 total) if they call KPWHRI to complete their scheduled survey directly, as opposed to waiting to be contacted by the study staff. Standard tracing techniques are being used to locate participants with bad mailing addresses or disconnected phone numbers. Incentives are mailed following the completion of each assessment.
3. Planned Analyses
3.1 Study Hypotheses
We hypothesize that, compared to people in the control group, participants in the experimental intervention group will:
Be more likely to quit smoking as evidenced by 7 day PPA rates at 6 month follow-up (primary outcome) and at 2 month follow-up (secondary outcome).
Be more likely to see a dental care professional between enrollment and the 6 month follow-up (primary outcome), or at least be more likely to have either completed a dental care visit or scheduled a future appointment (secondary outcome).
Exhibit more positive change in oral health knowledge, beliefs and attitudes (e.g., self-efficacy, motivation) that could influence future behavior change (secondary outcomes).
We expect that positive change in participants’ knowledge, attitudes, and beliefs at 2 months will mediate positive change in the primary outcome behaviors at 6 months; and that the Oral Health 4 Life program will be acceptable to quitline callers. We will also explore change in self-reported oral health behaviors (e.g., daily brushing, flossing). We will use a 0.05 significance level for each of the two primary outcomes and consider the intervention to be a success if the comparison for either primary outcome is significant at 6 months and that neither outcome indicates the intervention group to be inferior. Comparisons of outcomes at 2 months will be considered secondary. Analyses of both outcomes at all follow-up times will be reported, imposing a more stringent requirement than simply reporting a sole significant outcome.[37]
3.2 Analytic samples
At the request of our DSMB, main outcomes will be analyzed using two analytic samples. One will include all 737 initially enrolled participants, regardless of study eligibility or study retention. Since no follow-up data is being collected on 19 individuals who were ineligible and mistakenly enrolled (see discussion in section 2.5), primary outcome data for these individuals will be treated as missing data. The second analytic sample (n = 718) will be limited to those individuals who were eligible to participate and included in the study. Analytic findings based on this second analytic sample will be considered the primary study results, but we will present the results from both analyses if results for the primary outcomes differ between the two samples. However, since missing data will be imputed as smokers and non-utilizers of dental care, and the 19 individuals not included in the primary analytic sample were equitably distributed across groups (9 vs. 10), we do not anticipate their exclusion will alter the primary findings. All analyses will use an ITT methodology such that subjects are analyzed according to their assigned treatment group, regardless of noncompliance, protocol deviation or withdrawal from treatment utilization.
3.3 Primary and secondary analyses
To assess the difference between the experimental and control groups for smoking abstinence, the regression model will include data from both follow-up time points in a single model to improve power and adjust for possible correlation within individuals using generalized estimating equations.[38] To assess differences in dental care utilization, we will compute whether participants saw a dentist post-enrollment using data from each follow-up, then run a single logistic regression model. We will specify an independent working correlation structure and estimate robust standard errors to avoid making model assumptions about the variance. Models will use a logit link function for the binary primary outcomes and odds ratios will be used to quantify differences between groups. To improve precision and power, analyses will be adjusted for the following pre-specified baseline characteristics that are expected, based on prior knowledge, to be important predictors of outcome or missing data: sex, age, pharmacotherapy use (e.g., NRT), baseline cigarettes per day, depression history, self-efficacy, and motivation for quitting. Models will also adjust for variables used to stratify randomization (dental insurance coverage and state quitline).
To estimate the effects of the intervention on secondary outcomes (e.g., completing or scheduling a dental appointment, brushing, self-efficacy, motivation, etc.), we will apply similar regression models as described for the primary outcomes, using appropriate link functions dependent on outcome type, and further adjust for baseline outcome value when appropriate.
To handle missing follow-up data for the primary outcomes we will assume that missing subjects are smokers and are non-users of dental service. In sensitivity analyses, we will use modern imputation methods to impute missing outcome data, based on the observed pattern of missingness.[39] We also will conduct a respondent only missing data sensitivity analysis that only includes subjects with observed outcome data (i.e., a complete case analysis).
3.4 Mediation Analyses
If the experimental intervention is more effective than control for either primary outcome at 6 months, we will then conduct mediator analyses to assess whether relevant secondary outcomes measured at 2 months (self-efficacy, motivation, knowledge,) mediate at least part of this effect. Mediation will be explored separately for each significant main outcome. These analyses will be conducted using the framework recommended by Baron and Kenny [40], but using more recent statistical methods developed to better quantify and decompose different aspects of the mediation effect.[41]
3.5 Process Analyses
Summary statistics will be used to characterize quantitative acceptability ratings and scores. T-tests will be used to compare groups on continuous ratings and chi-squares for proportions. Open-ended qualitative responses will be reviewed by investigators and may be transcribed and coded thematically, as needed in order to provide insight into the participants’ satisfaction with the intervention programs and their components.
3.6 Economic Analyses
We will calculate the incremental cost of adding the OH4L program, from the perspective of a future payer such as a state contractor. If warranted based on the outcomes, we will produce a decision support tool that will allow states to determine whether adding the OH4L program makes economic sense to state quitlines in light of the treatment effects on cessation and oral health care utilization. The decision support tool will have three components. The first is a detailed model of the cost associated with the oral health program, using activity accounting methods.[42] We will use micro-costing to assign unit costs to every physical and human resource required to deliver the program (e.g., training time, supplies, additional time spent per call). The second component of the decision tool is the probability of success for each positive outcome (cessation, dental visit, both) associated with a quitline program incorporating OH4L. This information will be based on trial results and we will incorporate uncertainty by using confidence intervals derived from the analyses of our trial data as described above. The third component will be a user entered factor based on their willingness to pay so that a potential sponsor of OH4L can assess, based on alternative assumptions about cost, programmatic success and willingness to pay, whether investing in the OH4L program makes financial sense to them. Each quitline purchaser user will have the ability to vary each model parameter to determine the circumstances under which they would be willing to invest in purchasing the OH4L program.
3.7 Sample Size Considerations
This is the first study to examine whether an integrated oral health promotion-smoking cessation program delivered through tobacco quitlines will increase abstinence rates or dental care utilization, so the potential effect sizes of the intervention are unknown. There is no prior literature to guide expectations. Given this, we chose a sample size that would provide sufficient power to detect what would be clinically meaningful differences for either primary outcome. A sample size of n=718 has 90% power to detect a 9% difference in smoking abstinence (12% control vs 21% intervention) and a 6.7% difference in dental care utilization (5% vs. 11.7%); we have 80% power to detect a difference of 7.6% in smoking abstinence and 5.6% in dental care utilization.
Lesser effect sizes could be statistically significant, but since the goal of this work is to inform public health practice and policy, we sought to identify differences which would provide clear and unambiguous evidence that the effects warrant future dissemination. For example, with an appropriate sample size, differences of 2%–3% are statistically significant, but based on our discussions with Quitline service providers and their contractors (e.g., state departments of health), it would be difficult to argue for adoption of the OH4L program if the observed effects were this small. More substantive differences such as can be detected in the current study, would provide strong support for the program adoption. In fact, increasing smoking cessation or treatment utilization by ~6% or greater in this high risk population could have an important public health impact given the broad reach of the tobacco quitlines (impact = reach x effectiveness) [43].
Power estimates assume a two-sided alpha=0.05 Wald Z test statistic for a difference between proportions using a normal approximation with pooled variance [44]. We assumed a 7-day PPA rate of 12% based on observed data from the target quitlines (range 11%–12.5%), which is also consistent with estimated abstinence rates of tobacco quitlines based on a meta analysis of the literature (average cessation rate at 6-months 12.7% (95% CI 11.3–14.2)). Although no participants will have seen a dentist in the past 6 months at enrollment as a condition of eligibility, it is possible a few will seek dental care over the course of the study. Thus, we assume a rate of 5% for this outcome in the control group at 6-month follow-up. Sample sizes used in the power analysis are based on complete case follow-up which will occur by design for all primary outcome analyses since we will be imputing missing values for the primary outcomes (missing cases will be assumed to be smokers and to not have visited the dentist).
4. Summary and Discussion
This manuscript provides an overview of the rationale, design, and methods for the OH4L study, a semi-pragmatic randomized clinical trial. This protocol may be useful to other researchers interested in promoting oral health among smokers, those interested in partnering with tobacco quitlines to extend standard services to address other high risk health behaviors among smokers, or those interested in semi-pragmatic trial design.
In addition to detailing our study methods, our experience speaks to some of the real world challenges of conducting semi-pragmatic trials within state quitline services. For example, service eligibility can change over time based on state’s financial ability to provide this care. When resources are limited, eligibility criteria can tighten unexpectedly, limiting the pool of available participants. This affected one of our three state quitlines during the one year recruitment period. Similarly, we had originally planned to work with a fourth state quitline, but were forced to drop this plan prior to fielding the study when the state unexpectedly suspended their multi-call quit program due to limited financial resources. Another challenge we encountered was a lack of control over the implementation and timing of system changes, such as reprogramming AW systems to automatically screen out ineligible callers when changes were made to the service eligibility criteria in one state. As a result of this, we enrolled 12 people who were not eligible for this study and subsequently were removed from the trial. If the programming changes could have been implemented quickly, this error would not have happened, but when working with real world service providers, researchers must accept less experimental control.
Despite this and the other inherent limitations of pragmatic trials, we contend that this type of embedded research is important. The trade-off for relinquishing some experimental control is that interventions are tested under real-world conditions, resulting in a more valid assessment of their effectiveness if they are disseminated. Because OH4L was designed to be disseminated through quitlines and is being evaluated in the context of three state quitlines, the study design provides greater confidence that the observed results will be reproducible. Thus, the results of this study will not only inform the effectiveness of the OH4L intervention, it will allow interested states to make informed decisions about future adoption of this program. Study results are not yet available, but will be presented following completion of this trial.
Acknowledgments
This research is supported by the National Institute for Dental and Craniofacial Research (NIDCR; U01DE024462). NIDCR program staff were involved in the design of this trial, but were not directly involved in the preparation of this manuscript. We are grateful to the contributions of the many study team members at KPWHRI, AW, and UCD who are making this trial possible.
This includes the KPWHRI Survey Research Program and Ellen Schartz for follow-up data collection; Mary Shea for mailing study incentives and materials; Eric Baldwin for overseeing data management and participant tracking systems at KPWHRI; Andrew Baer and DT Tran for oversight of the OH4L website; Deborah King and Paula Blasi for fidelity monitoring; Erica Salmon and Mark Campbell for overseeing operations at AW; Mona Deprey and Sue Zbikowski, PhD for their assistance implementing this study at AW; and Annie Shaffer for her assistance with the manuscript preparation. We also thank our DSMB and NIDCR program staff for ongoing oversight of this trial. Finally, we thank all of the people who graciously agreed to participate in this study.
Abbreviations
- OH4L
Oral Health for Life
- QD
quit date
- CBT
cognitive behavioral therapy
- PPA
point prevalent abstinence
- NRT
nicotine replacement therapy
- KPWHRI
Kaiser Permanente Washington Health Research Institute
- AW
Alere Wellbeing
- UCD
University of California, Davis
- DSMB
Data and Safety Monitoring Board
- NIDCR
National Institute of Dental and Craniofacial Research
Footnotes
Conflicts of Interest
Dr. Bush is employed by Alere Wellbeing, a subsidiary of Optum. Alere is the leading provider of state-supported tobacco quitlines in the U.S. All other authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Disease Control and Prevention. Oral health: Preventing cavities, gum disease, tooth loss, and oral cancer--At a glance 2010. Atlanta, GA: Division of Oral Health, National Center for Chronic Disease Prevention and Health Promotion; 2010. [Google Scholar]
- 2.US Department of Health and Human Services. Oral Health in America: A Report of the Surgeon General. Rockville, MD: US Department of Health and Human Services, National Institute of Dental and Craniofacial Research, National Institutes of Health; 2000. [Google Scholar]
- 3.Drilea SK, Reid BC, Li CH, Hyman JJ, Manski RJ. Dental visits among smoking and nonsmoking US adults in 2000. Am J Health Behav. 2005;29:462–71. doi: 10.5555/ajhb.2005.29.5.462. [DOI] [PubMed] [Google Scholar]
- 4.Mucci LA, Brooks DR. Lower use of dental services among long term cigarette smokers. J Epidemiol Community Health. 2001;55:389–93. doi: 10.1136/jech.55.6.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McClure JB, Riggs KR, St John J, Cerutti B, Zibikowski S. Understanding oral health promotion needs and opportunities among tobacco quitline callers. Public Health Rep. 2012;127:401–6. doi: 10.1177/003335491212700408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McClure JB, Divine G, Alexander G, Tolsma D, Rolnick SJ, Stopponi M, et al. A Comparison of Smokers’ and Nonsmokers’ Fruit and Vegetable Intake and Relevant Psychosocial Factors. Behav Med. 2009;35:14–22. doi: 10.3200/BMED.35.1.14-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Difranza JR, Guerrera MP. Alcoholism and smoking. J Stud Alcohol. 1990;51:130–5. doi: 10.15288/jsa.1990.51.130. [DOI] [PubMed] [Google Scholar]
- 8.Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States - Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61:1107–15. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- 9.Falk DE, Yi HY, Hiller-Sturmhofel S. An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders - Findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Res Health. 2006;29:162–71. [PMC free article] [PubMed] [Google Scholar]
- 10.Kahler CW, Strong DR, Papandonatos GD, Colby SM, Clark MA, Boergers J, et al. Cigarette smoking and the lifetime alcohol involvement continuum. Drug Alcohol Depend. 2008;93:111–20. doi: 10.1016/j.drugalcdep.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watt RG. Emerging theories into the social determinants of health: implications for oral health promotion. Community Dentist Oral Epidemiol. 2002;30:241–7. doi: 10.1034/j.1600-0528.2002.300401.x. [DOI] [PubMed] [Google Scholar]
- 12.Renz A, Ide M, Newton T, Robinson P, Smith D. Psychological interventions to improve adherence to oral hygiene instructions in adults with periodontal diseases. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD005097.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Yevlahova D, Satur J. Models for individual oral health promotion and their effectiveness: A systematic review. Aust Dent J. 2009;54:190–7. doi: 10.1111/j.1834-7819.2009.01118.x. [DOI] [PubMed] [Google Scholar]
- 14.Satur JG, Gussy MG, Morgan MV, Calache H, Wright C. Review of the evidence for oral health promotion effectiveness. Health Education Journal. 2010;69:257–66. [Google Scholar]
- 15.Watt R, Fuller S, Harnett R, Treasure E, Stillman-Lowe C. Oral health promotion evaluation - time for development. Community Dentist Oral Epidemiol. 2001;29:161–6. doi: 10.1034/j.1600-0528.2001.290301.x. [DOI] [PubMed] [Google Scholar]
- 16.Newton JT. Psychological models of behaviour change and oral hygiene behaviour in individuals with periodontitis: a call for more and better trials of interventions. J Clin Periodontol. 2010;37:910–1. doi: 10.1111/j.1600-051X.2010.01591.x. [DOI] [PubMed] [Google Scholar]
- 17.Zbikowski SM, Hapgood J, Smucker Barnwell S, McAfee T. Phone and web-based tobacco cessation treatment: real-world utilization patterns and outcomes for 11,000 tobacco users. J Med Internet Res. 2008;10:e41. doi: 10.2196/jmir.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu SH, Anderson CM, Tedeschi GJ, Rosbrook B, Johnson CE, Byrd M, et al. Evidence of real-world effectiveness of a telephone quitline for smokers. N Engl J Med. 2002;347:1087–93. doi: 10.1056/NEJMsa020660. [DOI] [PubMed] [Google Scholar]
- 19.Orleans CT, Schoenbach VJ, Wagner EH, Quade D, Salmon MA, Pearson DC, et al. Self-help quit smoking interventions: effects of self-help materials, social support instructions, and telephone counseling. J Consult Clin Psychol. 1991;59:439–48. doi: 10.1037//0022-006x.59.3.439. [DOI] [PubMed] [Google Scholar]
- 20.Swan GE, McClure JB, Jack LM, Zbikowski SM, Javitz HS, Catz SL, et al. Behavioral counseling and varenicline treatment for smoking cessation. Am J Prev Med. 2010;38:482–90. doi: 10.1016/j.amepre.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.North American Quitline Consortium. A Promising Practices Report. Quitlines and Priority Populations: An Update on Our Progress to Reach and Serve Those Most Impacted by Tobacco’s Harm. 2016 [updated 2016; cited 2016 October 26]. Available from: http://c.ymcdn.com/sites/www.naquitline.org/resource/resmgr/links/QuitlinesandPriorityPopulati.pdf.
- 22.McClure JB, Riggs K, St John J, Catz SL. [More] evidence to support oral health promotion services targeted to smokers calling tobacco quitlines in the United States. BMC Public Health. 2013;13:336. doi: 10.1186/1471-2458-13-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McClure JB. Leveraging the US tobacco quitline infrastructure to promote oral health: feedback from key stakeholders. Prev Med. 2014;60:134–5. doi: 10.1016/j.ypmed.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ. 2015;350:h2147. doi: 10.1136/bmj.h2147. [DOI] [PubMed] [Google Scholar]
- 25.Ford I, Norrie J. Pragmatic Trials. N Engl J Med. 2016;375:454–63. doi: 10.1056/NEJMra1510059. [DOI] [PubMed] [Google Scholar]
- 26.Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Clinical practice guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service; 2008. Treating tobacco use and dependence: 2008 update. [Google Scholar]
- 27.Bandura A. Human agency in social cognitive theory. Am Psychol. 1989;44:1175–84. doi: 10.1037/0003-066x.44.9.1175. [DOI] [PubMed] [Google Scholar]
- 28.Miller WR. Motivational interviewing: research, practice, and puzzles. Addict Behav. 1996;21:835–42. doi: 10.1016/0306-4603(96)00044-5. [DOI] [PubMed] [Google Scholar]
- 29.Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3. New York: Guilford Press; 2012. [Google Scholar]
- 30.Kahneman D, Tversky A. Prospect theory: An analysis of decsion under risk. Econometrica. 1979:47. [Google Scholar]
- 31.O’Keefe DJ, Jensen JD. The relative persuasiveness of gain-framed and loss-framed messages for encouraging disease prevention behaviors: a meta-analytic review. J Health Commun. 2007;12:623–44. doi: 10.1080/10810730701615198. [DOI] [PubMed] [Google Scholar]
- 32.SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–59. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention. National Health Interview Survey. 2017 [updated 2017; cited 2017 March 29]. Available from: https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm.
- 34.Jones EE, Sigall H. The bogus pipeline: A new paradigm for measuring affect and attitude. Psychol Bull. 1971;76:349–64. [Google Scholar]
- 35.Brennan D, Spencer J, Roberts-Thomson K. Dental knowledge and oral health among middle-aged adults. Australian and New Zealand Journal of Public Health. 2010;34:472–5. doi: 10.1111/j.1753-6405.2010.00592.x. [DOI] [PubMed] [Google Scholar]
- 36.Humphris GM, Morrison T, Lindsay SJ. The Modified Dental Anxiety Scale: validation and United Kingdom norms. Community Dent Health. 1995;12:143–50. [PubMed] [Google Scholar]
- 37.Li QH. Biometrical Journal. 2009. Evaluating co-primary endpoints collectively in clinical trials; p. 51. [DOI] [PubMed] [Google Scholar]
- 38.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- 39.Enders CK. Applied Missing Data Analyses. New York: Guilford Press; 2010. [Google Scholar]
- 40.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 41.VanderWeele TJ. Marginal structural models for the estimation of direct and indirect effects. Epidemiology. 2009;20:18–26. doi: 10.1097/EDE.0b013e31818f69ce. [DOI] [PubMed] [Google Scholar]
- 42.Frick KD. Microcosting Quantity Data Collection Methods. Med Care. 2009;47:S76–S81. doi: 10.1097/MLR.0b013e31819bc064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abrams DB, Orleans CT, Niaura RS, Goldstein MG, Prochaska JO, Velicer W. Integrating individual and public health perspectives for treatment of tobacco dependence under managed health care: a combined stepped-care and matching model. Ann Behav Med. 1996;18:290–304. doi: 10.1007/BF02895291. [DOI] [PubMed] [Google Scholar]
- 44.D’Agostino RB, Chase W, Belanger A. The appropriateness of some common procedures for testing the equality of two independent binomial populations. The American Statistician. 1988;42:198–202. [Google Scholar]