Abstract
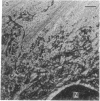
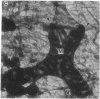
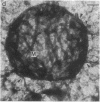
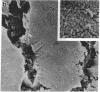
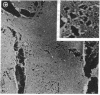
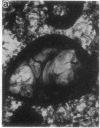
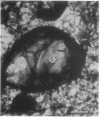
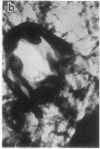
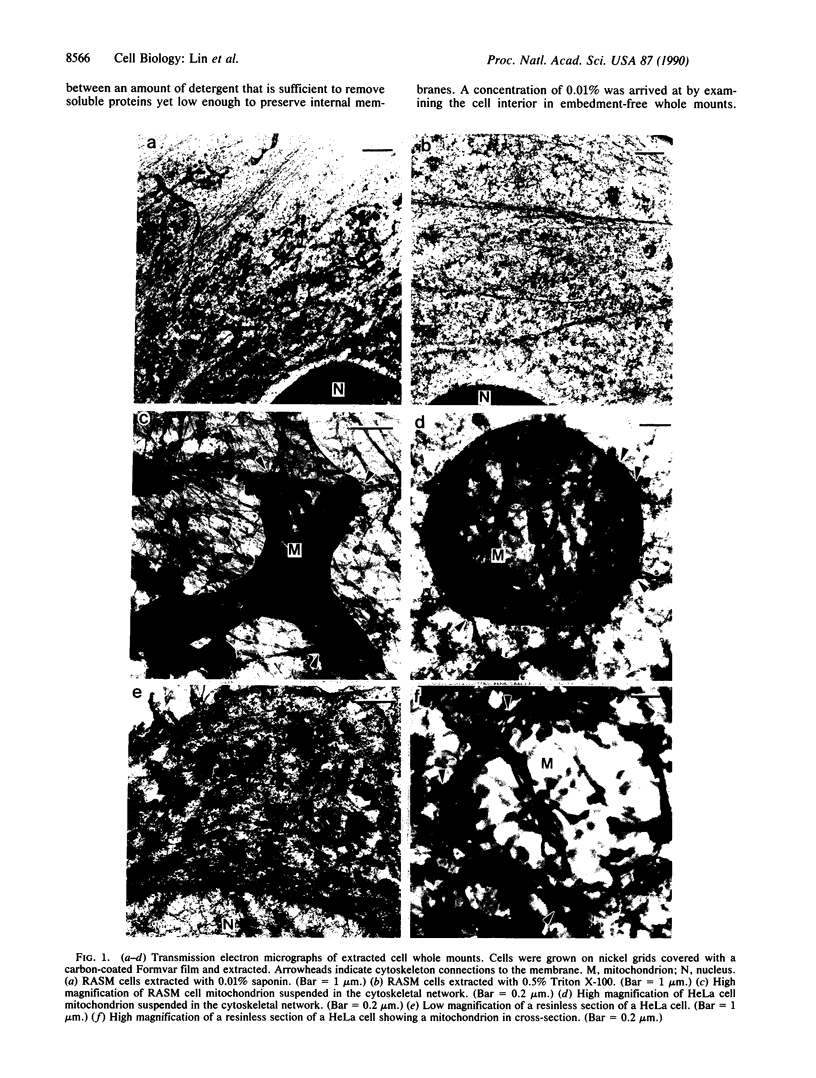
Embedment-free electron microscopy images the cytoskeleton and nuclear matrix, which are very difficult to visualize in conventional electron micrographs. However, to be effective, cell structures must be depleted of soluble proteins, which otherwise shroud cell architecture. Nonionic detergents effect this extraction, releasing soluble proteins but also destroying all membranes. Saponin can permeabilize plasma membranes, releasing soluble proteins while preserving many cytoplasmic membranes. Stereoscopic electron microscopy of resinless sections shows the many connections of the cytoskeleton to mitochondrial membranes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariano M. A., Matus A. I. Ultrastructural localization of cyclic GMP and cyclic AMP in rat striatum. J Cell Biol. 1981 Oct;91(1):287–292. doi: 10.1083/jcb.91.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANGHAM A. D., HORNE R. W., GLAUERT A. M., DINGLE J. T., LUCY J. A. Action of saponin on biological cell membranes. Nature. 1962 Dec 8;196:952–955. doi: 10.1038/196952a0. [DOI] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Duerr A., Solomon F., Penman S. The outer boundary of the cytoskeleton: a lamina derived from plasma membrane proteins. Cell. 1979 Aug;17(4):859–865. doi: 10.1016/0092-8674(79)90326-x. [DOI] [PubMed] [Google Scholar]
- Capco D. G., Krochmalnic G., Penman S. A new method of preparing embeddment-free sections for transmission electron microscopy: applications to the cytoskeletal framework and other three-dimensional networks. J Cell Biol. 1984 May;98(5):1878–1885. doi: 10.1083/jcb.98.5.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey E. G., Krochmalnic G., Penman S. The nonchromatin substructures of the nucleus: the ribonucleoprotein (RNP)-containing and RNP-depleted matrices analyzed by sequential fractionation and resinless section electron microscopy. J Cell Biol. 1986 May;102(5):1654–1665. doi: 10.1083/jcb.102.5.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh H., Takenaka T., Shozushima M. The fibrillar network in the cytoplasm of squid giant axon extracted with saponin. Cell Struct Funct. 1983 Mar;8(1):11–18. doi: 10.1247/csf.8.11. [DOI] [PubMed] [Google Scholar]
- He D. C., Nickerson J. A., Penman S. Core filaments of the nuclear matrix. J Cell Biol. 1990 Mar;110(3):569–580. doi: 10.1083/jcb.110.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Kirschner M. W. Filament organization revealed in platinum replicas of freeze-dried cytoskeletons. J Cell Biol. 1980 Jul;86(1):212–234. doi: 10.1083/jcb.86.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii M., Miyazaki Y., Otsuki M., Suzuki H., Goto Y. The intermediate filaments in human hepatocytes. Tohoku J Exp Med. 1985 Dec;147(4):317–329. doi: 10.1620/tjem.147.317. [DOI] [PubMed] [Google Scholar]
- Jackson D. A., Cook P. R. Visualization of a filamentous nucleoskeleton with a 23 nm axial repeat. EMBO J. 1988 Dec 1;7(12):3667–3677. doi: 10.1002/j.1460-2075.1988.tb03248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsumoto T., Kurimura T. Ultrastructural localization of concanavalin A receptors in the plasma membrane: association with underlying actin filaments. Biol Cell. 1988;62(1):1–10. [PubMed] [Google Scholar]
- Lenk R., Penman S. The cytoskeletal framework and poliovirus metabolism. Cell. 1979 Feb;16(2):289–301. doi: 10.1016/0092-8674(79)90006-0. [DOI] [PubMed] [Google Scholar]
- Lenk R., Ransom L., Kaufmann Y., Penman S. A cytoskeletal structure with associated polyribosomes obtained from HeLa cells. Cell. 1977 Jan;10(1):67–78. doi: 10.1016/0092-8674(77)90141-6. [DOI] [PubMed] [Google Scholar]
- Maupin P., Pollard T. D. Improved preservation and staining of HeLa cell actin filaments, clathrin-coated membranes, and other cytoplasmic structures by tannic acid-glutaraldehyde-saponin fixation. J Cell Biol. 1983 Jan;96(1):51–62. doi: 10.1083/jcb.96.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter K. R., Stearns M. E. Stereomicroscopy of whole cells. Methods Cell Biol. 1981;22:53–75. doi: 10.1016/s0091-679x(08)61870-1. [DOI] [PubMed] [Google Scholar]
- Porter K. R. The cytomatrix: a short history of its study. J Cell Biol. 1984 Jul;99(1 Pt 2):3s–12s. doi: 10.1083/jcb.99.1.3s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter K. R., Wolosewick J. J. Application of high voltage electron microscopy in biological sciences. J Electron Microsc (Tokyo) 1977;26 (Suppl):15–20. [PubMed] [Google Scholar]
- Schliwa M. Action of cytochalasin D on cytoskeletal networks. J Cell Biol. 1982 Jan;92(1):79–91. doi: 10.1083/jcb.92.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliwa M., van Blerkom J. Structural interaction of cytoskeletal components. J Cell Biol. 1981 Jul;90(1):222–235. doi: 10.1083/jcb.90.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John P. A., Froehner S. C., Goodenough D. A., Cohen J. B. Nicotinic postsynaptic membranes from Torpedo: sidedness, permeability to macromolecules, and topography of major polypeptides. J Cell Biol. 1982 Feb;92(2):333–342. doi: 10.1083/jcb.92.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Yamada S. S., Pastan I. Ultrastructural antibody localization of alpha2-macroglobulin in membrane-limited vesicles in cultured cells. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4359–4363. doi: 10.1073/pnas.75.9.4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosewick J. J. The application of polyethylene glycol (PEG) to electron microscopy. J Cell Biol. 1980 Aug;86(2):675–661. doi: 10.1083/jcb.86.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]