Abstract
Purpose
To determine the therapeutic efficacy of a novel rare sugar, L-psicose, for the treatment of HSV-1 induced herpetic stromal keratitis (HSK) in a mouse eye model.
Methods
One rare sugar L-psicose was assayed for HSV-1 inhibition of in vitro virus adsorption. The IC50 and IC90 values of L-psicose were determined using plaque reduction assay (PRA) in CV-1 cell. Female Balb/c mice were corneally infected with HSV-1, strain KOS-GFP; A topical eye drop treatment of L-psicose was started 24 h after infection and continued four times daily for ten consecutive days. The severity of HSK was monitored by slit lamp examination in a masked fashion and Infectious HSV-1 shedding was determined by PRA.
Results
L-psicose was found to have anti-viral activity in vitro at an IC50 dose of 99.5 mM and an IC90 dose of 160 mM. Topical eye drop treatment with 200 mM L-psicose in PBS solution significantly reduced the severity of HSK compared to the mock treatment group. The in vivo mouse ocular model results of L-psicose therapy correlated with accelerated clearance of virus from eye swabs.
Conclusion
The results suggest that topical treatment with rare sugar L-psicose has efficacy against HSK through inhibition of HSV-1.
Keywords: Rare sugar, Herpes simplex keratitis, Eye drop, L-psicose, HSV-1
1. Introduction
Monosaccharides are essential component of cell surface receptors and play a very important role in cell signaling pathways (1). As a result, carbohydrates that can disrupt binding of molecules to the cell surface receptors or interrupt cell-signaling pathways may have important therapeutic potentials (2).
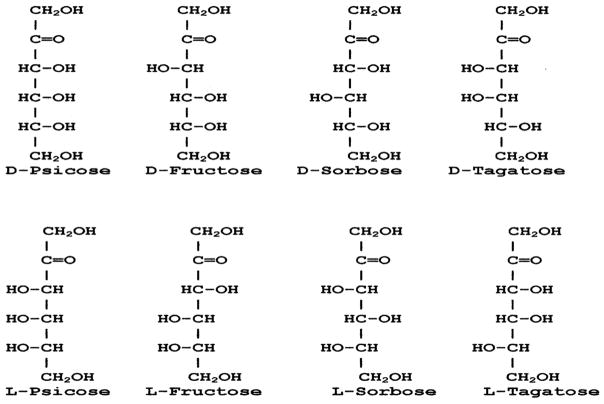
On the basis of availability we can classify monosaccharide into two groups, natural and rare. According to International Society of Rare Sugars (ISRS) rare sugars are monosaccharides and their derivatives that rarely exist in the nature. The practical application or usefulness of rare carbohydrates has not been well investigated because of high costs and unavailability. However, because of the recent introduction of “Izumoring” schemes (3) it is now possible to produce many of these rare monosaccharides in large quantities by using microbial and enzymatic transformations. As a result, functional studies of rare sugars have gained momentum in recent years. Utilization of rare sugars for various purposes still awaits extensive exploration. Despite their costs and low availability, these rare carbohydrates are very important since they have the potential for use in many areas such as food additives, diet sugars, antioxidants, antiviral agents, nucleoside analogs, glycosidase inhibitors, and anticancer agents (4–11). In a previous study Muniruzzaman et al found that some simple rare ketoses (L-Xylulose and L-Fructose) were specific inhibitors of alpha-glucosidase and glucosidase in vitro and in vivo (4). This study also reported that some rare sugars inhibited the removal of glucoses from N-linked oligosaccharides of the influenza viral hemagglutinin in the cell culture systems. Activities of glucosidases are required for the right processing of glycoproteins so that correct folding and sorting can be maintained (12). Our knowledge about the physiological effects of rare carbohydrates is very limited. In a previous study, since some rare keto-sugars appear to be inhibitory to glycoprotein processing enzymes (4), it was of interest to screen ketohexoses to determine whether any of them would inhibit the entry of HSV1 to cell. Among the eight ketohexoses D-tagatose, L-tagatose, D-psicose, L-psicose, D-sorbose and L-fructose are considered to be rare as they are found in nature very low quantities. We screened all these ketohexoses (Fig. 1) in vitro for their anti-HSV-1 activity and found L-psicose to have relatively strong activity. In the present study, we show that L-psicose is effective in inhibiting HSV-1 in vitro. The mechanism of HSV-1 inhibition by L-psicose is mediated through blocking of viral adsorption to cell surface. We also show that in vivo, L-psicose is effective in inhibiting HSV-1 induced corneal opacity and ocular virus shedding in mouse eye model.
Fig. 1.
Structure of eight ketohexoses screened in this study for anti-HSV-1 activity.
2. Materials and methods
2.1. Antiviral compounds
All ketohexoses used in this study was produced in Dr. Izomuri’s lab. Anti-viral peptide apoEdp was commercially synthesized (GeneMed, Texas, US) and used as described before (13). ApoEdp is a short 18 aa peptide derived from receptor binding region of human apolipoprotein E effectively inhibits HSV-1 adsorption in vitro and herpetic stromal keratitis (HSK) in vivo (13).
2.2. Cells, viruses and treatment
CV-1 cells (African green monkey kidney cells) were purchased from American Type Culture Collection, Manassas, VA) were propagated in Eagle’s minimum essential medium (EMEM) containing 0.15% Na2CO3 supplemented with 10% fetal bovine serum (FBS), penicillin G (100 U/ml), and streptomycin (100 mg/ml). HSV-1 strain KOS-GFP (gift from Timothy P Foster, LSU Health Sciences Center, New Orleans, LA, USA) was used and titrated in CV-1 cells.
2.3. Virus adsorption assay
CV-1 cells prepared with a final density of 4 × 106 cells/mL added with HSV-1 KOS-GFP at 1 PFU (Plaque forming Units)/cell and varying concentrations of L-psicose was incubated at 4 °C for 1 h to allow adsorption but to prevent penetration. Following adsorption, unbound virus and sugar was removed by washing three times with cold PBS (4 °C). Cell pellets were serially diluted and plated on 24-well monolayer of CV-1 cells with an overlay of 0.5% methylcellulose. Following 48 h of incubation at 37 °C, cells were then fixed with 5% buffered formalin (5% formalin in PBS) and stained with 0.05% crystal violet. Positive control well did contain apoEdp peptide and performed in parallel. The number of HSV-1 infected plaques was counted for each concentration of L-psicose or peptide and for no drug controls. The inhibitory effect of the L-psicose on plaque reduction was calculated by following formula. Percentage inhibition (number) = (1 – plaque number in antiviral well/plaque number in no drug control well) × 100. The 50% and 90% dose of inhibitory concentration was hand calculated from the dose response curves generated from the data (14). For each experiment, three independent replicates were performed.
2.4. Mice
For the animal experiment, Xavier University of Louisiana Institutional Animal Care and Use Committee approved procedure was followed. We used female C57BL/6 (Taconic Farms, NY) from 5 to 6 weeks of age.
2.5. Ocular infection
Mice were anesthetized using a mixture of Xylazine (6.6 mg/kg of body weight) and Ketamine (100 mg/kg) through intraperitoneal route of injection. Scarification of eyes was performed in a 2X2 cross-hatch pattern and then 4 μL of virus was inoculated in each eye containing 5 × 105 PFU of virus/inoculum.
2.6. Quantification of viral titer from eye swabs
Eye swabs were collected to perform plaque assays to quantify the virus using CV-1 as indicator cell. Sterile filter paper strips were used as eye swabs and mixed with I ml of cold EMEM containing 10% FBS. Serial dilution of eye swabs was done before adding on CV-1 cell monolayer and incubated at 37 °C for 1 h for virus adsorption. Following adsorption, medium was aspirated and fresh EMEM was added to each well. After 2 days, viral plaques were counted in each well for quantification of PFU/mL.
2.7. Treatment groups
Safety and toxicity of L-psicose was done in the eyes of naïve mice. The 200 mM concentration of L-psicose was well tolerated without any corneal toxicity when applied five times a day for ten consecutive days. Corneal opacity and neovascularization were evaluated as a measure of toxicity. One day after corneal infection with virus, mice were tropically treated with 4 μL of 200 mM L-psicose in each eye. Placebo was applied with PBS for mock-treatment groups.
2.8. Slit lamp examination and quantitation of corneal opacity and neovascularization
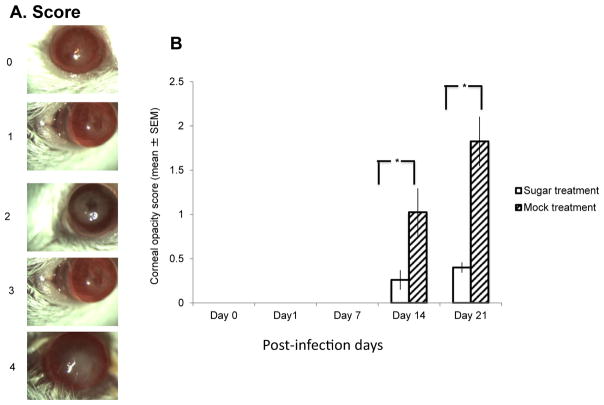
Mice eyes were examined under a slit lamp microscope (Eye Cap; Haag-Streit International, Mason OH, USA) in a masked fashion. Severity of the HSK infection was determined by measuring corneal opacity (13). Corneal opacity was evaluated on a parameter as follows: 0, no opacity; 1, mild cloudiness with visible iris; 2, moderate cloudiness with obscured iris; 3, total corneal cloudiness with invisible iris; 4, total opacity with no posterior view.
2.9. Statistics
The potentiality of the sugar and peptide for treatment against HSV-1 induced corneal opacity and HSV-1 titer in the eyes were determined by the mean ± SEM and considered significant at P values <0.05, calculated with students t-test.
3. Results
3.1. L-Psicose inhibits HSV-1 adsorption in CV-1 cells
Effect of L-psicose against HSV-1 adsorption was evaluated in CV-1 cells. The IC50 and IC90 dose of inhibition was calculated as described by others (14). L-psicose was found to have anti-HSV-1 activity at an IC50 dose of 99.51 mM and IC90 dose of 166.48 mM (Table 1). As a positive control drug, we used a peptide (apoEdp) previously reported by us to have peptidomimetic blocking effect on HSV-1 adsorption (13).
Table 1.
L-psicose inhibits HSV-1 adsorption to CV-1 cell.
| Methods | Criteria | L-psicose(mM) | ApoEdp peptide (mM) |
|---|---|---|---|
| Adsorption | IC50 | 99.51 | 0.4 |
| IC90 | 166.48 | 0.55 |
3.2. Eye drop treatment of L-psicose inhibits corneal opacity induced by HSV-1
Starting at day 1 post-infection, topical eye drop treatment of 200 mM L-psicose applied 4 μL per eye, 4 times a day for 10 consecutive days. Corneal opacity was measured by slit lamp examination and the severity was graded as described in the materials and methods. Corneal opacity was not evident until day 14 post-infection. As evident in Fig. 2, L-psicose treatment resulted significant (p < 0.05) inhibition of corneal opacity compared to mock treated eyes.
Fig. 2.
Female C57Bl/6 mice were corneally infected with HSV-1 KOS-GFP. Topical eye drop treatment with 200 mM solution of L-psicose in PBS started next day after corneal inoculation of virus. Eye drops applied four times daily for ten consecutive days. Mock treatment was done by using PBS as eye drop. (A.) Representative photographs of corneal opacity examined with slit lamp microscope and graded from 0 to 4 as described in materials and methods. Corneal opacity was seen only at days 14 and 21. L-psicose treatment significantly (*P < 0.05) inhibited the severity of corneal opacity compared to mock-treated eyes. Ten mice were used per experimental group. Each mouse was treated in their both eyes with sugar eye drop or mock-treated in three independent experiments. Results are expressed as the mean ± SEM and are considered significant, with *P < 0.05 determined by Student’s t-test.
3.3. Eye drop treatment of L-psicose inhibits infectious HSV-1 shedding
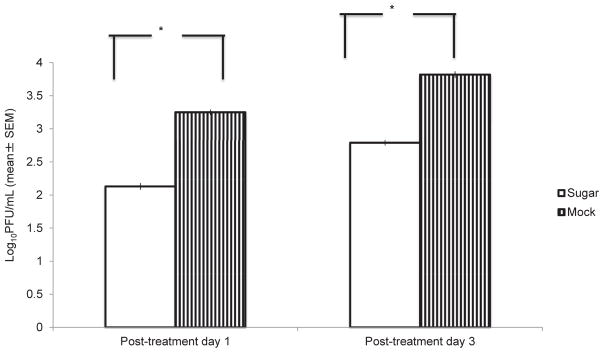
Ocular swabs were assayed to determine the antiviral effect of L-psicose eye drop treatment. Infectious virus shedding was calculated by determining PFU/mL. Shedding of infectious virus from L-psicose treated eyes were found significantly (p < 0.05) reduced compared to mock-treated (Fig. 3).
Fig. 3.
Eye swabs collected at day 1 and 3 following L-psicose treatment used for PRA in monolayer of CV-1 cells. The 200 mM concentration of L-psicose as eye drop treatment significantly inhibited infectious virus shedding compared to mock treatment. Ten mice were used per experimental group. Each mouse was treated in their both eyes with sugar eye drop or mock-treated in three independent experiments. Results are expressed as the mean ± SEM and are considered significant, with *P < 0.05 determined by Student’s t-test.
4. Discussion
Current drug discovery efforts for HSV focus primarily on nucleoside based inhibition of viral genome synthesis. However, drug-resistant mutations in the viral genome can arise during therapy and, subsequently, reduce the effectiveness of specific viral enzyme inhibitors. Unfortunately, incorporation of anti-metabolite (nucleoside analog) often reported to get incorporated into DNA of normal host cells that may lead to the development of toxicity during topical and systemic therapy (15). Herpes infections continue to be prevalent and resistance to nucleoside analogs has been reported (16, 17), especially in immunocompromised transplant recipients and AIDS patients. Therefore, it is important to explore new treatment options with alternative mechanisms of antiviral action (18). An alternative and complementary strategy is to target the cellular receptors that are also required for viral attachment (adsorption), entry, and cell-to-cell spread. We focused on virus adsorption to susceptible CV-1 cells, which is an early stage rather than late stage of penetration and intracellular replication of virus life cycle. As a positive control for virus adsorption assay we used a peptide called apoEdp previously reported by us (13) antiviral through peptidomimetic inhibition of early stage HSV-1 replication. Acyclovir (ACV) is a common choice of nucleoside-based treatment of HSV-1 infection. Our rationale of not to use ACV as positive control because ACV as a nucleoside analog inhibits late stage of viral nucleic acid synthesis but not early stage of virus adsorption.
Initially, in this study we have screened eight ketohexoses (Fig. 1) in vitro for their anti-HSV-1 activity using plaque reduction assay. Among the eight ketohexoses L-psicose has the maximum inhibitory effect followed by L-fructose (data not shown). The mechanism of HSV-1 inhibition by these two rare ketohexoses is mediated through the blocking of viral adsorption to the cell surface. Fig. 1 shows the structural formula of the eight ketohexoses. One interesting observation here is that the two active ketohexoses (L-psicose and L-fructose) has structural similarity at the carbon number 4 and 5. We hypothesize that position of hydroxyl group at carbon number 3, 4 and 5 is important for anti-HSV-1 activity. Based on our observation, we predict that ketopentose L-ribulose may show similar anti-HSV-1 activity because of it’s structural similarity to L-psicose and L-fructose.
This is our initial study regarding anti-HSV-1 activity of rare monosaccharide L-psicose. Very little is known about the biological function of this rare ketohexose. We have done cell viability testing in Vero (CV-1) cells using a dye reduction assay. L-psicose appeared to be non-toxic at the maximum concentration used in this study. However, further studies needed to be done to reduce the IC50/IC90 of L-psicose. HSK evident through appearance of stromal opacity in the cornea is the result of virus replication inside the cell and subsequent invasion of immune cells in the stroma resulting corneal cloudiness or opacity. Topical eye drop treatment dose of L-psicose was chosen 200 mM which is higher than IC90 (167 mM) but found safe and nontoxic to naïve mice eyes. Our experimental data of corneal opacity and infectious virus shedding suggest that L-psicose treatment results inhibition of virus entry and lower load of virus inside the cell may have diminished infectious virus shedding and development of less severe corneal opacity.
Studies regarding structural and functional impact of carbohydrates are very important in the development of carbohydrate-based drugs. During the last two decades, several imino sugars and nucleotide analogs with antiviral properties have been reported (19–22). However, to our knowledge this report is the first report of a simple sugar with in vitro and in vivo antiviral property.
Acknowledgments
Support is provided in part by grant number 2G12MD007595-06 from the National Institute on Minority Health and Health Disparities (NIMHD), National Institutes of Health (NIH), Department of Health and Human Services (DHHS) and its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIMHD or NIH.
Footnotes
Peer review under responsibility of Japanese Pharmacological Society
Conflict of interest
None
References
- 1.Aoki K, Porterfield M, Lee SS, Dong B, Nguyen K, McGlamry, et al. The diversity of O-Linked glycans expressed during Drosophila melanogaster development reflects stage- and tissue-specific requirements for cell signaling. J Biol Chem. 2008;283:30385–30400. doi: 10.1074/jbc.M804925200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horne G, Wilson FX. Therapeutic applications of iminosugars: current perspectives and future opportunities. Prog Med Chem. 2011;50:135–176. doi: 10.1016/B978-0-12-381290-2.00004-5. [DOI] [PubMed] [Google Scholar]
- 3.Izumori K. Izumoring: a strategy for bioproduction of all hexoses. J Biotechnol. 2006;124:717–722. doi: 10.1016/j.jbiotec.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 4.Muniruzzaman S, Pan YT, Zeng Y, Atkins B, Izumori K, Elbein AD. Inhibition of glycoprotein processing by L-fructose and L-xylulose. Glycobiology. 1996;6:795–803. doi: 10.1093/glycob/6.8.795. [DOI] [PubMed] [Google Scholar]
- 5.Livesey G, Brown JC. D-Tagatose is a bulk sweetener with zero energy determined in rats. J Nutr. 1996;126:1601–1609. doi: 10.1093/jn/126.6.1601. [DOI] [PubMed] [Google Scholar]
- 6.Matsuo T, Suzuki H, Hashiguchi M, Izumori K. D-Psicose is a rare sugar that provides no energy to growing rats. J Nutr Sci Vitaminol (Tokyo) 2002;48:77–80. doi: 10.3177/jnsv.48.77. [DOI] [PubMed] [Google Scholar]
- 7.Hossain MA, Wakabayashi H, Goda F, Kobayashi S, Maeba T, Maeta H. Effect of the immunosuppressants FK506 and D-allose on allogenic orthotopic liver transplantation in rats. Transpl Proc. 2000;32:2021–2023. doi: 10.1016/s0041-1345(00)01540-2. [DOI] [PubMed] [Google Scholar]
- 8.Levin GV. Tagatose, the new GRAS sweetener and health product. J Med Food. 2002;5:23–36. doi: 10.1089/109662002753723197. [DOI] [PubMed] [Google Scholar]
- 9.Levin GV, Zehner LR, Saunders JP, Beadle JR. Sugar substitutes: their energy values, bulk characteristics, and potential health benefits. Am J Clin Nutr. 1995;62:1161S–1168S. doi: 10.1093/ajcn/62.5.1161S. [DOI] [PubMed] [Google Scholar]
- 10.Hossain A, Yamaguchi F, Hirose K, Matsunaga T, Sui L, Hirata Y, et al. Rare sugar D-psicose prevents progression and development of diabetes in T2DM model Otsuka Long-Evans Tokushima Fatty rats. Drug Des Devel Ther. 2015;9:525–535. doi: 10.2147/DDDT.S71289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malm SW, Hanke NT, Gill A, Carbajal L, Baker AF. The anti-tumor efficacy of 2-deoxyglucose and D-allose are enhanced with p38 inhibition in pancreatic and ovarian cell lines. J Exp Clin Cancer Res. 2015;34(1):31. doi: 10.1186/s13046-015-0147-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helenius A, Aebi M. Roles of N-linked glycans in the endoplasmic reticulum. Annu Rev Biochem. 2004;73:1019–1049. doi: 10.1146/annurev.biochem.73.011303.073752. [DOI] [PubMed] [Google Scholar]
- 13.Bhattacharjee PS, Neumann DM, Foster TP, Clement C, Singh G, Thompson HW, Kaufman HE, Hill JM. Effective treatment of ocular HSK with a human apolipoprotein E mimetic peptide in a mouse eye model. Invest Ophthalmol Vis Sci. 2008;49:4263–4268. doi: 10.1167/iovs.08-2077. [DOI] [PubMed] [Google Scholar]
- 14.Luganini A, Nicoletto SF, Pizzuto L, Pirri G, Giuliani A, Landolfo S, Gribaudo G. Inhibition of herpes simplex virus type 1 and type 2 infections by peptide-derivatized dendrimers. Antimicrob Agents Chemother. 2011 Jul;55(7):3231–3239. doi: 10.1128/AAC.00149-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boisjoly HM, Pavan-Langston D, Kenyon KR, Baker AS. Superinfections in herpes simplex keratitis. Am J Ophthalmol. 1983 Sep;96(3):354–361. doi: 10.1016/s0002-9394(14)77827-4. [DOI] [PubMed] [Google Scholar]
- 16.Kimberlin DW, Whitley RJ. Antiviral resistance–an emerging problem. Antivir Res. 1995 Apr;26(4):365–368. doi: 10.1016/0166-3542(95)00026-i. [DOI] [PubMed] [Google Scholar]
- 17.Coen DM. Acyclovir-resistant, pathogenic herpesviruses. Trends Microbiol. 1994 Dec;2(12):481–485. doi: 10.1016/0966-842x(94)90652-1. [DOI] [PubMed] [Google Scholar]
- 18.Martinez CM, Luks-Golger DB. Cidofovir use in acyclovir-resistant herpes infection. Ann Pharmacother. 1997 Dec;31(12):1519–1521. [PubMed] [Google Scholar]
- 19.Wu SF, Chyan-Jang Lee CJ, Liao CL, Dwek RA, Zitzmann N, Lin YL. Antiviral effects of an imino sugar derivative on flavivirus infections. J Virol. 2002;76:3596–3604. doi: 10.1128/JVI.76.8.3596-3604.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zitzmann N, Mehta AS, Carrouee S, Butters TD, Platt FM, McCauley J, et al. Imino sugars inhibit the formation and secretion of bovine viral diarrhea virus, a pestivirus model of hepatitis C virus: implications for the development of broad spectrum anti-hepatitis virus agents. PNAS. 1999;96:11878–11882. doi: 10.1073/pnas.96.21.11878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang J, Wang L, Ma D, Qu X, Guo H, Xu X, et al. Novel imino sugar derivatives demonstrate potent antiviral activity against flaviviruses. Antimicrob Agents Chemother. 2009;76:1501–1508. doi: 10.1128/AAC.01457-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathé C, Gosselin G. L-Nucleoside enantiomers as antivirals drugs: a mini-review. Antivir Res. 2006;71:276–281. doi: 10.1016/j.antiviral.2006.04.017. [DOI] [PubMed] [Google Scholar]