Abstract
Background and Purpose
We conducted a randomized exploratory study to assess safety and the probability of a favorable outcome with adjunctive argatroban, a direct thrombin-inhibitor, administered to rt-PA treated ischemic stroke patients.
Methods
Patients treated with standard-dose rt-PA, not receiving endovascular therapy, were randomized to receive no argatroban or argatroban (100-μg/kg bolus) followed by infusion of either 1 μg/kg/min (low-dose) or 3 μg/kg/min (high-dose) for 48 hours. Safety was incidence of symptomatic intracerebral hemorrhage (sICH). Probability of clinical benefit (mRS 0–1 at 90-days) was estimated using a conservative Bayesian Poisson model (neutral prior probability centered at relative risk [RR]=1.0 and 95% prior intervals: 0.33–3.0).
Results
Ninety patients were randomized: 29 to rt-PA alone, 30 to rt-PA + low-dose argatroban, and 31 to rt-PA + high-dose argatroban. Rates of sICH were similar among control, low-dose and high-dose arms: 3/29 (10%); 4/30 (13%); and 2/31 (7%), respectively. At 90-days 6 (21%) rt-PA alone; 9 (30%) low-dose, and 10 (32%) high-dose patients were mRS 0–1. The RR (95% Credible Interval) for mRS 0–1 with low, high, and either low or high dose argatroban was 1.17 (0.57, 2.37), 1.27 (0.63, 2.53), and 1.34 (0.68, 2.76). The probability that adjunctive argatroban was superior to rt-PA alone was 67%, 74%, and 79% for low, high, and low or high dose, respectively.
Conclusions
In patients treated with rt-PA, adjunctive argatroban was not associated with increased risk of sICH and provides evidence that a definitive effectiveness trial is indicated.
Keywords: thrombolysis, thrombin inhibitor, acute stroke, anticoagulation, randomized controlled trial, argatroban, adjunctive therapy
Subject Terms: Clinical Studies, Cerebrovascular Disease/Stroke, Ischemic Stroke, Thrombosis
INTRODUCTION
Recombinant tissue plasminogen activator (rt-PA) is a highly effective treatment for acute ischemic stroke if patients can be treated within 4.5 hours.1 However, only about one third of patients receiving rt-PA completely recover by 3 months. The benefit of rt-PA has been linked to clot lysis, and only 20–30% of treated patients with documented arterial occlusion will have complete recanalization, with up to 60% having partial recanalization.2 Furthermore, clinical deterioration, perhaps due to reocclusion, occurs in at least 15% of patients. Moreover, rt-PA fails to reperfuse the brain in most patients with large thrombi.3 Administration of the direct thrombin inhibitor argatroban with rt-PA might improve no re-flow in microcirculation, increasing the speed and completeness of recanalization, preventing re-occlusion, and thereby reducing extent of infarction.4,5
To assess the safety of concomitant argatroban and standard dose rt-PA for ischemic stroke, we conducted a multicenter, single-arm study that verified that fewer than 10% of patients had symptomatic intracranial hemorrhage (sICH), the predefined safety threshold.6 In that study (n=65) three sICH cases occurred (4.6%; 95% CI, 0.9 –12.9); 40% had complete recanalization within two hours (compared to 18% of historical controls treated with rt-PA alone), and 36% of those assessed at 90 days had an excellent outcome (0–1 modified Rankin Scale score [mRS]).
These encouraging results prompted us to undertake an exploratory, phase IIb, randomized trial, with similar inclusion/exclusion criteria to test the same dose of argatroban in the single-arm study and a higher-dose of argatroban in combination with IV rt-PA. Our aims were to test safety, develop unbiased estimates of the treatment effects and use Bayesian analyses to estimate the probability that excellent functional recovery is increased by adjunctive low or high-dose argatroban with rt-PA.7–10
METHODS
Study design and participants
ARTSS-2 was a 3-arm, multicenter, randomized, blinded-outcome evaluation, exploratory trial performed at 16 US and UK sites. Acute ischemic stroke patients receiving IV rt-PA within 4.5 hours of symptom onset were included if: (1) age ≥18 and (2) NIHSS ≥10 or any NIHSS with proximal intracranial arterial occlusion (transcranial Doppler ultrasound – TCD or Computed Tomography-Angiogram – CTA) of: terminal internal carotid, middle cerebral (M1 or proximal M2), posterior cerebral (PCA, P1 or proximal P2), distal vertebral or basilar arteries. Exclusions were planned endovascular therapy. Complete exclusion criteria is located in the online-supplement. The study was approved by each center’s local review board and overseen by an independent physician safety monitor and data and safety monitoring committee. Data collection, monitoring and analysis were performed by an independent data coordinating center.
Randomization and blinding
Patients were randomized (1:1:1) to receive argatroban at two doses: (low-dose or high-dose), or rt-PA alone. Randomization was web-based and stratified using sequential minimization to balance three baseline characteristics: study site, presence of terminal ICA occlusion (3-level categorical variable: present, not-present or unknown), and HAT score (Hemorrhage After Thrombolysis).11,12 Treatment masking of bed-side clinicians and patients was not feasible due to the complexity of sham aPTT tests in a multicenter trial and the prohibitive costs of placebo manufacture and administration. Hospital clinical assessments were performed by vascular neurologists who were not blinded to treatment allocation. Neuroimaging was interpreted by a central image-core blinded to randomization group and clinical outcome. 90 day clinical assessments were performed in-person by study personnel blinded to randomization group.
Procedures
The study procedures are summarized in the online-supplement. All patients received intravenous rt-PA (0.9 mg/kg; maximum dose 90mg, 10% administered as 1 min bolus, the remaining infused over 1 hour) in the hospital emergency department or hyperacute stroke unit. Written informed consent from the patient or legally authorized representative was obtained. Patients randomized to argatroban received 100μg/kg intravenous bolus over 3–5 minutes within 1 hour of the rt-PA bolus followed by argatroban infusion of either 1.0μg/kg per minute (low-dose) or 3.0μg/kg per minute (high-dose) for 48 hours adjusted to a target activated partial thromboplastin time of 1.75 or 2.25 × baseline (± 10%) in low and high-dose arms, respectively. Infusion rates were adjusted in response to the activated partial thromboplastin time (aPTT) according to a dosing algorithm 2, 6, 12, and 24 hours after initiation of argatroban; the end of argatroban infusion; and in the event of major bleeding in which case the infusion was terminated immediately. In absence of hemorrhage, the algorithm required temporary cessation if the aPTT returned >100 or >130 seconds in the low or high-dose arm, respectively. Concomitant antithrombotics were not permitted during infusion.
Baseline examinations included routine laboratory tests, non-contrast CT, vessel imaging – transcranial Doppler ultrasound (TCD) or Computed Tomography Angiography (CTA) head ± neck if possible, NIHSS, and mRS. A repeat non-contrast CT was performed at 48 hours. Patients with baseline (pre-rt-PA) vascular imaging and no contraindication to repeat study, had a 2–3 hour repeat study of the same modality using standard definitions of occlusion location and recanalization.9 mRS, quality of life assessments, and NIHSS scores were obtained at 7 and 90 days. Study participants were asked at 90 days if they recalled the study treatment they had received.
Outcomes
The predefined primary outcome was the proportion of patients with a score of 0–1 on the modified Rankin scale, indicating an excellent clinical outcome of no clinically significant residual stroke deficits at 90 (±10) days.
Secondary outcomes were: symptomatic ICH within 48 hours of rt-PA bolus; 2–3 hours recanalization; NIHSS neurological improvement at 2, 24, 48 hours, day 7 and day 90; quality of life (standard gamble and EuroQol EQ-5D); and costs from a health system perspective. We plan to report the economic evaluation separately. Other safety outcomes included: parenchymal hematoma (PH); hemorrhagic transformation (HT); major systemic bleeding defined as a drop in hemoglobin of ≥2g/dL and transfusion of ≥2 units of blood.
An independent physician safety monitor and data-safety board monitored the trial. sICH was defined as any evidence of bleeding on CT scan that in the opinion of the clinical investigator or independent safety monitor was associated with clinically significant neurological worsening. In the event of any neurological deterioration (NIHSS ≥1 point increase) with any ICH, central CT/MRI findings, and clinical summaries were reviewed by the physician safety monitor. To avoid abandoning a beneficial therapy based on chance findings in a small number of patients, the DSMB utilized a pre-determined four-stage safety algorithm. Argatroban arm termination was considered only if the lower limit of the 95% confidence interval for sICH rate exceeded an absolute rate of 10% and the Bayesian posterior probability of benefit (a minimum of 3% absolute increase in 90-day mRS 0–1) was <20% compared to the control arm (see online-supplement for full protocol including the safety stopping rule).13,14
Statistical Analysis
The trial was designed to enroll 105 patients with 35 patients per arm. Typical of exploratory studies, the sample size was maximized according to available trial funding and with the objective of gaining useful preliminary safety and efficacy information. Each dose of argatroban was compared with rt-PA alone and in combination (low+high argatroban + rt-PA vs. rt-PA alone).
The primary clinical outcome, mRS 0–1 at 90-days, was analyzed with a Bayesian Poisson regression model to generate relative risks (RR) adjusted for stratification variables. The Bayesian approach was performed to estimate the probability of argatroban benefit compared to usual care (rt-PA-alone). A RR >1 indicated the “risk” of an excellent clinical outcome (mRS of 0–1) in argatroban+rt-PA. Conversely, a RR of <1 indicated worse outcomes (less patients with mRS of 0–1). A neutral prior distribution centered at RR=1.0 was used with a 95% prior interval (PrI) of 0.33–3.0 (based on the largest likely effect size identified for major outcomes in randomized trials and recommended over vague or flat priors) – see online supplement for details of the Bayesian approach.15 The same prior was used for safety termination of argatroban arms. We prespecified an exploratory analysis of the primary outcome adjusting for stratification as well as age and NIHSS.
Secondary outcomes were also analyzed (adjusted for stratification variables) using the same Bayesian Poisson regression model, but with different prior distributions. Since we expected escalating doses of argatroban to likely increase the risk for sICH, we chose a skeptical prior: RR=1.5 with 95% PrI 1.16–2.50 for low-dose and RR=2.0 with 95% PrI 1.33–4.0 for high-dose. For sICH, a RR of >1 indicates the increased risk of sICH. Rates of arterial recanalization used a neutral prior (RR=1.0, 95% PrI 0.75–1.75) for both argatroban groups. NIHSS neurological improvement at 2, 24, and 48 hours, used a neutral prior (RR=1.0, 95% PrI 0.33–3.0) for both Argatroban groups. For the NIHSS at days 7 and 90, a neutral prior with wider 95% CrI (RR=1.0, PrI 0.25–4.0) was used for both Argatroban groups. The wider PrIs reflect the increased uncertainty of treatment hazards at these later time points. Anticoagulation results, adverse event rates, and all other secondary outcomes were analyzed for treatment group differences using ANOVA, Chi-square, and non-parametric equivalents where appropriate. Analyses were performed using intention to treat (ITT).
Four post-hoc analyses (adjusted for stratification variables) were performed: 1) ordinal logistic regression of 90-day mRS outcome (scores 5 and 6 combined); 2) frequentist Poisson regression for RR and 95% CI for the primary mRS; 3) Bayesian Poisson regression using the same neutral prior as the primary outcome and 4) sICH (ITT and as-treated) outcomes as one patient randomized to the high-dose arm never received study drug, but suffered a sICH within 45 min of rt-PA) using both the trial definition of sICH and the SITS-MOST definition (PH-2 + increase in NIHSS ≥4 points).16 Unadjusted RR estimates were also analyzed. We fitted all Bayesian models via Markov Chain Monte Carlo methods (online-supplement). Analyses were conducted using SAS Version 9.4 (Cary, NC) and R (v2.11.1). ARTSS-2 was preregistered with ClinicalTrials.gov, number NCT01464788.
RESULTS
Between December 21, 2011, and March 23, 2015, 90 of 105 planned patients were randomized as the trial was stopped early after beneficial results of endovascular trials resulted in most eligible patients receiving thrombectomy which was a study exclusion. Study patient flow diagram is shown in Figure 1A. Baseline characteristics are displayed in Table 1. 72% percent were enrolled in the US and 28% in the UK. There were minor imbalances in NIHSS, stroke onset to rt-PA, history of atrial fibrillation and ASPECTS score across arms. Overall, 46 of 90 (51%) patients had baseline and follow-up intracranial arterial imaging demonstrating proximal occlusions with the lowest percentage (11/29, 38%) in the rt-PA alone arm. Stroke etiology was similar across arms (online-supplement).
Figure 1.
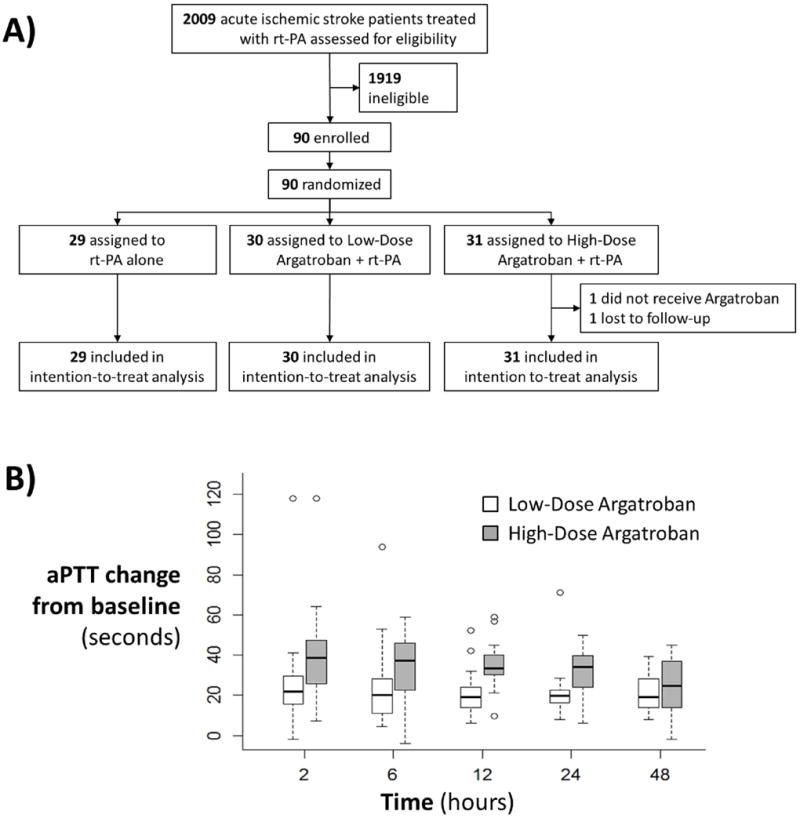
A) Patient flow diagram. One patient was lost-to-follow-up for 90-day assessment. B) Box-whisker plot of absolute aPTT changes (seconds) over the 48 hour infusion. Goal aPTT = 1.75 and 2.25 times the patient’s own baseline value in the low and high-dose argatroban arms, respectively.
Table 1.
Baseline characteristics. Abbreviations: HAT- hemorrhage after thrombolysis; ICA – internal carotid artery; US-United States; UK-United Kingdom.
| Variable | Control rt-PA-alone N=29 |
Low-Dose Argatroban + rt-PA N=30 |
High-Dose Argatroban + rt-PA N=31 |
|---|---|---|---|
|
| |||
| Age mean±SD | 69±15 | 71±15 | 67±13 |
|
| |||
| Male N(%) | 17(59) | 17(57) | 16(52) |
|
| |||
| Ethnicity N(%) | |||
| Caucasian | 15(52) | 13(43) | 15(48) |
| Hispanic | 2(7) | 4(13) | 4(13) |
| Black | 11(38) | 13(43) | 11(36) |
| Asian | 1(3) | 0(0) | 1(3) |
|
| |||
| Stroke Onset to rt-PA bolus, minutes mean±SD | 114±43 | 134±52 | 114±46 |
|
| |||
| Baseline NIHSS median (IQR), range | 15 (11,20), 4–26 | 16 (11,21), 2–29 | 13 (7,17), 3–33 |
|
| |||
| Glucose median, range | 123, 69–418 | 130, 88–309 | 125, 86–494 |
|
| |||
| aPTT, mean±SD, range | 27±5, 10–36 | 28±4, 13–36 | 29±3, 20–34 |
|
| |||
| Antithrombotic medications N(%) | |||
| Aspirin | 11(38) | 7(24) | 12(39) |
| Clopidogrel | 2(7) | 1(4) | 2(7) |
| Warfarin | 1(4) | 2(7) | 1(3) |
| Dabigatran | 0 | 0 | 1(3) |
| Past Medical History N(%) | |||
| Prior Stroke | 3(10) | 3(10) | 5(16) |
| Hypertension | 25(86) | 24(80) | 25(81) |
| Coronary Artery Disease | 1(4) | 3(10) | 1(3) |
| Diabetes mellitus | 6(21) | 10(33) | 0(29) |
| Congestive Heart Failure | 6(21) | 1(3) | 6(19) |
| Atrial Fibrillation | 5(17) | 7(23) | 11(36) |
| Hypercholesterolemia | 8(30) | 16(55) | 11(39) |
| Current Smoker | 8(28) | 5(18) | 6(20) |
|
| |||
| ASPECTS score median (IQR), range | 10 (8,10), 3–10 | 8 (6,10), 1–10 | 9 (8,10), 3–10 |
|
| |||
| Stratification Variables N(%) | |||
| HAT score | |||
| 0–2 (low-risk) | 25(86) | 25(83) | 26(84) |
| 3–5 (high-risk) | 4(14) | 5(17) | 5(16) |
| Terminal ICA occlusion | |||
| Present | 3(10) | 4(13) | 3(10) |
| Not-present | 18(62) | 18(60) | 21(68) |
| N/A - No vessel imaging | 8(28) | 8(27) | 7(23) |
| Site | |||
| US sites | 21(72) | 22(73) | 22(71) |
| UK sites | 8(28) | 8(27) | 9(29) |
| Vessel Occlusion N(%)* | N=11 | N=20 | N=15 |
| MCA (M1 or proximal M2) | 9(82) | 16(80) | 13(87) |
| Terminal ICA | 2(18) | 2(10) | 2(13) |
| Vertebrobasilar | 0 | 2(10) | 0 |
Vessel imaging required if baseline NIHSS was <10. Modalities: CT-Angiogram=80%; TCD=20%.
Of the patients who received argatroban, 44 (73%) had it started at or before the rt-PA infusion was completed with 13 ± 12 minutes drug overlap. The 16 (27%) remaining patients had argatroban started between 1–32 minutes after rt-PA infusion completion. Figure 1B and the online-supplement detail duration, and infusion adjustments, and coagulation results. At 90 days, 82% of patients were unable to accurately recall their study arm and 67%, if they had received any argatroban.
Primary Outcome
The proportion of patients with an excellent clinical outcome (0–1 on the modified Rankin Scale) was 21% (6/29) with rt-PA alone, 30% (9/30) with low-dose argatroban, and 10/31 (32%) with high-dose argatroban (Table 2 and Figure 2A). The single lost-to-follow-up patient’s (randomized to high-dose arm) day 7 mRS=4 was carried forward to 90-days as the local investigator confirmed the patient had not died at 90-days. Compared to rt-PA alone, the absolute difference in excellent clinical outcomes favored argatroban: 9% (low-dose argatroban) and 11% (high-dose).
Table 2.
Primary and secondary outcome results. All regression analyses adjusted for stratification variables. Abbreviations: CrI–credible interval, CI–confidence interval.
| Control rt-PA-alone N=29 |
Low-Dose Argatroban + rt-PA N=30 |
High-Dose Argatroban + rt-PA N=31 |
Low + High-Dose Argatroban + rt-PA N=61 |
|
|---|---|---|---|---|
|
| ||||
| PRIMARY OUTCOME | ||||
| mRS 0–1 at 90-days N(%) | 6(21) | 9(30) | 10(32) | 19(31) |
| RR (95% CrI), probability RR>1.0 | – | 1.17 (0.57, 2.37), 0.67 | 1.27 (0.63, 2.53), 0.74 | 1.34 (0.68, 2.76), 0.79 |
|
| ||||
| SECONDARY OUTCOMES | ||||
| Symptomatic Intracerebral Hemorrhage N(%) | 3(10) | 4(13) | 2(7) | 6(10) |
| RR (95% CrI), probability RR>1.0 | – | 1.55 (1.07, 2.25), 0.99 | 1.73 (1.04, 2.89), 0.98 | – |
| Recanalization at 2–3 hours | N=11 | N=20 | N=15 | N=35 |
| Complete N (%) | 2(18) | 3(15) | 4(27) | 7(20) |
| RR (95% CrI), probability RR>1.0 | – | 0.98 (0.61, 1.56), 0.46 | 1.05 (0.66, 1.68), 0.58 | 1.02 (0.64, 1.63), 0.53 |
| Complete + Partial N (%) | 6(55) | 4(20) | 7(47) | 11(31) |
| RR (95% CrI), probability RR>1.0 | – | 0.86 (0.55, 1.33), 0.25 | 1.06 (0.68, 1.65), 0.60 | 0.92 (0.59, 1.43), 0.35 |
| Neurological Improvement, NIHSS, median (IQR) | ||||
| RR (95% CrI), probability RR>1.0 | ||||
| 2-hours | 11 (6,19) N=29 | 14.5 (5,18) N=28 | 11 (4,16) N=31 | 13 (5,18) N=59 |
| – | 1.11 (0.78, 1.58), 0.73 | 0.94 (0.66, 1.33), 0.36 | 1.02 (0.74, 1.39), 0.55 | |
| 24-hours | 10 (5,18) N=29 | 14 (3,20) N=29 | 9 (4,16) N=31 | 11.5 (3.5,18) N=60 |
| – | 1.19 (0.79, 1.81), 0.79 | 0.94 (0.62, 1.42), 0.38 | 1.06 (0.72, 1.54), 0.62 | |
| 48-hours | 8 (3,18) N=27 | 10 (4,21) N=29 | 10 (2,17) N=31 | 10 (2.5,20) N=60 |
| – | 1.19 (0.76, 1.88), 0.78 | 0.97 (0.61, 1.53), 0.44 | 1.09 (0.71, 1.63), 0.65 | |
| Day 7 | 5 (3,14) N=27 | 10.5 (3,18) N=30 | 6 (2,14) N=29 | 9 (2,16) N=59 |
| – | 1.17 (0.7, 1.98), 0.72 | 0.9 (0.53, 1.53), 0.34 | 1.03 (0.64, 1.66), 0.55 | |
| Day 90 | 2.5 (1,8) N=24 | 5.5 (1.5,12.5) N=24 | 2 (1,7) N=25 | 3 (1,12) N=49 |
| – | 1.33 (0.73, 2.42), 0.83 | 1.02 (0.55, 1.88), 0.52 | 1.21 (0.68, 2.1), 0.75 | |
| Quality of Life at 90-days, median (IQR) | ||||
| EuroQol EQ-5D utility | 0.62 (0,0.82) N=29 | 0.38 (0.05,0.79) N=28 | 0.64 (0.18,0.84) N=29 | 0.48 (0.12,0.83) N=57 |
| Standard Gamble utility | 0.83 (0.45,0.98) N=27 | 0.68 (0.05,0.94) N=27 | 0.88 (0.50,0.95) N=28 | 0.78 (0.45,0.94) N=55 |
| Visual Analogue Scale (converted to 0–1 scale) | 0.50 (0.28,0.75) N=28 | 0.42 (0.22,0.80) N=28 | 0.60 (0.50,0.85) N=29 | 0.50 (0.30,0.80) N=57 |
Figure 2.
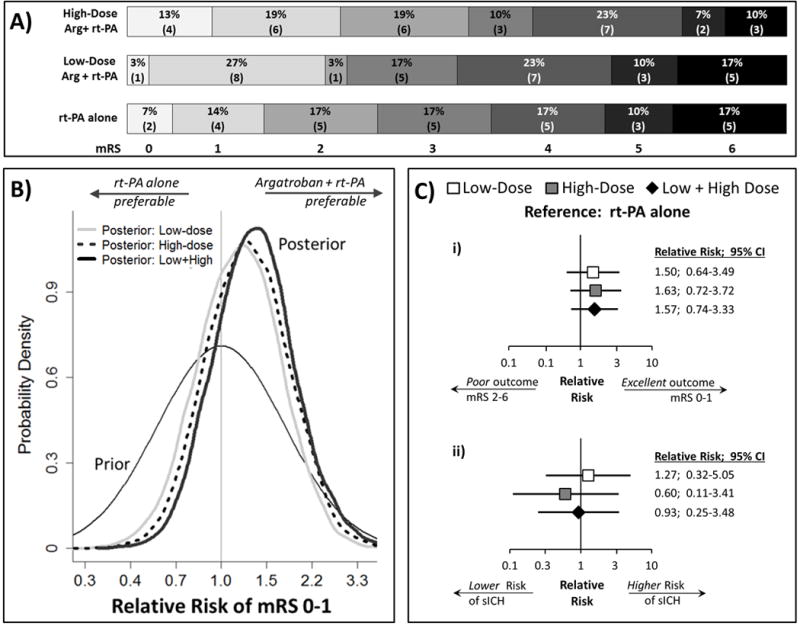
A) Distribution of 90-day modified Rankin Scale scores. B) Graphical depiction of Bayesian results. The neutral prior is centered at Relative Risk=1.0 indicating a 50:50 chance of argatroban+rt-PA superiority or inferiority. The area under the curve located on the right-hand side of RR=1.0 for each posterior, represents the probability that combination is superior (i.e., RR >1.0) to rt-PA-alone (67%-low; 74%-high and 79%-low+high dose). Note: the x-axis is set to logarithmic scale. C) Forest-plots of i) 90-day excellent clinical outcome; and ii) symptomatic ICH within 48-hours of rt-PA, analyzed using frequentist approach and adjusted for stratification variables.
Bayesian RR (95% CrI) for low, high, and either low or high dose argatroban was 1.17 (0.57, 2.37), 1.27 (0.63, 2.53), and 1.34 (0.68, 2.76), see Table 2. As depicted in Figure 2B, these results can be interpreted that the odds that argatroban is effective have improved from 1:1 as set in our prior (i.e., the prior probability that argatroban is effective is 50%) to about 2:1 (67% in terms of the probability) for low-dose; about 3:1 (74% in terms of the probability) for high-dose; and about 4:1 (79% in terms of the probability) for combined low+high doses.
Secondary and Safety Outcomes
Secondary outcomes are listed in Table 2. Incidence of sICH, rates of recanalization, NIHSS improvement and quality of life utilities were similar across study arms. Rates and types of asymptomatic ICH were comparable across arms and no major systemic bleeding adjudicated as argatroban-related occurred. A complete list of adverse events is located in the online-supplement. Mortality at 90 days occurred in 5 (17%, 95% CI 6–36), 5 (17%, 95% CI 6–35), and 3 (10%, 95% CI 2–26) for rt-PA alone, low-dose argatroban, and high-dose argatroban, respectively.
Post-Hoc Analyses
Results of pre-specified exploratory and post-hoc analyses are displayed in Table 3. Including covariates age and NIHSS score to the primary outcome regression analysis reduced the point estimate and probability of treatment superiority in the high-dose arm. Frequentist RR for mRS and sICH are displayed in Table 3, Figure 2Ci and 2Cii. Using the SITS-MOST definition, overall rates of sICH were lower and nearly identical across arms. Substituting a neutral prior (identical to the primary outcome analysis) for the conservative, the probability that adjunctive argatroban increased sICH risk was 63%, 34%, and 48% for low, high, and low or high dose, respectively. All unadjusted outcomes are in the online-supplement.
Table 3.
Results of pre-specified exploratory and post-hoc analyses. Unless specified otherwise, all analyses are adjusted for stratification variables. Abbreviations: SITS-MOST–Safe Implementation of Thrombolysis in Stroke-Monitoring Study. Abbreviations: CrI–credible interval, CI–confidence interval.
| Control rt-PA-alone N=29 |
Low-Dose Argatroban + rt-PA N=30 |
High-Dose Argatroban + rt-PA N=31 |
Low + High-Dose Argatroban + rt-PA N=61 |
|
|---|---|---|---|---|
|
| ||||
| PRESPECIFIED EXPLORATORY ANALYSIS | ||||
| mRS 0–1 at 90-days adjusted for stratification variables, age and NIHSS | ||||
| RR (95% CrI), probability RR>1.0 | – | 1.17 (0.56, 2.4), 0.66 | 1.07 (0.53, 2.16), 0.58 | 1.18 (0.59, 2.43), 0.67 |
|
| ||||
| POST HOC ANALYSES | ||||
| mRS 0–1 at 90-days | ||||
| 1) * Ordinal Logistic Regression OR (95% CI), p-value | – | 1.23 (0.49, 3.07), 0.66 | 2.03 (0.8, 5.1), 0.13 | 1.58 (0.71, 3.50), 0.26 |
| 2) * Poisson Regression RR (95% CI), p-value | – | 1.50 (0.64, 3.49), 0.35 | 1.63 (0.72, 3.72), 0.24 | 1.57 (0.74, 3.33), 0.24 |
| Symptomatic Intracerebral Hemorrhage | ||||
| 3) Intention To Treat (ITT) N(%), 95% CI | 3(10), 2.2–27.4 | 4(13), 3.8–30.7 | 2(7), 0.8–21.4 | 6(10), 3.7–20.2 |
| * RR (95% CI), p-value | – | 1.27 (0.32-5.1), 0.74 | 0.60 (0.11-3.41), 0.56 | 0.93 (0.25-3.48), 0.91 |
| As Treated N(%), 95% CI | 4(13), 3.8–30.7 | 4(13), 3.8–30.7 | 1(3), 0.1–17.2 | 5(8), 2.8–18.4 |
| * RR (95% CI), p-value | – | 0.98 (0.28–3.47), 0.97 | 0.24 (0.03–2.07), 0.19 | 0.60 (0.17–2.11), 0.43 |
| SITS-MOST Definition (ITT) N(%), 95% CI | 0 | 1(3), 0.1–17.2 | 2(7), 0.8–21.4 | 3(5), 1.0–13.7 |
| SITS-MOST Definition (As Treated) N(%), 95% CI | 1(3), 0.1–17.2 | 1(3), 0.1–17.2 | 1(3), 0.1–17.2 | 2(3), 0.4–11.5 |
| 4) †Neutral Prior Bayesian RR (95% CrI), probability RR>1.0 | – | 1.17 (0.48, 2.75), 0.63 | 0.82 (0.33, 2.0), 0.34 | 0.98 (0.42, 2.4), 0.48 |
Frequentist Poisson Regression.
Neutral Prior: RR=1.0 and 95% predictive interval: 0.33–3.0.
DISCUSSION
ARTSS-2 is the first randomized trial of concurrent IV thrombolysis and anticoagulation. We found no evidence that the addition of argatroban increased hemorrhage or death. Moreover, the findings for the clinical outcomes were encouraging. Despite the limited number of patients studied, conservative Bayesian analyses indicated a 79% probability that adjunctive argatroban increased the percent of patients with a score of 0–1 on the modified Rankin scale at 90 days.
Despite a paucity of events and wide 95% confidence intervals, escalating levels of thrombin-inhibition did not suggest an increased risk of symptomatic ICH. Combined with our previous study of 65 patients (mean age=63±14; median NIHSS=13) treated with low-dose argatroban + rt-PA6, a total of 125 thrombolysed moderate-to-severe acute ischemic strokes have received adjunctive argatroban with a total of eight sICH (6.4%). Importantly, both studies used a conservative sICH definition that did not mandate the presence of PH-2 or neurological worsening of 4 or more NIHSS points. Since the SITS-MOST definition of sICH accounts for the largest hemorrhage-related worsening in 90-day functional outcomes, it has increasingly become the standard used in recent stroke trials.17 In the current study, SITS-MOST sICH incidence was the same (3.3%) in rt-PA alone compared with patients that received argatroban (as treated analysis). Combining both adjunctive argatroban + rt-PA studies, three of 125 (2.4%, 95% CI by the exact Clopper-Pearson method: 0.5%–6.9%) patients that received argatroban + rt-PA suffered sICH according to SITS-MOST criteria. Our incidence is comparable to the 3.7% sICH rate reported in a recent meta-analysis of 3,391 rt-PA treated strokes from nine randomized trials.18 Despite more severe strokes in the current study, the percentage of patients with mRS 0–1 are similar to our previous cohort study (36%).
We used Bayesian methods to calculate estimates of the probability of adjunctive treatment benefit.8,9 These probabilities provide clinical investigators the best current evidence of a therapy’s potential benefit and are one of the main inputs for decision-making. For example, if the posterior probability of benefit is deemed large enough then investigators will plan a future larger trial. However, these probabilities are not obtainable from frequentist analyses. In their statement on p-values, the American Statistical Association states that studies should not simply rely on a p-value or statistical significance since neither is a good measure of evidence of benefit.19 They state that where appropriate, results should be supplemented with other approaches including Bayesian methods. These methods are uncontested for evaluation of diagnostic tests and have been recommended by the FDA for studies of medical devices.20 Use of Bayesian methods in oncology21 is widespread, and have also been adopted in NIH funded neurological trials (with FDA oversight), including an interventional trial for status epilepticus.22
Concerns about Bayesian analyses have largely been related to choosing an overly optimistic prior probability (or a prior based on weak evidence), thus producing overly optimistic posterior probabilities of treatment benefit. This concern did not apply to this trial because we utilized a neutral estimate of treatment effect (RR=1.0). This use of a prior RR of 1.0 in Bayesian analyses “shrinks” the RR estimate at the study conclusion closer to the null, resulting in more conservative estimates of the treatment effect than with conventional frequentist estimates (online-supplement). Further, our choice of 95% prior intervals for the prior allows for considerable uncertainty within a range that encompasses the RR observed for virtually all therapies between those that are quite beneficial (RR=0.3) or quite harmful (RR=3.3).15
We analyzed outcomes using relative risk because its interpretation is more straightforward than odds ratios (OR) and better understood by clinicians. Moreover, OR estimates in randomized trials always overestimate the point estimate for RR and this difference becomes greater with increasing incidence of the outcome (online-supplement). The more traditional frequentist and ordinal “shift” results were consistent with the Bayesian.
Vessel imaging was optional to avoid delays in administering argatroban and include patients who were unable to undergo arterial imaging for various reasons (a small-bore IV, renal contraindications to iodinated contrast, lack of emergent neuroradiology interpretation, lack of support for the second imaging study, IRB concerns, etc.). Since half of the patients did not undergo arterial imaging, caution should be exercised when interpreting recanalization results.
There is strong rationale for combination medical therapy in acute ischemic stroke. Combination lytics, anticoagulants and antiplatelets are frequently used for myocardial infarction with demonstrable impact on reperfusion and clinical outcomes. Medical therapies are widely available and can be rapidly administered in any emergency department or stroke unit; treatment regimens are needed that reduce the frequency of re-occlusion after rt-PA. Therefore, amplification and maintenance of recanalization remains a crucial target for reperfusion therapy.
Despite recent important endovascular advances, a large percentage of patients remain disabled which may be in part explained by significant delays to endovascular facilities as well as moderately low rates of complete TICI-3 reperfusion. The latter may contribute to microcirculation thrombosis and no-reflow that concurrent antithrombotics may prevent. Importantly, although low absolute risk (5%), new infarctions in unaffected territories are known to occur during endovascular therapy and are associated with worse patient outcomes.23
Endovascular therapy was excluded because at the time of the trial it was considered experimental. We feel this is an advantage of the design as it adds to the building evidence of medical adjunctive antithrombotic amplification of IV-rt-PA.6,24 After terminating the trial at 90 patients, we tested the feasibility and explored safety and reperfusion outcomes in a small cohort treated with IV-rt-PA, high-dose argatroban and endovascular therapy in 0–6 hour AIS with large vessel occlusion (clinicaltrials.gov NCT02448069). The results will be reported separately but demonstrated the feasibility and safety of combining the ARTSS-2 protocol with endovascular thrombectomy.
Study limitations include the open-label design that was necessary due to prohibitive costs of placebo manufacture and sham aPTT tests. By appointing an independent physician monitor, blinded image core and clinical outcome evaluators, we have minimized this limitation. Given that vessel imaging was not mandatory, a meaningful analysis of early recanalization rates was not possible.
CONCLUSIONS
The results of this randomized trial, like those in our cohort study, support the safety of adjunctive argatroban in the doses assessed for ischemic stroke patients. This evidence plus our findings suggesting an increased likelihood of an excellent clinical outcome justify assessing argatroban with rt-PA in a large effectiveness trial.
Supplementary Material
Acknowledgments
None.
SOURCES OF FUNDING
This study was supported by grant P50NS044227 from the National Institute of Neurological Disorders and Stroke (NINDS), supplement from the American Recovery and Revitalization Act, training grant T32NS07412 from the NIH, and philanthropic support from the Diane and Harold Farb Stroke Research (DHFSR) Fund to the Department of Neurology at the University of Texas Health Science Center at Houston (UTHSC). This work was also supported by the Center for Clinical and Translational Sciences, which is funded by NIH Clinical and Translational Award UL1 RR024148 [TL1 RR024147 for the T32 program; KL2 RR0224149 for the K12 program] from the National Center for Research Resources (NCRR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCRR or the NIH. Glaxo-Smith-Kline Inc. and Mitsubishi Pharma EU provided study medication to US and UK sites respectively without charge. Funders and medication suppliers had no role in study design, data collection, analysis, interpretation, or writing. The data core had full access to all the data in the study.
APPENDIX
ARTSS-2 investigators: Zahra Ajani, Andrei V. Alexandrov, Igor Cherches, Bruce Coull, Jesse Dawson, Debra del Junco, Andrew Demchuk, Joseph Devine, Aisha S. Dickerson, Anand Dixit, James L. Frey, Martin James, Usman Khan, Steven Levine, Claire MacDonald, Marc Malkoff, Elaine McColl, Vivek Misra, Michael Mullen, Richard Perry, Bartlomiej Piechowski-Jozwiak, Christine Roffe, Navi Sangha, April Sisson, Georgios Tsivgoulis, John J. Volpi.
ADB and JCG conceived the study, obtained funding, and were co-principal investigators. GAF conceived the study, and was the UK chief investigator. ADB, JT, CP, MHR, LS, and JCG designed and managed the trial with help from JLM and TM. ADB, JCG, and GAF drafted and report. LS, CM, ASD and EM coordinated study medication allotment, monitored data, imaging collection, and regulatory requirements for all study sites. MHR, CC, and CP were the study statisticians who take responsibility for the integrity of the data, accuracy and preparation of the analysis for this report. JT advised on study design and statistical aspects. MHR was the PI of the independent data coordinating center. ADB, MHR, GAF, and JCG were members of the trial steering committee. BC, DdJ, and IC served on the Data Safety and Monitoring Board. AD and GT provided independent neuroimaging interpretation. MMa was the independent physician safety monitor. All authors commented on the draft and approved the final version.
Footnotes
Clinical Trial Registration-URL: http://www.clinicaltrials.gov. Unique Identifier: NCT01464788.
DISCLOSURES
Dr. Barreto reports grants from NINDS and other from DHFSR to UTHSC, during the conduct of the study. Dr. Grotta reports grants from NINDS, other from DHFSRF, during the conduct of the study. Dr. Tyson reports grants from UTHSC, during the conduct of the study. Drs. Ford, Cai, Rahbar, Pedroza, and Loren Shen have nothing to disclose.
*List of ARTSS-2 investigators is provided in the Appendix.
References
- 1.Lees KR, Emberson J, Blackwell L, Bluhmki E, Davis SM, Donnan GA, et al. Effects of alteplase for acute stroke on the distribution of functional outcomes: a pooled analysis of 9 trials. Stroke. 2016;47:2373–2379. doi: 10.1161/STROKEAHA.116.013644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexandrov AV, Grotta JC. Arterial reocclusion in stroke patients treated with intravenous tissue plasminogen activator. Neurology. 2002;59:862–867. doi: 10.1212/wnl.59.6.862. [DOI] [PubMed] [Google Scholar]
- 3.Saqqur M, Uchino K, Demchuk AM, Molina CA, Garami Z, Calleja S, et al. Site of arterial occlusion identified by transcranial Doppler predicts the response to intravenous thrombolysis for stroke. Stroke. 2007;38:948–954. doi: 10.1161/01.STR.0000257304.21967.ba. [DOI] [PubMed] [Google Scholar]
- 4.Tamao Y, Kikumoto R. Effect of argatroban, a selective thrombin inhibitor, on animal models of cerebral thrombosis. Semin Thromb Hemost. 1997;23:523–530. doi: 10.1055/s-2007-996130. [DOI] [PubMed] [Google Scholar]
- 5.Lyden P, Pereira B, Chen B, Zhao L, Lamb J, Lei IF, et al. Direct thrombin inhibitor argatroban reduces stroke damage in 2 different models. Stroke. 2014;45:896–899. doi: 10.1161/STROKEAHA.113.004488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barreto AD, Alexandrov AV, Lyden P, Lee J, Martin-Schild S, Shen L, et al. The argatroban and tissue-type plasminogen activator stroke study: final results of a pilot safety study. Stroke. 2012;43:770–775. doi: 10.1161/STROKEAHA.111.625574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lilford RJ, Thornton JG, Braunholtz D. Clinical trials and rare diseases: a way out of a conundrum. BMJ. 1995;311:1621–1625. doi: 10.1136/bmj.311.7020.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spiegelhalter DJ, Myles JP, Jones DR, Abrams KR. Methods in health service research. An introduction to bayesian methods in health technology assessment. BMJ. 1999;319:508–512. doi: 10.1136/bmj.319.7208.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wijeysundera DN, Austin PC, Hux JE, Beattie WS, Laupacis A. Bayesian statistical inference enhances the interpretation of contemporary randomized controlled trials. J Clin Epidemiol. 2009;62:13–21. doi: 10.1016/j.jclinepi.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Rahbar MH, Dickerson AS, Cai C, Pedroza C, Hessabi M, Shen L, et al. Methodological issues for designing and conducting a multicenter, international clinical trial in acute Stroke: experience from ARTSS-2 trial. Contemp Clin Trials. 2015;44:139–148. doi: 10.1016/j.cct.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;1:103–115. [PubMed] [Google Scholar]
- 12.Lou M, Safdar A, Mehdiratta M, Kumar S, Schlaug G, Caplan L, et al. The HAT Score: a simple grading scale for predicting hemorrhage after thrombolysis. Neurology. 2008;71:1417–1423. doi: 10.1212/01.wnl.0000330297.58334.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeMets DL, Pocock SJ, Julian DG. The agonising negative trend in monitoring of clinical trials. Lancet. 1999;354:1983–1988. doi: 10.1016/S0140-6736(99)03464-9. [DOI] [PubMed] [Google Scholar]
- 14.Tyson JE, Pedroza C, Wallace D, D’Angio C, Bell EF, Das A. Stopping guidelines for an effectiveness trial: what should the protocol specify? Trials. 2016;17:240. doi: 10.1186/s13063-016-1367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pedroza C, Han W, Truong VT, Green C, Tyson JE. Performance of informative priors skeptical of large treatment effects in clinical trials: A simulation study. Stat Methods Med Res. 2015 doi: 10.1177/0962280215620828. [DOI] [PubMed] [Google Scholar]
- 16.Wahlgren N, Ahmed N, Davalos A, Ford GA, Grond M, Hacke W, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007;369:275–282. doi: 10.1016/S0140-6736(07)60149-4. [DOI] [PubMed] [Google Scholar]
- 17.Rao NM, Levine SR, Gornbein JA, Saver JL. Defining clinically relevant cerebral hemorrhage after thrombolytic therapy for stroke: analysis of the National Institute of Neurological Disorders and Stroke tissue-type plasminogen activator trials. Stroke. 2014;45:2728–2733. doi: 10.1161/STROKEAHA.114.005135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whiteley WN, Emberson J, Lees KR, Blackwell L, Albers G, Bluhmki E, et al. Risk of intracerebral hemorrhage with alteplase after acute ischaemic stroke: a secondary analysis of an individual patient data meta-analysis. Lancet Neurol. 2016;15:925–933. doi: 10.1016/S1474-4422(16)30076-X. [DOI] [PubMed] [Google Scholar]
- 19.Wasserstein RL, Lazar NA. The ASA’s statement on p-values: context, process, and purpose. Am Stat. 2016;70:129–133. [Google Scholar]
- 20.Bonangelino P, Irony T, Liang S, Li X, Mukhi V, Ruan S, et al. Bayesian approaches in medical device clinical trials: A discussion with examples in the regulatory setting. J Biopharm Stat. 2011;21:938–953. doi: 10.1080/10543406.2011.589650. [DOI] [PubMed] [Google Scholar]
- 21.Lee JJ, Chu CT. Bayesian clinical trials in action. Stat Med. 2012;31:2955–2972. doi: 10.1002/sim.5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bleck T, Cock H, Chamberlain J, Cloyd J, Connor J, Elm J, et al. The established status epilepticus trial. Epilepsia. 2013;54:89–92. doi: 10.1111/epi.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ganesh A, Al-Ajlan FS, Sabiq F, Assis Z, Rempel JL, Butcher K, et al. Infarct in a new territory after treatment administration in the ESCAPE randomized controlled trial (endovascular treatment for small core and anterior circulation proximal occlusion with emphasis on minimizing CT to recanalization times) Stroke. 2016;47:2993–2998. doi: 10.1161/STROKEAHA.116.014852. [DOI] [PubMed] [Google Scholar]
- 24.Adeoye O, Sucharew H, Khoury J, Vagal A, Schmit PA, Ewing I, et al. Combined approach to lysis utilizing eptifibatide and recombinant tissue-type plasminogen activator in acute ischemic stroke-full dose regimen stroke trial. Stroke. 2015;46:2529–2533. doi: 10.1161/STROKEAHA.115.010260. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


