Abstract
Polymer nano/micro fibers have found many applications including 3D cell culture and the creation of wound dressings. The fibers can be produced by a variety of techniques that include electrospinning, the primary disadvantage of which include the requirement for a high voltage supply (which may cause issues such as polymer denaturation) and lack of portability. More recently, solution blow spinning, where a high velocity sheath gas is used instead of high voltage, has been used to generate polymer fibers. In this work, we used blow spinning to create nano/microfibers for microchip-based 3D cell culture. First, we thoroughly investigated fiber generation from a 3D printed gas sheath device using two polymers that are amenable to cell culture (polycaprolactone, PCL and polystyrene, PS) as well as the parameters that can affect PCL and PS fiber quality. Using the 3D printed sheath device, it was found that the pressure of the sheath N2 and the concentration of polymer solutions determine if fibers can be produced as well as the resulting fiber morphology. In addition, we showed how these fibers can be used for 3D cell culture by directly depositing PCL fibers in petri dishes and well plates. It is shown the fibers have good compatibility with RAW 264.7 macrophages and the PCL fiber scaffold can be as thick as 178 ± 14 μm. PCL fibers created from solution blow spinning (with the 3D printed sheath device) were then integrated with a microfluidic device for the first time to fabricate a 3D cell culture scaffold with a flow component. After culturing and stimulating macrophages on the fluidic device, it was found that the integrated 3D fibrous scaffold is a better mimic of the extracellular matrix (as opposed to a flat, 2D substrate), with enhanced nitrite accumulation (product of nitric oxide release) from macrophages stimulated with lipopolysaccharide. PS fibers were also made and integrated in a microfluidic device for 3D culture of endothelial cells, which stayed viable for at least 72 hours (48 hours under the flowing conditions). This approach will be useful for future studies involving more realistic microchip-based culture models for studying cell-to-cell communication.
Introduction
When polymers are processed to create fine fibers on the micrometer to nanometer scale, they start to demonstrate unique characteristics such as high surface area-to-mass ratio and high porosity.1, 2 These properties make polymer micro/nanofibers an optimal material for many applications such as biomedical uses, sensors and filters.3-6 From the perspective of biomedical applications, polymer micro/nano fibers can be a good substrate for wound dressing materials7 as well as being used as a mimic of the in vivo extracellular matrix (ECM) that provides mechanical support to organs and tissues and regulates cellular activities.8, 9 In vitro cell/tissue culture has become an important research tool for investigations involving drug discovery and physiological studies. However, conventional two dimensional (2D) cell culture in flasks does not reproduce the physiological environments that cells encounter in vivo.10 Numerous biocompatible polymer fibers have been reported as ECM analogue scaffolds for the culture of multiple cells/tissues.11, 12 For example, Masaeli and colleagues recently reported their study of nerve tissue engineering on a nanofiber scaffold.13 Moreover, polymer fiber-based tissue prostheses such as skin5, 14 and blood vessels15 have also been developed, which can be potentially used for implantation surgeries in the future. Polymer fibers can also be used as a medium for wound dressings.7
Polymer micro/nanofibers can be generated by several techniques, including: electrospinning,16 melt-blowing,17 phase separation,18 self-assembly,19 and template synthesis.20 Among these techniques, electrospinning is commonly used due to its relatively simple setup, cost effectiveness and adaptability for large-scale continuous fiber production.21 Electrospinning is a technique that utilizes polymer solutions to fabricate non-woven fibers with diameters on the micrometer to nanometer scale (ranging from single digit nanometer to hundreds of microns21). A standard electrospinning system consists of a syringe with a metal cannula, a syringe pump, a high voltage power supply and a grounded collector. Briefly, when a polymer solution is drawn into the needle and charged with a large potential of ∼20 kV, the electric field between the charged needle and the grounded collector electrode helps to overcome the surface tension of the droplet and generates a charged Taylor cone, which can be elongated by the electrostatic force. This cone whips through the air towards the collector, creating dry fibers through evaporation of the solvent.22 The fibers are typically collected on the grounded collector as a mat of widely spread fibers (process is depicted in Figure S1 of the Supplementary Information).
Electrospinning has shown great success in fabricating fibers from an array of polymers.14, 22 However, the primary disadvantages of electrospinning are the requisite usage of high voltages to promote solvent evaporation and fiber formation and lack of portability. Another technique termed solution blown spinning has recently emerged as way to generate fibers without the use of high voltage.23, 24 In this approach, a high velocity sheath gas is used to apply a focusing force to a pumped polymer solution, resulting in polymer stream that can subsequently expand into fine fibers that can be collected onto a non-grounded substrate.23, 24 Medeiros and colleagues described how the cone and fibers are formed through a gas flow focus during solution blow spining.23 Fiber generation from several polymers such as poly(methyl methacrylate) (PMMA) and poly(lactic acid) (PLA) via solution blow spinning has also been investigated.23, 25 Daristotle and colleagues summarized some applications of solution blow spun fibers such as tissue engineering, regenerative medicine and wound dressings in a recent review.26 In this current work, we advanced the technology by creating a customized gas sheath device using 3D-printing and using this device to generate nano/micro scale fibers from two polymers (polycaprolactone, PCL and polystyrene, PS) that are commonly used as cell culture substrates Furthermore, we present here the first combination of this technique and microfluidics to make a biomimetic device that enables 3D cell culture under flowing conditions, the results of which suggest that macrophages cultured on such a device (with PCL fibers) respond more dramatically during an immunological stimulation (as compared to more traditional 2D culture). We also tested 3D culture of endothelial cells on similar fluidic devices (with PS fibers), which demonstrated good cell viability for at least 48 hours of continuous flow over the cells.
Experimental
Solution blow spinning Setup
As shown in Figure 1A, the setup for solution blow spinning mainly consists of three parts: the polymer solution supply, the gas sheath apparatus, and the target that collects fibers. Two types of polymers were studied in this work: PCL and PS. For the PCL study, a PCL solution was made by dissolving PCL beads (MW = 80,000, Sigma-Aldrich, MO, US) in 1,1,1,3,3,3-hexafluoro-2-propanol (HFP), and this solution was loaded in a 500 μL glass syringe. Multiple concentrations (w/v) of PCL were investigated in this study, as will be described in following parts. A piece of Tygon tubing (0.02″ i.d. × 0.06″ o.d., Cole-Parmer, IL, US) was connected to the syringe by Luer adapters (IDEX, CA, US). At the other end of the Tygon tubing, a steel cannula (300 μm i.d. × 550 μm o.d., New England Small Tube Corporation, NH, US) was connected as the outlet for the polymer solutions. The cannula was then placed in a 3D-printed gas sheath device, which is the key part in the solution blow spinning technique. The gas sheath device consists of a cone with a side connection port to a gas tank, and an open cap. The cap and the cone were joined with the printed threads, with a piece of septa in between, as shown in Figure 1C (Figure S2 in the supplementary information shows the device design details and dimensions). After the steel cannula was placed through the gas sheath device, the side port of the sheath device was connected to a N2 tank to apply a sheath flow surrounding the cannula, which tapers the solution to form a cone. Different sheath flow velocities were tested for different PCL concentrations in this study, as will be described subsequently. A syringe pump (Harvard apparatus, MA, US) was used to push PCL solutions at 10 μL/min through the cannula, where the sheath gas sharpened the polymer solution to form a cone, which then bursts into fine fibers. Unlike electrospinning, the collector substrate does not need to be grounded in this technique. Therefore, any substrate can be used as the collector. Substrates such as card paper and petri dishes can be used to collect fibers in this study.
Figure 1.
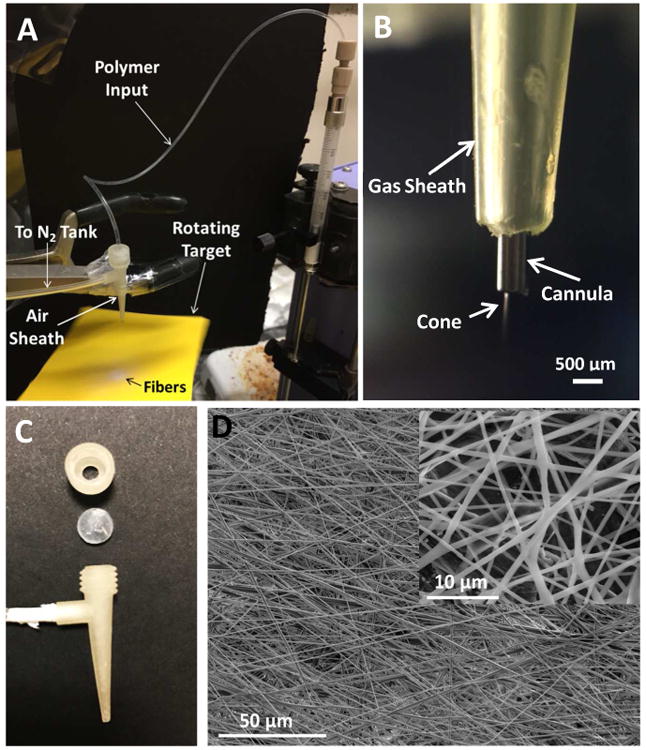
(A) An image of the solution blow spinning setup using the 3D printed gas sheath device. A PCL solution was pushed by a syringe pump through a piece of Tygon tubing to the steel cannula, which was surrounded by a 3D-printed gas sheath device. N2 was introduced via the side port to taper the polymer solution to form a cone. The cone then burst into fibers that were deposited (the white circular spot) on a piece of rotating cardboard. (B) An image of the cannula, the gas sheath and the formed cone. (C) The 3D-printed gas sheath device. This device contains two parts, a cap and a cone with a side port. The two parts can be joined by the printed threads, with a piece of septa in between. The top of the cap is open so that the steel cannula can be placed through the cone. The side port can be connected to a gas tank to provide sheath flow to generate nanofibers. (D) SEM images of fibers produced by solution blow spinning. In this example, 12 % PCL (in HFP) solution was spun under N2 sheath flow at 3.1 m/s (12.5 psi). The inset was taken from the same fiber sheet, but at a smaller scale.
For the PS studies, polystyrene beads (MW = 280,000, Sigma-Aldrich, MO, USA) were first dissolved in dimethylformamide (DMF, Sigma-Aldrich, MO, USA), with this 20% (w/v) solution being used to make more dilute solutions of different concentrations. To form the fibers, a PS solution was then drawn in to a 3 mL syringe, which was fitted with a 20 gauge blunt tip needle. A steel cannula (300 μm i.d. × 550 μm o.d.) was then inserted into the tip of 20 gauge needle as the outlet of the polymer solution. The syringe was placed in a syringe pump that was situated vertically and the same gas sheath device was fitted over the cannula. The PS solution was then delivered at a flow rate of 75 μL/min. With applied sheath N2 around the cannula, PS fibers can be generated and collected.
Determination of sheath N2 flow velocity windows for different polymer concentrations
The sheath N2 is the key to generating fibers from polymer solutions. It was observed that only optimal sheath flow velocities can result in fiber formation. The highest PCL concentration investigated in this study was 12% (w/v); more concentrated solutions were too viscous to be delivered by a regular syringe pump. Sheath N2 with decreased pressures (from 20 psi, at a decreasing interval of 2.5 psi) was applied to the 12% PCL solution. The highest pressure at which a stable cone can be formed was recorded as the upper limit of the sheath N2 velocity window. As the sheath N2 pressure decreased, the polymer solution tended to form droplets instead of cones at the end of the cannula. The lowest pressure at which a cone can still form (below which, droplets will form) was recorded as the lower limit of the sheath N2 window. The concentration of PCL was then serially lowered by 2% (w/v), and the same sheath tests were performed, until the solution was too diluted to generate fibers even at a low sheath N2 velocity, which indicated the threshold concentration to generate fibers. The sheath N2 pressure values (in psi) were converted to linear velocity values (in m/s) by using a tube flowmeter (model 7205-0062-A, King Instrument Company, CA, US). Different polystyrene solutions (12.5%, 15%, 20% (w/v)) were examined in a similar way to acquire the optimal sheath flow velocity range for them.
Characterization of fibers
Solution blow spun fibers generated from different polymer concentrations and at different sheath N2 flow velocities were characterized by scanning electron microscope imaging (SEM, FEI Inspect-50 model, OR, USA). After the fiber containing substrates were cut into about 1 cm × 1 cm squares, samples were sputter coated with gold at 30 mA for 40 sec (Denton Vacuum LLC, NJ, USA) to increase conductivity. After a sample was loaded in the SEM, an accelerating voltage of 20 kV was applied. At least 3 images from each sample were taken from randomly chosen spots. The SEM images were analyzed using the ImageJ program. For each image, at least 50 fiber diameter measurements were taken. Repeating measurements on the same fiber were avoided to the maximum extent.
Fabrication of the fiber-containing microfluidic device
Investigating the effect of a 3D scaffold on macrophage culture started with the design of a 3D-printed microfluidic device, onto which fibers could be solution blow spun for 3D cell culture under flowing conditions. 3D-printing has emerged as a powerful tool for fabricating fluidic devices in recent years.27-30 The device was printed with a Mojo 3D-printer (Stratasys, MN, USA) with Acrylonitrite Butadiene Styrene (ABS, Sigma-Aldrich, MO, USA) material. Figure 2A shows the assembling of the device. First, PCL fibers were spun onto an ABS insert (5 mm width × 25 mm length × 2.5 mm thick; “Fiber coated insert” in the figure), after which the insert was fitted into a holder (10 mm wide × 30 mm long × 4.5 mm thick with a 2.5 mm; “Holder” in the figure). Finally, the holder with the insert was plugged into the bottom slot of the flow device (the upward arrow in the figure). The flow device has another slot on the top side, where a piece of transparency film was placed (epoxy was used to affix film; the downward arrow in the figure). As shown in the side view (the right panel of the figure), a rectangular space between the two slots of the flow device defines the channel when both slots were sealed. The assembled flow device's channel has dimensions of 1 mm tall, 5 mm wide, and 25 mm long (5 mm2 cross-sectional area). In order to visualize the fibers and cells in the device, the flow device was fitted with a window made from transparency film and was set in place with epoxy (Permatex, CT, USA). The flow device has a steel pin (20 gauge, CML Supply, KY, USA) inserted into a hole designed within such that tubing can be attached. As a control, fibronectin (1 mg/L in PBS) coated inserts were also prepared and assembled in the fluidic device. Figure S3 in the SI shows the fluidic parts and their design details.
Figure 2.
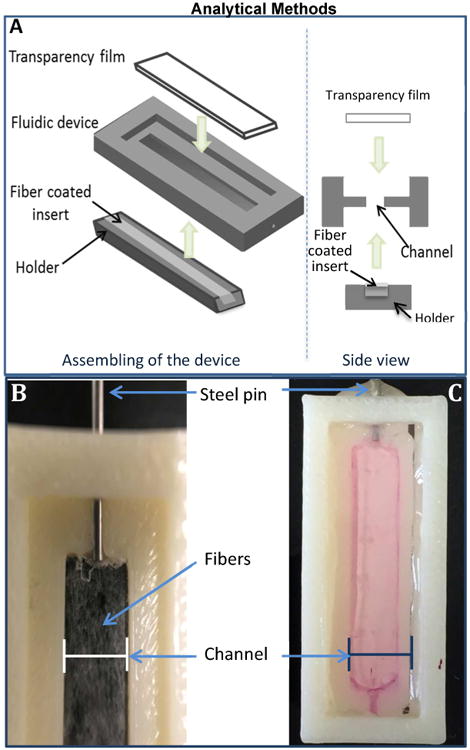
(A) Assembling of the device. A 3D-printed rectangular insert was first coated with solution blow spun fibers and then fit into a holder. A fluidic device was also fabricated by 3D-printing, which has two slots on top and bottom sides (only the top slot is visible in the figure). The holder was then plugged into the bottom slot; while a piece of transparency film was placed in the top slot (super glue was used to fix the film on the device). The right panel shows the side view of the process. There is a void space between the slots, which will form the channel when both sides are sealed. As a control, a device with a flat (no fibers coated) insert was also prepared. (B) A zoomed in view of the fluidic channel with white fibers clearly seen in the channel area. To take this picture, black tape was placed in the channel to better show the fibers coated on it but this was not used in cell culture experiments (the tape is for contrast in this picture only). (C) An assembled device filled with DMEM media that is ready for cell culture.
Macrophage studies on the fiber-containing microfluidic device
The mouse macrophage cell line RAW 264.7 (ATCC) was used in this study. After scraping a near confluent layer of the cells off a 35 mm petri dish, the cells were centrifuged at 500 g for 5 min and then re-suspended in DMEM media containing 10% fetal bovine serum (FBS) and 1% antibiotic (pen-strep). The cell suspension (∼2 × 106 cells/mL) was then introduced into the assembled fluidic device via tubing and a syringe until the channel was completely filled, after which, the device was placed in a petri dish and incubated (37 °C, 5% CO2) for 24 hours. After the 24 hour cell culture, macrophages were stimulated by circulating media containing lipopolysacchride (LPS). A piece of Tygon tubing (2 feet, 0.02″ i.d. × 0.06″ o.d., Cole-Parmer, IL, US) was attached to the steel pin and fed through the peristaltic pump. The assembled flow device with cells was situated on the top of a 15 mL centrifuge tube and the opposite end of the tubing was placed in the bottom of the tube. A solution of 0.1 mg/mL of LPS in DMEM (phenol red free; Life Technologies, CA, USA) was prepared and 1.3 mL was placed into the centrifuge tube. The device was placed into the incubator and the media was circulated at 400 μL/min for 24 hours. The experimental setup can be seen in Figure 2.
Detection of nitrite released by macrophages cultured on the microfluidic device
Nitrite (NO2-) was detected as a pro-inflammatory biomarker of macrophages31 using UV-vis spectroscopy with Griess reagents.32 An aliquot of 200 μL of the circulated media was pipetted into a clear 96 well plate (Greiner, Sigma Aldrich, MO, US), with 50 μL of Griess reagent for nitrite (Fluka, Sigma Aldrich, MO, US) being added and mixed. After 15 minutes of reaction, the well plate was read using a plate reader (Molecular Devices, CA, US) using absorbance at 530 nm. A calibration curve was obtained for each run using a nitrite standard solution (Sigma-Aldrich, MO, US) diluted with fresh stimulation media. Because cell count on each device may vary, the nitrite release from each device was normalized in terms of the total cell count. In order to quantify the amount of cells cultured on each device, after the LPS stimulation, the device was soaked in 1 mL DI water in a 1.7 mL centrifuge vial, which was then vigorously vortex mixed for approximately 10 minutes to ensure complete cell lysis. The Hoescht assay was then prepared to quantify the amount of DNA, which can be a measurement of cell count.33 Stock Hoesct 33258, pentahydrate (Life Technologies, OR, US) was first diluted from 10 mg/mL to 0.02 mg/mL with TNE buffer (42 mM Tris-HCl, 4.2 mM EDTA, and 8.4 M NaCl; all chemicals are from Sigma-Aldrich, MO, USA). In a 96 well plate, 40 μL of the prepared Hoescht assay and 160 μL of the cell lysate was added together immediately before analysis with the plate reader under fluorescence mode (excitation = 350 nm, emission = 460 nm) A calibration curve was constructed for this by counting cells with a hemocytometer first, lysis in DI water, and analysis with the Hoescht method.
Endothelial cell culture on the fiber-containing microfluidic device and viability measurement
PS fibers were coated on the 3D-printed ABS insert mentioned above via solution blow spinning (20% PS; sheath N2 pressure=15 psi). Four fiber-coated inserts were sterilized by soaking them in 70% ethanol and subsequent drying in UV, which were then placed in a 5 cm petri dish, followed by adding 5mL of 1.6 × 106/mL endothelial cell suspension (in fresh DMEM) in a sterile hood. The petri dish was then placed in a 37° C incubator for 24 hours for the cells to adhere on the fibers (static culture), after which, the inserts were assembled into the fluidic devices as have described above. Fresh media was then circulated through each device via the connected Tygon tubing using a peristaltic pump at 400 μL/min for designated periods of time (dynamic culture) for up to 48 hours. Cells were examined after staining with acridine orange at pre-determined time points with a fluorescent microscope and counted using the Hoescht method as described above.
Results and Discussion
Production of fibers with solution blow spinning
Figure 1A demonstrates the process that was used to solution blow spin PCL nanofibers (using the 3D printed gas sheath device). When the PCL solution was pumped through the Tygon tubing and the steel cannula, sheath N2 was applied around the cannula through a 3D-printed gasr sheath device. The sheath N2 tapers the PCL solution at the tip of the cannula to form a cone (Figure 1B). Like the Taylor cone formed in electrospinning, the cone formed by the sheath N2 can also expand at the tip to generate fibers. Unlike the whipping movement of fibers generated by electrospinning (depicted in Figure S1), the fibers can be focused by the sheath N2 as they travel to the collecting substrate.
In this work, we created a 3D-printed gas sheath device (Figure 1C) that plays three important roles in the process of solution blow spinning: 1) tapering the polymer solution to form a cone, 2) confining the generated fibers to be deposited as a focused sheet, and 3) aiding in the evaporation of the solvent to produce dry fibers on the collector. The specific design details of the sheath device can be found in Figure S2 in the supplementary information. The size of the tip of the sheath device was also optimized. The o.d. of the steel cannula was 550 μm, and the optimal gas sheath tip size was determined to be 800 μm in diameter. A smaller tip size led to limited space between the gas sheath and the cannula, causing N2 flow problems out of the device, while the N2 flow out of a larger tip size did not taper the polymer solution effectively. As shown in Figure 1A, the white circular area on the yellow cardboard was used to collect a fiber sheet. With a rotating collection stage, a round fiber sheet with diameter of about 1 cm can be collected reproducibly. The sheath N2 also dries the solvent of the polymer solution. If the gap distance (distance between the cannula and the collector) is larger than 4 cm, all the collected fibers collected are dry, with no welded fibers being observed. Figure 1D shows the SEM images of fibers spun from a 12% (w/v) PCL solution under sheath N2 flow at 3.1 m/s (12.5 psi), which clearly demonstrates the creation of clean, non-welded PCL nanofibers with the solution blow spinning technique using the 3D printed sheath device.
The effects of sheath pressure and polymer concentration on fiber formation and morphology
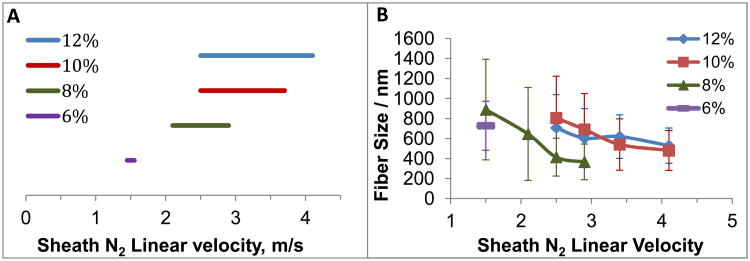
The sheath N2 velocity highly affects the formation of cones and subsequent fibers. Too high of a sheath N2 velocity led to unstable cones, where the cone/fibers drifted in random directions, while too low of a velocity did not taper the solution into a sufficient cone, with the solution instead forming droplets when exiting the cannula. Therefore, the optimal sheath N2 velocity windows for polymer solutions were investigated using the 3D printed gas sheath device. We investigated both PCL and PS in this work, which are two commonly used polymers for cell culture purposes.34, 35 The most concentrated PCL used in this study was 12%, because use of higher concentrations led to viscous solutions that were difficult to pump. A 4.1 m/s (20 psi) N2 sheath flow was initially applied to this PCL solution, which was then serially decreased at an interval of 1.5 m/s (2.5 psi) until the first stable cone was observed, with this indicating the upper limit of the window for fibers from a 12% PCL solution. As the sheath N2 velocity decreased to a point where cone formation failed to occur (and only droplet formation was observed), the lower limit of the window was determined. The PCL concentration was serially lowered by 2%, and the sheath N2 velocity window for each concentration was determined, the results of which are summarized in Figure 3A. It can be seen that a higher concentration of PCL has a larger sheath N2 velocity window. The 6% PCL solution (viscosity = 505.2 ± 0.76 cP) was determined as the lower threshold concentration, below which no fibers can be generated even with a very low sheath N2 velocity. The viscosity of different PCL concentrations is shown in Figure S5 of the supplementary information. These viscosity values are consistent with what has been reported in the literature.36-38 The cone was formed by the balance between sheath N2 focusing force and the viscous force of the polymer solution, which may explain why with the 3D printed device and these sheath velocities PCL solutions with low concentrations (low viscosities) were unable to generate fibers. The sizes of fibers generated from different concentrations of PCL at their specific sheath N2 velocity windows were measured on SEM images using ImageJ. Figure 3B demonstrates that solution blow spinning can generate fine fibers on the scale of hundreds of nanometers for all 4 PCL concentrations used here. With increased sheath N2 velocity, the fiber size tends to decrease. We also measured the viscosity of different PCL concentrations (Figure S5), which showed good agreement with literature values.37, 39-42 The sheath N2 flow velocities used in this technique can be easily achieved with commonly used gas cylinders and associated tubing, which have been routinely used in applications such as sample dehydration43 and polymer production.44
Figure 3.
(A) Sheath N2 velocity windows for different PCL concentrations using the 3D printed sheath device for fiber generation. The threshold concentration of PCL to generate solution blow spun fibers was determined as 6%. A lower concentration cannot generate fibers with solution blow spinning. (B) Widths of fibers from different PCL concentrations at their specific sheath N2 velocity windows. (N=9 samples, error= standard deviation). PCL solutions with higher concentrations have larger sheath N2 velocity windows. For each concentration of PCL, the fiber size tends to decrease with increased sheath N2 flow velocity.
To better visualize how the sheath N2 affects PCL fiber size/morphology in the 3D printed gas sheath device, SEM images of fibers generated from 8% PCL under 1.5, 2.1, 2.5 and 2.9 m/s sheath N2 flow (corresponding pressure values are 2.5, 5.0, 7.5, and 10 psi) were captured (from left to right in Figure 4). As indicated by the numbers below the images, which are the measured sizes for corresponding fibers (mean of 9 samples ± standard deviation), the fiber size tends to decrease with increased sheath N2 flow. The fiber sizes shown in Figure 4C (2.5 m/s sheath N2) and Figure 4D (2.9 m/s sheath N2) are statistically smaller than that in Figure 4A (1.5 m/s sheath N2) at 95% and 99% confidence levels, respectively. These images also suggest that with a higher sheath N2 velocity, the fiber size becomes more uniform, as can be indicated by the decreasing standard deviation values. The optimal N2 sheath velocity ranges for the 3D printed gas sheath device with different PS solutions were also investigated, the results of which are summarized in Figure S6.
Figure 4.
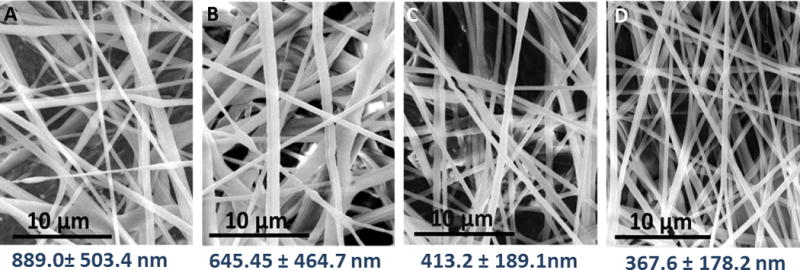
SEM images of solution blow spun fibers from 8 % PCL solutions at different sheath N2 linear velocities. The numbers under each image indicate the measured fiber sizes (mean of 9 samples ± standard deviation). From left to right, the sheath N2 flow velocities are 1.5, 2.1, 2.5, 2.9 m/s, respectively (corresponding pressure values are 2.5, 5.0, 7.5, and 10 psi). The pictures show that the fiber size keeps decreasing with increased sheath N2 linear velocity, so does the deviation of fiber sizes.
As aforementioned, micro/nano fibers have applicability in a number of fields. As opposed to the use of electrospinning, some unique characteristics of solution blow spinning can make certain applications simpler and easier. Figure 5A shows “SLU” (the abbreviation for Saint Louis University) written with fibers produced by the 3D printed gas sheath onto a piece of cardboard. The width of the letters was focused within a few millimeters by the sheath N2, whereas deposition spot size and focusing can become an issue with highly charged electrospun fibers. Because the collector does not need to be grounded, any substrate such as metals, paper, and plastics can be coated with fibers directly by solution blow spinning. For all the applications in this work, 12% PCL at 10 psi N2 sheath pressure were used. The fiber deposition process lasts 2 min, which generates a fiber film of around 20 μm. The thickness can increase with deposition time, until the upper limit is reached, beyond which, the newly formed fibers start to delaminate off the film and thus cannot be further deposited. With the 3D printed gas sheath device it was found that the maximum thickness of the PCL fiber film under these conditions is 178 ± 14 μm (Figure 5B). As shown in Figure 5C, some fibers were directly coated on a 35 mm petri dish by solution blow spinning, which can then be used as a scaffold for in vitro cell/tissue culture. Because petri dishes and other plastic cell culture containers are not conductive, such fibers cannot be directly coated on them by the conventional electrospinning technique. Instead, a commonly used method is to electrospin fibers onto a grounded metal substrate (usually forms a wide spread fiber sheet), which can be peeled off, cut, and placed in a dish for cell culture. The technique of solution blow spinning obviously simplifies this process. We have also successfully coated PCL fibers in a 6-well cell culture plate by solution blow spinning (Figure S8). The fluorescent image in Figure 5D shows RAW 264.7 cells cultured in such a petri dish (stained with acridine orange), which demonstrates good adherence of the cells on the solution blow spun fibers.
Figure 5.
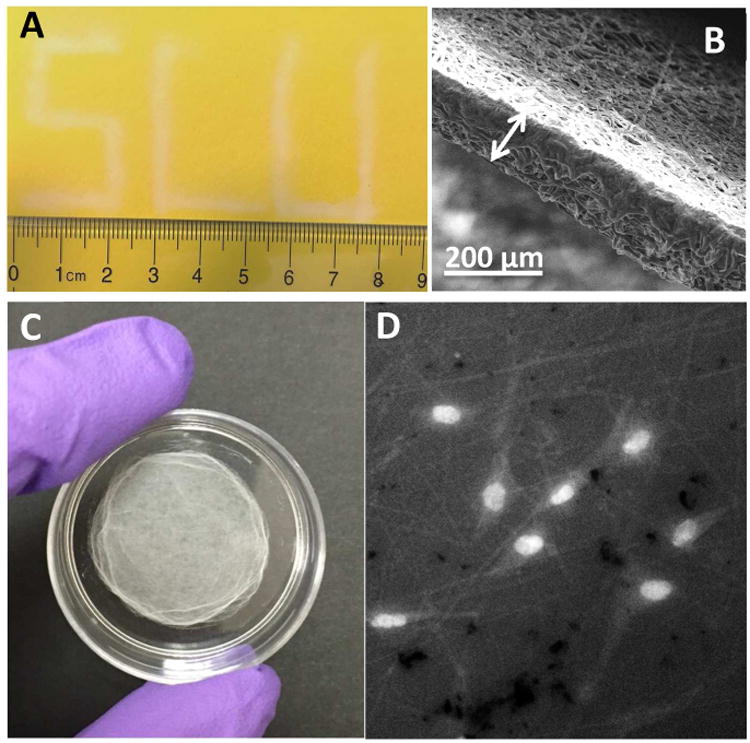
Examples of the unique characteristics of solution blow spinning. (A) Solution blow spinning enables depositing fibers onto focused areas. In contrast to electrospinning which produces a widely spread fiber sheet, the sheath gas in solution blow spinning generates the cone and fibers and focuses the fibers onto desired areas. Solution blow spinning can be a suitable technique for the application of accurate wound dressings. This technique does not need a high voltage supply, which makes it more portable and potentially safer. (B) The SEM image of the thickest fiber film that can be generated from 12 % PCL at 10 psi sheath N2. The thickness of the film was determined to be 178.4 ± 13.6 μm (n=3, mean ± standard deviation). (C) A 35 mm petri dish coated with solution blow spun fibers. The fiber collector in solution blow pinning does not need to be grounded, and thus solution blow spun fibers can be directly deposited on any substrate. Because the setup of solution blow spinning is simple and low cost, it can be potentially used by many laboratories to spin fibers on cell culture containers (i.e. petri dishes) for 3D cell scaffold fabrication. (D) Fluorescent image of cultured macrophages in such a petri dish as in (C). The bright spots show the nuclei of the cells stained with acridine orange. It can be clearly seen that the cells were adhered to the solution blow spun fibers.
Integration of solution blow spun fibers in a fluidic device for microchip-based 3D cell culture
The primary objective of this work was to integrate solution blow spun fibers into a microfluidic device for 3D cell culture. Fllow-based cell studies on microfluidics have been trending in recent years.45 Our recent findings suggest that 3D cell culture scaffold (i.e., fiber) integration can enhance the capability of a cell laden microfluidic device by more closely mimicking the native environment (ECM + flow conditions).46 In our previous work, electrospinning was used to direct fibers completely through an open channel network, with the center part of the enclosed channel not containing any fibers. Because of the simplicity of the solution blow spinning technique described here and the ability to use non-grounded targets, we herein report a novel microfluidic device design that contains solution blow spun fibers for 3D cell culture, with the fibers being directed directly onto the desired surface (with the substrate being perpendicular to the fiber spray). As in Figure 2A, a 3D-printed slim insert was coated by solution blow spinning (which takes 2 min to generate a fiber film 20 μm in thickness) that was then fitted into a rectangular holder. A fluidic device was also fabricated by 3D-printing, which has two rectangular slots on both top and bottom sides. The holder (with the combined insert) was then plugged into the bottom side of the device, while a piece of transparency film was placed in the top slot. As shown in the side view (the right panel of the figure), there is a void rectangular space between the two slots, which defines the channel when both slots were sealed (Figures 2B and 2C). As a control, a device that has a flat insert (no fibers coated on it) was also prepared.
To demonstrate the applicability of the blow spun fibers for microchip-based 3D cell culture, macrophages were utilized. After culturing macrophages on the devices for 24 hours (static condition), LPS stimulation was introduced to the macrophage cells under flowing conditions. The accumulation of NO2- from macrophages cultured on both devices (fibrous insert and flat insert) was measured off-chip using Griess reagent32. As macrophages are stimulated, they will transition the phenotype from M0 (native state) to M1 (pro-inflammatory) and then M2 (pro-healing), with the release of various cytokines and other molecules. Reactive nitrogen species (i.e., nitric oxide) is an indicator of the M1 pro-inflammatory state, which will become NO2- in cell culture (due to oxidation of the NO). In other words, the amount of accumulated NO2- can be a biomarker of the pro-inflammatory response of macrophages. Because the cell count on different devices may vary, the amount of cells on each device was also determined using a Hoescht assay,33 as described in the Experimental section (cell count data in Figure S7 of SI). The measured NO2- from each device was then normalized by cell count on that specific device (presented as μM NO2-/105 cells). Figure 6B shows the normalized NO2- accumulation from macrophages cultured on fibers and on the flat, fibronectin-coated surface. It can be seen that after LPS stimulation, there is significantly more NO2- release (blue bars in Figure 6B; 13.4 ± 2.0 μM vs. 8.6 ± 1.0 μM; n=6, ± SEM; p< 0.005) from macrophages cultured on the fibers in a fluidic channel than those on the flat surface, which suggested that the fibrous scaffold in the fluidic device enhanced the immune response of macrophages upon LPS stimulation. However, the NO2- production from unstimulated macrophages is at a low concentration for both the fibrous and the flat culture conditions, with no significant difference between the two (red bars in Figure 6B; 1.1 ± 0.2 μM vs. 1.0 ± 0.1 μM; n=4, ± SEM). It has been reported that the interaction of macrophages with in vivo ECM is an important part of the processes involving macrophage stimulation, such as wound healing.47, 48 Our results highly indicate that the integrated solution blow spun fibers in the fluidic device is a much improved in vitro ECM mimic for macrophages, as compared to more traditional 2D culture. In addition to fiber integration, some other unique characteristics of this fluidic system make it a versatile tool for future cell studies. Theoretically, many other cell types can be cultured on the scaffold under flowing conditions. By modulating the insert size and flow rate, specific shear stress can be expected to be applied on the cells. For example, we tested endothelial cell culture on a similar fluidic device with solution blow spun PS fibers (Figure S6C), which showed good viability of the cells. Figures 7A shows the fluorescent image of the endothelial cells on the PS fibers after a 24-hour static culture (in a petri dish, with initial seeding being very concentrated), after which, the cells were cultured for another 24 and 48 hours under flowing conditions (Figures 7B and 7C, respectively). A rough comparison between the three fluorescent images did not indicate difference of cell counts, meaning that no cells were lost during this pumping experiment. The ability to keep the cells adherent for this long period of time in the microchip-based 3D scaffold is a good testament of the improved culture conditions provided by this approach. After accurately quantifying the cells cultured under the three conditions using the Hoechst assay, it was confirmed that the cells can be viable on the fibers in the flowing media for at least 48 hours (after the initial 24-hour static culture step, see Figure 7D). The 3D printed microchip device is also modular, with the insert being removable and easy to reassemble, which enables reusability of the fibrous scaffolds. Although the device used in this work has a relatively big channel size (1 mm tall, 5 mm wide, and 25 mm long), it is easy to make smaller versions. For example, we have made a 500 μm wide by 1mm tall (cross section) channel for future work (Figure S9).
Figure 6.
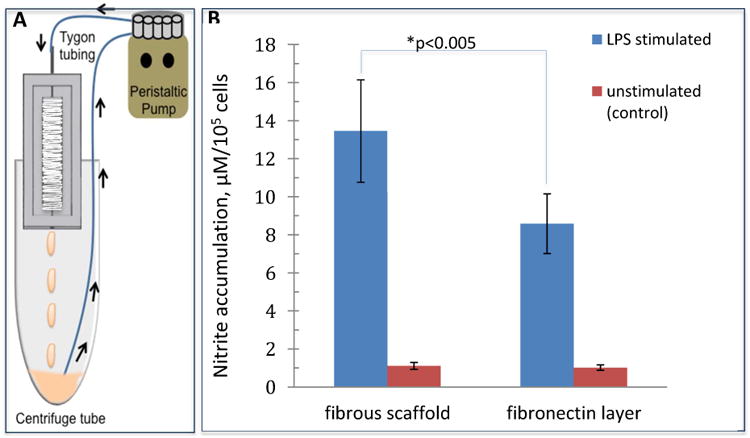
(A) Experimental setup for macrophage stimulation. Tygon tubing was fixed over the steel pin in the flow device. The Tygon tubing was fed through a peristaltic pump and the loose end was placed into a centrifuge tube. The flow device was positioned in the top of the centrifuge tube and 1.3 mL of 0.1 mg/mL LPS in complete DMEM without phenol red was dispensed into the centrifuge tube for 24 hour circulation. (B) Comparison of nitrite release of macrophages cultured on PCL fibers and fibronectin coated inserts. After being stimulated by LPS, macrophages in the microfluidic devices produced significantly more nitrite per 105 cells when cultured on PCL fibers relative to those cultured on a a flat surface (13.4 ± 2.0 vs. 8.6 ± 1.0μM NO2-, blue bars, p<0.005, n=6, error=SEM). As a control, macrophages in a device that were not stimulated with LPS produced a small amount of nitrite (1.1 ± 0.2 vs. 1.0 ± 0.1 μM NO2-, red bars, n=4, error=SEM).
Figure 7.
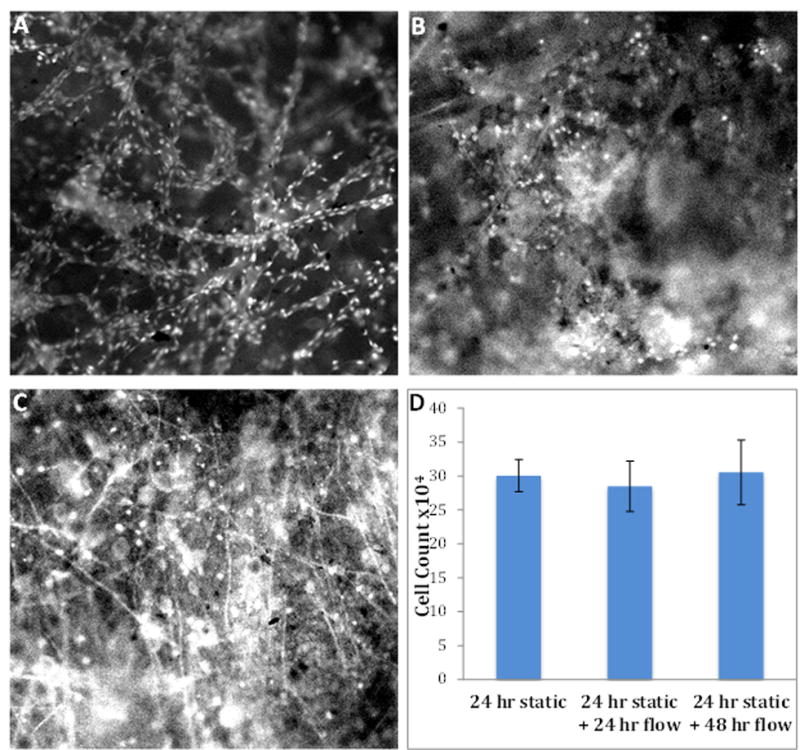
Fluorescent images and cell counting of endothelial cells cultured on PS fibers integrated in the microfluidic device. (A) Endothelial cells after a 24-hour static culture in a petri dish. (B) Endothelial cells cultured for 24 hours under flowing media (after the 24 hour static culture step). (C) Endothelial cells cultured for 48 hours under flowing media (after the 24 hour static culture step). Bright spots indicate cells stained by acridine orange, although the topography of the fibers prevents obtaining a uniform focal plane and some cells may be out of focus. (D) Cell counting results for the endothelial cells cultured under the three conditions mentioned above. It suggests that after a 72-hour culture (24 hour static culture + 48 hour culture in flowing media), the cells were still viable on the PS fibers without losing cell numbers.
Conclusion
In this work, we developed a 3D printed gas sheath device and used it to study the solution blow spinning of nano/micro PCL and PS fibers. A systematic investigation into determining how sheath N2 flow rate and polymer solution concentration (viscosity) can affect fiber formation and fiber size was completed. With increased sheath N2 velocity, the fiber size tends to decrease, as does the fiber size distribution. Compared to conventional electrospinning, solution blow spinning provides a simpler and lower cost way to generate polymer fibers, which has been used to create 3D cell culture scaffolds in petri dishes and well pates directly. Importantly, we used solution blow spinning to coat PCL fibers on a 3D printed insert, which can be integrated into a fluidic channel for 3D cell culture and stimulation under flowing conditions. It was found that macrophages cultured on the fibers can release more pro-inflammatory markers under flowing LPS stimulation, which suggest that the solution blown spun cell culture scaffold enhanced the immunological response of the cells in the fluidic device. Endothelial cells were also cultured on a similar fluidic device with PS fibers, which stayed viable for at least 72 hours (48 hours under the flowing conditions). Overall, this work investigated the solution blow spinning technique in a quantitative and systematic way to generate nanofibers, as well as the application of the fibers in 3D cell culture in a microfluidic device. Due to the robustness and customizability of 3D-printed fluid devices,30 future work will focus on integrating this device with other modules such as separation and detection chips for (near) real time monitoring cell activity/communication.
Supplementary Material
Acknowledgments
Support from the National Institute of General Medical Sciences (Award Number R15GM084470-04) is acknowledged.
References
- 1.Cipitria A, Skelton A, Dargaville TR, Dalton PD, Hutmacher DW. J Mater Chem. 2011;21:9419–9453. [Google Scholar]
- 2.Long YZ, Li MM, Gu CZ, Wan MX, Duvail JL, Liu ZW, Fan ZY. Prog Polym Sci. 2011;36:1415–1442. [Google Scholar]
- 3.Aleshin AN. Adv Mater. 2006;18:17–27. [Google Scholar]
- 4.Frenot A, Chronakis IS. Curr Opin Colloid In. 2003;8:64–75. [Google Scholar]
- 5.Venugopal J, Ramakrishna S. Tissue Eng. 2005;11:847–854. doi: 10.1089/ten.2005.11.847. [DOI] [PubMed] [Google Scholar]
- 6.Zhang YZ, Lim CT, Ramakrishna S, Huang ZM. J Mater Sci-Mater M. 2005;16:933–946. doi: 10.1007/s10856-005-4428-x. [DOI] [PubMed] [Google Scholar]
- 7.Khil MS, Cha DI, Kim HY, Kim IS, Bhattarai N. J Biomed Mater Res B. 2003;67b:675–679. doi: 10.1002/jbm.b.10058. [DOI] [PubMed] [Google Scholar]
- 8.Sell S, Barnes C, Smith M, McClure M, Madurantakam P, Grant J, Mcmanus M, Bowlin G. Polym Int. 2007;56:1349–1360. [Google Scholar]
- 9.Sell SA, Wolfe PS, Garg K, McCool JM, Rodriguez IA, Bowlin GL. Polymers-Basel. 2010;2:522–553. [Google Scholar]
- 10.Baker BM, Chen CS. J Cell Sci. 2012;125:3015–3024. doi: 10.1242/jcs.079509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mo XM, Xu CY, Kotaki M, Ramakrishna S. Biomaterials. 2004;25:1883–1890. doi: 10.1016/j.biomaterials.2003.08.042. [DOI] [PubMed] [Google Scholar]
- 12.Pham QP, Sharma U, Mikos AG. Tissue Eng. 2006;12:1197–1211. doi: 10.1089/ten.2006.12.1197. [DOI] [PubMed] [Google Scholar]
- 13.Masaeli E, Morshed M, Nasr-Esfahani MH, Sadri S, Hilderink J, van Apeldoorn A, van Blitterswijk CA, Moroni L. Plos One. 2013;8:1932–1943. doi: 10.1371/journal.pone.0057157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venugopal J, Ramakrishna S. Appl Biochem Biotech. 2005;125:147–157. doi: 10.1385/abab:125:3:147. [DOI] [PubMed] [Google Scholar]
- 15.Xu CY, Inai R, Kotaki M, Ramakrishna S. Biomaterials. 2004;25:877–886. doi: 10.1016/s0142-9612(03)00593-3. [DOI] [PubMed] [Google Scholar]
- 16.Reneker DH, Chun I. Nanotechnology. 1996;7:216–223. [Google Scholar]
- 17.Ward GF. Filtr Separat. 2001;38:42–43. [Google Scholar]
- 18.Yang F, Murugan R, Ramakrishna S, Wang X, Ma YX, Wang S. Biomaterials. 2004;25:1891–1900. doi: 10.1016/j.biomaterials.2003.08.062. [DOI] [PubMed] [Google Scholar]
- 19.Hartgerink JD, Beniash E, Stupp SI. Science. 2001;294:1684–1688. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 20.Martin CR. Chem Mater. 1996;8:1739–1746. [Google Scholar]
- 21.Huang ZM, Zhang YZ, Kotaki M, Ramakrishna S. Compos Sci Technol. 2003;63:2223–2253. [Google Scholar]
- 22.Doshi J, Reneker DH. J Electrostat. 1995;35:151–160. [Google Scholar]
- 23.Medeiros ES, Glenn GM, Klamczynski AP, Orts WJ, Mattoso LHC. J Appl Polym Sci. 2009;113:2322–2330. [Google Scholar]
- 24.Polat Y, Pampal ES, Stojanovska E, Simsek R, Hassanin A, Kilic A, Demir A, Yilmaz S. J Appl Polym Sci. 2016;133 [Google Scholar]
- 25.Behrens AM, Casey BJ, Sikorski MJ, Wu KL, Tutak W, Sandler AD, Kofinas P. Acs Macro Lett. 2014;3:249–254. doi: 10.1021/mz500049x. [DOI] [PubMed] [Google Scholar]
- 26.Daristotle JL, Behrens AM, Sandler AD, Kofinas P. Acs Appl Mater Inter. 2016;8:34951–34963. doi: 10.1021/acsami.6b12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen CP, Wang YM, Lockwood SY, Spence DM. Analyst. 2014;139:3219–3226. doi: 10.1039/c3an02357e. [DOI] [PubMed] [Google Scholar]
- 28.Liu YL, Chen CP, Summers S, Medawala W, Spence DM. Integr Biol. 2015;7:534–543. doi: 10.1039/c4ib00243a. [DOI] [PubMed] [Google Scholar]
- 29.Mu RP, Chen CP, Wang YM, Spence DM. Anal Methods. 2016;8:6856–6864. [Google Scholar]
- 30.Chen CP, Mehl BT, Munshi AS, Townsend AD, Spence DM, Martin RS. Anal Methods. 2016;8:6005–6012. doi: 10.1039/C6AY01671E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iyengar R, Stuehr DJ, Marletta MA. Proc Natl Acad Sci U S A. 1987;84:6369–6373. doi: 10.1073/pnas.84.18.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ivanov VM. J Anal Chem. 2004;59:1002–1005. [Google Scholar]
- 33.Kim YJ, Sah RL, Doong JY, Grodzinsky AJ. Anal Biochem. 1988;174:168–176. doi: 10.1016/0003-2697(88)90532-5. [DOI] [PubMed] [Google Scholar]
- 34.Woodruff MA, Hutmacher DW. Prog Polym Sci. 2010;35:1217–1256. [Google Scholar]
- 35.Ni M, Tong WH, Choudhury D, Rahim NAA, Iliescu C, Yu H. Int J Mol Sci. 2009;10:5411–5441. doi: 10.3390/ijms10125411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van der Schueren L, De Schoenmaker B, Kalaoglu OI, De Clerck K. Eur Polym J. 2011;47:1256–1263. [Google Scholar]
- 37.Nezarati RM, Eifert MB, Cosgriff-Hernandez E. Tissue Eng Part C. 2013;19:810–819. doi: 10.1089/ten.tec.2012.0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sajeev US, Anand KA, Menon D, Nair S. B Mater Sci. 2008;31:343–351. [Google Scholar]
- 39.Beachley V, Wen XJ. Mat Sci Eng C-Bio S. 2009;29:663–668. doi: 10.1016/j.msec.2008.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fridrikh SV, Yu JH, Brenner MP, Rutledge GC. Phys Rev Lett. 2003;90 doi: 10.1103/PhysRevLett.90.144502. [DOI] [PubMed] [Google Scholar]
- 41.Sarazin P, Favis BD. Polymer. 2005;46:5966–5978. [Google Scholar]
- 42.Theron SA, Zussman E, Yarin AL. Polymer. 2004;45:2017–2030. [Google Scholar]
- 43.Tang ES, Wang L, Liew CV, Chan LW, Heng PW. Int J Pharm. 2008;350:172–180. doi: 10.1016/j.ijpharm.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 44.Czaja DE, Ivy CW. 1973;3:212. [Google Scholar]
- 45.Hung PJ, Lee PJ, Sabounchi P, Lin R, Lee LP. Biotechnol Bioeng. 2005;89:1–8. doi: 10.1002/bit.20289. [DOI] [PubMed] [Google Scholar]
- 46.Chen CP, Mehl BT, Sell SA, Martin RS. Analyst. 2016;141:5311–5320. doi: 10.1039/c6an01282e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wesley RB, Meng XP, Godin D, Galis ZS. Arterioscl Throm Vas. 1998;18:432–440. doi: 10.1161/01.atv.18.3.432. [DOI] [PubMed] [Google Scholar]
- 48.Schultz GS, Wysocki A. Wound Repair Regen. 2009;17:153–162. doi: 10.1111/j.1524-475X.2009.00466.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.