Abstract
The rostral-ventral subdivision of the anterior cingulate cortex (rACC) plays a key role in the regulation of emotional processing. Although rACC has strong anatomical connections with anterior insular cortex (AIC), amygdala, prefrontal cortex and striatal brain regions, it is unclear whether the functional connectivity of rACC with these regions changes when regulating emotional processing. Furthermore, it is not known whether this connectivity changes with deficits in emotion regulation seen in different kinds of anxiety and depression. To address these questions regarding rACC functional connectivity, non-patients high in self-reported anxious apprehension (AP), anxious arousal (AR), anhedonic depression (AD) or none (CON) indicated the ink color of pleasant, neutral and unpleasant words during functional magnetic resonance imaging. While ignoring task-irrelevant unpleasant words, AD and CON showed an increase in the functional connectivity of rACC with AIC, putamen, caudate and ventral pallidum. There was a decrease in this connectivity in AP and AR, with AP showing greater reduction than AR. These findings provide support for the role of rACC in integrating interoceptive, emotional and cognitive functions via interactions with insula and striatal regions during effective emotion regulation in healthy individuals and a failure of this integration that may be specific to anxiety, particularly AP.
Keywords: emotion, functional connectivity, fMRI, cingulate, insula, anxiety
The ability to actively detect sources of potential threat or reward is critical for adaptive interactions with the environment. However, when emotional stimuli are not task-relevant, it may be adaptive to down-regulate their processing and remain task-focused. The pregenual portion of rostral anterior cingulate cortex (rACC), corresponding to Brodmann's ‘precingulate’; architectonic areas 24, 32 and 33, has been shown to play a key role in the regulation of emotional processing. Human neuroimaging studies show that rACC is more active when participants are asked to regulate conflicting emotional information (Etkin et al., 2006; Egner et al., 2008), avoid attending to irrelevant emotional information (Whalen et al., 1998; Vuilleumier et al., 2001; Bishop et al., 2004; Mohanty et al., 2007), or exercise top-down control upon processing of emotional stimuli (Ochsner et al., 2004; Ochsner and Gross, 2005; Petrovic et al., 2005; Banks et al., 2007). In non-clinical populations high in anxiety (Engels et al., 2007; Bishop, 2009) and individuals diagnosed with anxiety disorders (Shin et al., 2001; Klumpp et al., 2012; Wheaton et al., 2014) rACC has been shown to be less active when attempting to ignore emotional stimuli in the context of a cognitive task but more active in individuals with depression (Elliott et al., 2002; Mitterschiffthaler et al., 2008; Eugène et al., 2010).
Remaining task-focused in the presence of emotional distractors involves accurate assessment of emotional information, resolution of interference from emotional information, and recruitment of appropriate cognitive and motor control, a series of functions that require active communication between limbic, striatal, prefrontal and sensorimotor regions (Bush et al., 2000; Pollatos et al., 2007; Heatherton and Wagner, 2011). An examination of rACC functional connectivity with limbic, striatal prefrontal and motor cortices can thus clarify how rACC contributes to the integration of emotional, cognitive and behavioral processes. This integration may play a critical role not only in the normal emotion regulation but also in emotion dysregulation in anxiety. Tracer and cytoarchitectural studies show that rACC has rich anatomical connections with limbic regions involved in emotional processing, particularly anterior insular cortex (AIC) and amygdala (Mesulam and Mufson, 1982;Vogt and Pandya, 1987; Carmichael and Price, 1995; Palomero-Gallagher et al., 2008, 2009). The rostral and ventral portions of ACC also have been shown to have anatomical connections with prefrontal and striatal regions involved in cognitive and motor control, including lateral prefrontal and medial orbitofrontal cortex (OFC; Pandya et al., 1981; Carmichael and Price, 1996), as well as brainstem motor nuclei such as periaqueductal grey (Müller-Preuss and Jürgens, 1976; Hardy and Leichnetz, 1981), and striatum, especially ventral striatum (Kunishio and Haber, 1994; Devinsky et al., 1995; Haber et al., 2006). However, there are regional variations in connectivity of rACC subregions (Morecraft et al., 1992). For instance pregenual portions of rACC, corresponding primarily to BA 32, shows stronger connectivity with midcingulate, medial OFC and frontopolar regions (Van Hoesen et al., 1993; Carmichael and Price, 1995, 1996). The subgenual portion of rACC, corresponding primarily to BA 25, shows denser anatomical connectivity with amygdala/hippocampus, hypothalamus, periaqueductal grey and nucleus accumbens (Devinsky et al., 1995; Freedman et al., 2000; Ghashghaei and Barbas, 2002; Haber et al., 2006; Klein et al., 2007; Johansen-Berg et al., 2008).
Although the complex anatomical connectivity of rACC makes it an ideal candidate for active communication with limbic regions involved in emotional evaluation and frontostriatal regions involved in cognitive and motor control, its task-related functional connectivity during the regulation of emotional interference is not well understood. It would be particularly valuable to identify the role of this connectivity in emotion dysregulation, such as anxiety and depression. Anxiety is characterized by an attentional bias toward threatening stimuli (McNally, 1998; Compton et al., 2000; Nitschke and Heller, 2002) and reduced recruitment of rACC during attentional and cognitive control in the presence of emotional distractors (Bishop et al., 2004; Etkin et al., 2006; Klumpp et al., 2012). However, anxiety is not a monolithic construct; different neural mechanisms are involved in anxious apprehension (AP), characterized by verbal rumination and worry (Barlow, 1991; Heller et al., 1997; Sharp et al., 2015), and anxious arousal (AR), characterized by physiological hyperarousal and tension (Nitschke et al., 1999). Although no study to our knowledge has directly compared rACC-functional connectivity in pure AP vs AR groups, prior studies show that there is lower rACC-limbic structural and functional connectivity in generalized anxiety disorder (Etkin et al., 2009; Tromp et al., 2012), and the pattern of rACC-amygdala responsivity predicts treatment response in GAD (Whalen et al., 2008; Holzel et al., 2013). Since both AP and GAD are characterized by worry, we expected that the functional connectvity of rACC during emotional regulation may differ not only for anxious vs non-anxious groups but also for AP vs AR groups.
Anxiety and depression frequently co-occur, but attentional biases toward unpleasant information tend to be specific to anxiety (Mogg et al., 1993, 1995; Bar-Haim et al., 2007). A few studies show increased recruitment of rACC during attentional control in the presence of emotional distractors in depression (Elliott et al., 2002; Mitterschiffthaler et al., 2008; Eugène et al., 2010). However, these studies did not carefully assess comorbid anxiety or directly compare groups that isolate the specific effects of anxiety and depression (e.g. that are carefully selected to have high depression and low anxiety scores or high anxiety and low depression scores). This is particularly important because studies show that the degree, severity and type of co-occurring anxiety may differentially affect patterns of brain activation in depression (George et al., 1997; Elliott et al., 2002; Mitterschiffthaler et al., 2008; Engels et al., 2010; Herrington et al., 2010). Overall, due to the high comorbidity of anxiety and depression as well as the dearth of studies examining different subtypes of anxiety, it remains unclear whether psychological and neural correlates of emotional interference are specific to certain subtypes of anxiety or depression. Although not representative of clinical samples, pure groups that are high only in one type of anxiety or depression overcome the problems of comorbidity seen in clinical samples and allow a careful examination of psychological of neural dysfunction specific to particular constructs of anxiety and depression, as championed by the National Institute of Mental Health (NIMH) Research Domain Criteria (RDoC) initiative (Kozak and Cuthbert, 2016; Sharp et al., 2015; Yee et al., 2015; Miller et al., 2016).
In the present study, functional magnetic resonance imaging (fMRI) data were recorded while non-patient groups differing in trait AP, AR, anhedonic depression (AD) or none (CON) performed a task requiring them to ignore task-irrelevant pleasant, neutral, or unpleasant distractors. We then used psychophysiological interaction (PPI) analysis (Friston et al., 1997) to examine group differences in rACC connectivity with (i) AIC and amygdala involved in interoceptive and emotional evaluation and (ii) frontal and striatal regions involved in cognitive and motor control for unpleasant or pleasant vs neutral condition. Behaviorally, it was hypothesized that individuals reporting higher anxiety would show larger interference effects due to unpleasant words than would a comparison group, specifically AP > AR > AD = CON. This pattern was expected because attentional biases to unpleasant stimuli are typically seen in anxiety but not depression (Gotlib and Joormann, 2010) and because worry, a characteristic of AP, impairs processing efficiency via distraction and/or impaired inhibition (Eysenck et al., 2007; Levin et al., 2007).
Neurally, it was hypothesized that functional coupling of rACC with AIC and amygdala for unpleasant vs neutral words would be AP < AR < AD = CON. This is based on evidence of (i) greater rACC–AIC resting-state functional connectivity (Margulies et al., 2007; Seeley et al., 2007; Taylor et al., 2009; Deen et al., 2011), task-based co-activation (Medford and Critchley, 2010; Gu et al., 2013), and rACC-amygdala task-based functional connectivity during successful resolution of emotional interference (Etkin et al., 2006) in non-anxious individuals and (ii) reduced rACC-amygdala functional connectivity in generalized anxiety disorder (Etkin et al., 2009). Next, it was hypothesized that functional coupling of rACC with prefrontal and striatal regions for unpleasant vs neutral words would be AP < AR < AD = CON. This is due to the evidence of (i) greater rACC-prefrontal cortex (PFC) resting-state and task-based functional connectivity in non-anxious individuals (Kerns et al., 2004; Mayer et al., 2011), (ii) greater rACC-striatal task-based co-activation (Postuma and Dagher, 2006) with frontostrial connectivity predicting individual differences in recruitment of cognitive control in non-anxious individuals (Liston et al., 2006; Shannon et al., 2009) and (iii) greater association of AP with impairments in error monitoring (Moser et al., 2013) and reduction in frontocingulate recruitment (Silton et al., 2011).
Methods
Participants
Sixty (27 females) paid volunteers (mean age = 19.44 years, s.d. = 4.06) were recruited based on questionnaire screening of 1099 college students. Participants completed the Penn State Worry Questionnaire (PSWQ; Meyer et al., 1990; Molina and Borkovec, 1994), which measures AP, and the Mood and Anxiety Symptom Questionnaire (MASQ; Watson et al., 1995a,b), which measures AR with the MASQ-AA subscale and AD with the MASQ-AD subscale. The present study used an eight-item subscale of the MASQ-AD scale that has been shown to reflect depressed mood (Nitschke et al., 2001). Based on their responses to these scales, participants were classified as high AP (N = 15), high AR (N = 14), high AD (N = 9) or CON (N = 22). The AP group scored below the 50th percentile on the MASQ-AA (M = 20.33, s.d. = 1.97) and AD (M = 13.22, s.d. = 2.57) scales and above the 80th percentile on the PSWQ (M = 68.83, s.d. = 3.41). The AR group scored below the 50th percentile on the PSWQ (M = 39.09, s.d. = 7.54) and MASQ-AD (M = 15.36, s.d. = 1.29) scale and above the 80th percentile on the MASQ-AA (M = 37.36, s.d. = 4.32) scale. The AD group scored below the 50th percentile on the MASQ-AA (M = 21.22, s.d. = 2.86) scale and PSWQ (M = 33.11, s.d. = 10.19), and above the 80th percentile MASQ-AD (M = 18.56, s.d. = 3.17) scale. The CON group scored below the 50th percentile on MASQ-AD (M = 12.39, s.d. = 2.52), MASQ-AA (M = 20.33, s.d. = 1.85) and PSWQ (M = 36.50, s.d. = 8.62). Percentile scores were based on the initial screening samples used in the present study (see Supplementary data). Correlations between MASQ-AA and MASQ-AD, r = 0.21; MASQ-AA and PSWQ, r = −0.21; as well as MASQ-AD and PSWQ, r = −0.22 were not significant in the sample (all P > 0.05).
A histogram of each scale obtained from 5095 participants along with cut-offs confirmed the generalizability of present scores (see Supplementary data). Specifically, scores are consistent with scores reported in other studies conducted using the USA samples. For example, in one study that used the same percentile cut-offs (Larson et al., 2007), the mean MASQ-AA score in the AP group was M = 22.21, s.d. = 3.36; in the AD group M = 22.79, s.d. = 4.64 and in the CON group M = 21.31, s.d. = 3.96. These are very similar to the MASQ-AA scores for present sample. Another laboratory using similar methods reported a MASQ-AA mean of M = 23.90, s.d. = 6.13, in an unselected sample (Moser et al., 2012). This mean is also very similar to the present means reported and reflected in our larger sample (see Supplementary data). Although present scores are higher than those of Schulte-van Maaren et al., (2012), this likely reflects a fundamental difference in the sample populations (American vs Dutch), because studies indeed show lower prevalence of anxiety symptoms in the Netherlands (Bijl et al., 2003; Kessler et al., 2007). Furthermore, the consistency among scores of the studies described above strongly suggests that our scores are generalizable, at least to an American population.
The PSWQ, MASQ-AA and MASQ-AD were administered again when the participants visited for the imaging session. The groups maintained their significant differences on all three scales. The groups did not differ in age, F(3, 60) = 0.50, P = 0.61, or gender, χ2 (3, N = 60) = 5.15, P = 0.16. Participants were right-handed, native speakers of English with self-reported normal color vision. All participants were given a tour of the laboratory, had the study procedures explained to them and were screened for any contraindications for the MRI participation. Six participants (2 AP, 1 AR and 3 CON) were excluded from fMRI data analyses due to scanner artifacts, leaving a total of 54 participants. Subsets of the present participant group have been used for previous publications focusing on task-related activation differences (Engels et al., 2007; Mohanty et al., 2007); however, the analyses and questions asked in the present research are novel and have not previously been reported.
Stimuli and experimental design
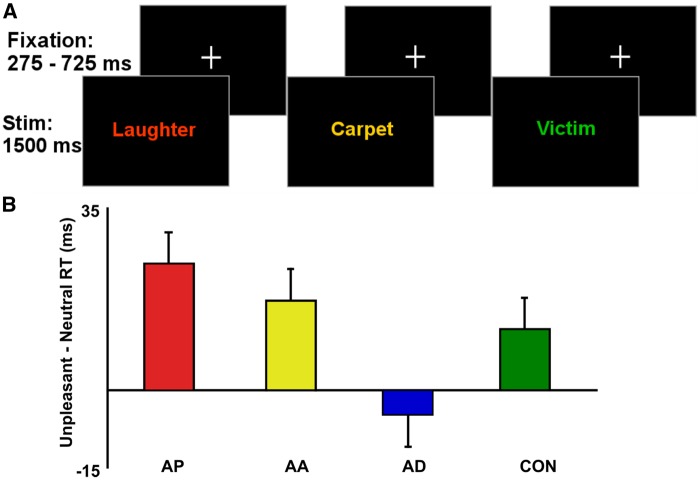
In line with methods reported earlier (Engels et al., 2007; Mohanty et al., 2007), the stimuli consisted of 256 words selected from the Affective Norms for English Words set (ANEW: Bradley and Lang, 1999). Sixty-four pleasant (e.g. birthday, ecstasy, laughter), 2 sets of 64 neutral (e.g. hydrant, moment, carpet) and 64 unpleasant (e.g. suicide, war, victim) words were carefully selected on the basis of established norms for arousal, valence and frequency of usage in the English language (Bradley and Lang, 1999; Toglia and Battig, 1978) as well as the number of letters. The pleasant and unpleasant words were higher in arousal with differing valences, whereas the neutral words were low in arousal and valence. All words ranged from three to eight letters long. Each trial consisted of a word presented in capital letters on a black background for 1500 ms, Tahoma 72-point font, in 1 of the 4 ink colors (red, yellow, green and blue), followed by a fixation cross presented randomly varying from 275 to 725 ms. Participants were instructed to press one of the four buttons (two per hand) to indicate the color of the word on the screen as quickly and accurately as possible while ignoring the meaning of the word (Figure 1).
Fig. 1.
(A) Participants reported the ink-color of words presented in alternating blocks of pleasant, neutral and unpleasant words. Only performance in unpleasant and neutral conditions was examined in the present study. (B) RT for unpleasant minus neutral words for the groups scoring high on anxious apprehension (AP), anxious arousal (AR), anhedonic depression (AD) and neither (CON). Error bars represent standard error of mean.
Trials were presented in blocks of pleasant, neutral or unpleasant words. Participants received 256 trials over the course of 16 blocks (4 pleasant, 8 neutral, 4 unpleasant) of 16 trials. Trials were blocked because pilot studies for the current project as well as published studies showed that a blocked design is more effective in eliciting interference due to emotional words than is an intermixed design (Dalgleish, 1995; Holle et al., 1997; Compton et al., 2000; Engels et al., 2007). Furthermore, pilot studies showed that in a block design there is an influence of emotional word blocks on the reaction time (RT) of immediately subsequent neutral word blocks (Engels et al., 2007). Hence, the order of presentation of blocks was counterbalanced across participants to ensure that the emotional and neutral blocks preceded each other equally often.
After every fourth block, participants were given a brief rest period. In addition to the 16 word blocks, four fixation blocks were included, with one at the beginning, one at the end, and two in the middle of the experiment. For fixation blocks, in the place of a word a brighter fixation cross was presented for 1500 ms, followed by the standard fixation cross. To control for stimulus familiarity, no word was repeated throughout the experiment (although some participants saw some of the same words in a parallel EEG session on a different day). During the course of a block, each color appeared only four times, and trials were pseudorandomized so that a color could occur consecutively no more than twice. STIM software was used to control word presentation and reaction-time measurement (James Long Company, Caroga Lake, NY, USA). MRI-compatible LCD goggles were used to display stimuli (Magnetic Resonance Technologies, Willoughby, OH, USA). Since pleasant stimuli are often less potent distractors than unpleasant stimuli (Hansen and Hansen, 1988), and because attentional biases in anxiety are typically seen for unpleasant stimuli (Bradley et al., 1998; Bradley, Mogg, White, Groom, & Bono, 1999; Fox et al., 2002), present hypotheses focus on attention in the presence of unpleasant and neutral word conditions. However, data from pleasant and unpleasant word conditions were analyzed to confirm that hypothesized differences are seen for the unpleasant but not the pleasant word condition.
Image acquisition
Structural and functional MRI data were acquired using a 3T Siemens Allegra. Functional volumes (N = 373) were acquired parallel to the axial plane of the anterior and posterior commissure with an interleaved echoplanar imaging sequence using the following parameters: 2000 ms repetition time (TR), 25 ms echo time (TE), 20 slices, 7 mm slice thickness, 3.75 × 3.75 mm2 in-plane resolution, 240 mm field of view (FOV), 60° flip angle. Although 7 mm is a relatively large slice thickness it has successfully been used to detect activity in limbic regions related to emotional processing (Stein et al., 2002; Shin et al., 2005). Structural images were acquired via a sagittal magnetization prepared rapid gradient echo (MPRAGE) sequence, TR 2000 ms, TE 25 ms, 60° flip angle, 240 mm FOV, 1.3 mm slice thickness, 1 × 1 mm2 in-plane resolution.
fMRI data preprocessing
The first six volumes were discarded in order to allow the signal to reach a steady state. The fMRI data were then preprocessed using SPM8 software (available at: http://www.fil.ion.ucl.ac.uk/spm) implemented in MATLAB (Mathworks, Inc., Natick, MA, USA). Images were spatially realigned to correct for motion with a fourth degree B-spline, co-registered to the participant’s mean functional image and high-resolution anatomical T1 scan, spatially normalized to a canonical T1 image, and smoothed with an 8 mm full-width half-maximum Gaussian kernel. No participants exhibited head motion of more than 3 mm in any direction. To test if there were differences between participant groups due to different patterns of movement, a one-way analysis of variance (ANOVA) was performed on average movement in each of the x, y and z dimensions, as well as for pitch, roll and yaw. None of the ANOVAs yielded significant group differences in any dimension, all F values <2.8, all P values >0.05.
Functional connectivity analyses
Given that rACC plays a crucial role in regulating task-irrelevant emotional interference, the present study examined its functional connectivity during pleasant, neutral or unpleasant distractors. A PPI analysis (Friston et al., 1997; Gitelman et al., 2003; McLaren et al., 2012) was conducted to examine how the difference between task conditions (pleasant, neutral or unpleasant distractors) changes the relationship of rACC with each voxel in the whole brain. PPI analyses estimate the contribution of an interaction between a psychological factor (change in experimental condition) and a physiological factor (activity in the seed region) to the activity in each voxel in the brain. This basic analysis method is extended to the generalized form of context-dependent PPI analyses (gPPI; http://brainmap.wisc.edu/PPI; McLaren et al., 2012), which enables modeling of connectivity differences by group and condition, thus increasing flexibility of statistical modeling over standard PPI methods. Statistical testing of gPPI comparing it to standard PPI methods found that gPPI improved model fit and sensitivity to true positive findings (McLaren et al., 2012; Cisler et al., 2013).
The rACC seed region for connectivity analyses was identified functionally as the region that was most responsive to unpleasant vs neutral words in the CON group (Figure 2; see Supplementary data). Furthermore, across a range of fMRI studies a similar region of the rACC emerged as sensitive to emotion-related interference or conflict (Whalen et al., 1998; Vuilleumier et al., 2001; Bishop et al., 2004; Etkin et al., 2006; Mohanty et al., 2007; Egner et al., 2008). Additionally, we also confirmed our results using a purely anatomically defined rACC seed region. The anatomical rACC seed region was defined based on a meta-analysis across nearly 10 000 studies to comprehensively map psychological states to discrete sub-regions in medial frontal cortex using relatively unbiased, data-driven methods (De La Vega et al., 2016). This approach revealed three distinct zones that differed substantially in function, each of which was further subdivided into two to four smaller subregions that showed additional functional variation. The region corresponding to rACC was selected as the seed region that was identified as mapping onto emotional function. This anatomical seed region corresponded primarily to BA 32 and also included some parts of BA 24.
Fig. 2.
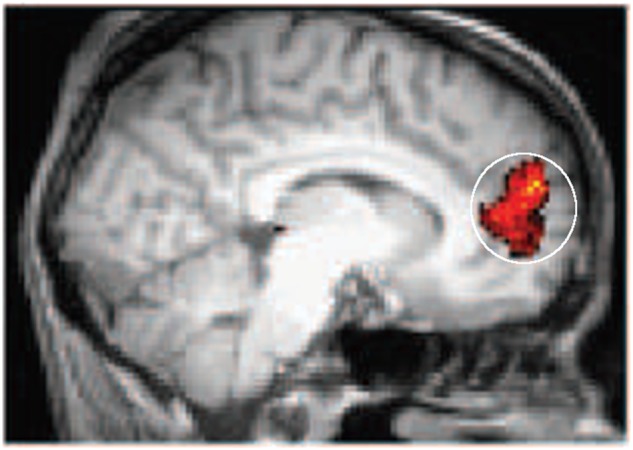
Higher rostral anterior cingulate (rACC) activation for unpleasant vs neutral words in CON participants constituted the seed region for functional connectivity analyses.
PPI analyses
For each subject, the ‘psychological’ term was computed by convolving the condition onset times for pleasant, neutral, and unpleasant conditions separately with the canonical HRF, and the ‘physiological’ term was estimated as the first eigenvariate time series of the BOLD signal extracted from rACC seed region (described above). This represents the average BOLD signal weighted by the voxel significance. To compute the ‘psychophysiological’ interaction term, time series was first de-convolved with the hemodynamic signal (Gitelman et al., 2003) to model out the effects of the canonical hemodynamic response function (HRF). The deconvolved physiological factor was multiplied by the psychological variable and again re-convolved with the HRF, giving the interaction term. PPI analyses were conducted by regressing activity in each voxel against the interaction term while controlling for variance associated with the psychological and physiological main effects. This generated the per-voxel parameter estimate (β) maps representing the magnitude of functional connectivity between the rACC seed region and voxel-wise activation in the brain as a function of unpleasant vs neutral and pleasant vs neutral word condition.
To assess how groups differed in rACC functional connectivity as a function of unpleasant vs neutral words, the PPI interaction term β maps for the unpleasant vs neutral word contrast were subjected to a one-way ANOVA with groups (AP, AR, AD and CON) as a factor, implemented in SPM8 (Penny et al., 2002). Similar analyses were conducted using pleasant vs neutral contrasts. For the hypothesis-driven examination of rACC connectivity with AIC and amygdala, an ROI was created by drawing a 12 mm sphere in bilateral AIC (±32, 10, −6) whose location was obtained from an in-depth examination of insular connectivity through cluster analysis (Deen et al., 2011) and an 8 mm sphere in bilateral amygdala (left: −21, −5, −16; right: 22, −4, −15) whose location was obtained from a broad meta-analysis of amygdala functional connectivity during emotional tasks (Sergerie et al., 2008) and combining them into a single ROI mask using WFU PickAtlas (Maldjian et al., 2003, 2004). The 3dClustSim program (December 2015 version) was used to control multiple voxelwise statistical testing in the ROI mask (Forman et al., 1995; Cox, 1996). A corrected significance level of P < 0.05 was achieved with a minimum cluster-size threshold of 32 contiguously activated voxels derived via Monte Carlo simulations. For all other results, a gray-matter mask taken from WFU PickAtlas was used, with a minimum size of 66 voxels derived via Monte Carlo simulations, resulting in a corrected threshold of P < 0.05. Next, orthogonal planned contrasts comparing experimental groups (AP and AR vs CON and AD, AP vs AR, and AD vs CON) were conducted in regions determined to be significant in the group-wise ANOVA. Results were examined at a-corrected threshold of P < 0.05. Finally, the MarsBaR toolbox (MARSeille Boîte À Région d’Intérêt; http://marsbar.sourceforge.net/) was used to extract beta values from significant clusters of activation for display purposes only.
Results
Behavioral data
All participants demonstrated greater than 80% accuracy on the task. The interference effect due to unpleasant words was calculated as the difference in RT for unpleasant minus neutral words. Across all subjects, there was a significant interference effect due to unpleasant words, t(53) = 3.06, P = 0.003, and pleasant words, t(53) = 2.68, P = 0.01, indicating that task-relevant processing of color is impaired in the presence of pleasant and unpleasant distractors. Figure 1 shows that the unpleasant word-related interference effect was AP > AR > CON > AD. A one-way between-groups ANOVA comparing the interference effect due to unpleasant words for the four groups was marginally significant, F(3, 51) = 2.541, P = 0.07. Dissecting this with orthogonal planned comparisons showed an interference effect due to unpleasant words that was greater for AP and AR vs AD and CON, t(51) = 2.18, P = 0.03, not different for AP vs AR, t(51) = −0.96, P = 0.34, and marginally less for AD vs CON, t(51) = −1.78, P = 0.08. A one-way between-groups ANOVA confirmed that these group differences in interference effect were not driven by group differences in RT for neutral words, F(3, 51) = 0.62, P = 0.61. Finally, a one-way between-groups ANOVA confirmed that there was no significant effect of group on interference due to pleasant distractors, F(3, 51) = 0.253, P > 0.5.
Group differences in rACC functional connectivity for unpleasant vs neutral words
Neurally, it was hypothesized that differential functional coupling of rACC with AIC and amygdala during unpleasant vs neutral words would be AP < AR< AD = CON. A voxelwise between-group ANOVA yielded group differences in rACC connectivity with AIC for unpleasant vs neutral words at peak MNI coordinates (−28, 6, 4; peak z-score = 3.44; Figure 3A). Planned comparisons among the groups showed weaker rACC–AIC connectivity for AP and AR vs CON and AD and AP vs AR, but no significant difference for AD vs CON. Contrary to the hypothesis, a voxelwise between-group ANOVA on rACC-amygdala connectivity for unpleasant vs neutral words showed no significant differences between groups.
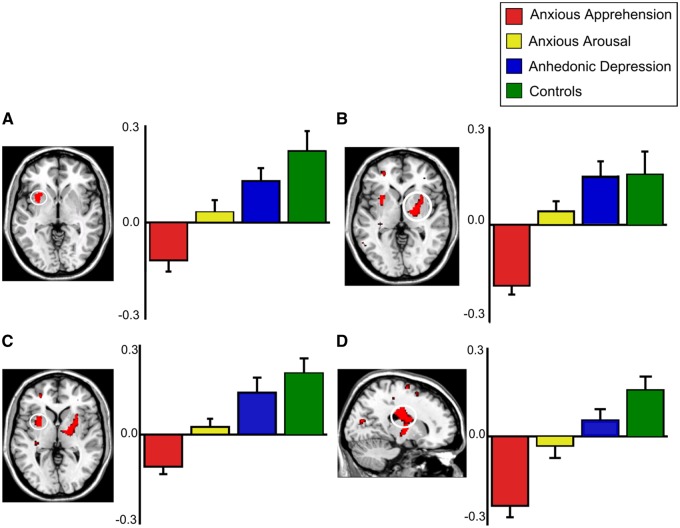
Fig. 3.
Higher functional connectivity between rACC seed region and (A) anterior insular cortex (AIC), (B) putamen, (C) rostral caudate and (D) pallidum and thalamus for unpleasant than for neutral words. Error bars represent standard error of mean.
Next, it was hypothesized that the differential functional coupling of rACC with prefrontal and striatal regions for unpleasant vs neutral words would be AP < AR < AD = CON. Using whole-brain analyses, the differential connectivity of rACC with rostral putamen (Figure 3B) and rostral caudate (Figure 3C) showed the predicted pattern. The pattern for rACC-ventral pallidum and thalamus (Figure 3D) was also as predicted, AP < AR < AD = CON. Significant group-related differences in rACC connectivity were found for rostral putamen (−28, 4, 2; peak z-score = 3.38), rostral caudate (16, −12, 26; peak z-score = 3.28), and ventral pallidum and thalamus (18, −6, 0; peak z-score = 3.02). Planned comparisons among the groups showed weaker rACC connectivity with rostral putamen, rostral caudate and ventral pallidum for AP and AR vs CON and AD and AP vs AR, respectively, but no significant difference for AD vs CON. An ANOVA on neutral trials only was conducted to confirm that they did not carry the connectivity differences. rACC connectivity to AIC and striatal regions for neutral words did not differ for the regions outlined above, indicating that group-related differences were driven primarily by unpleasant distractors. Finally, in order to determine that no subject in particular drove the results, a between-subjects ANOVA was performed removing each subject from the AD group one at a time showed no difference in significance (all ps < 0.01).
Next, we examined hypotheses regarding differential rACC functional connectivity for groups using an anatomically defined rACC seed (de la Vega et al., 2016). Overall, results were very similar to those obtained with the functionally defined rACC seed. A voxelwise between-group ANOVA yielded group differences in rACC connectivity with AIC for unpleasant vs neutral words at peak MNI coordinates (−28, 6, 4; peak z-score = 3.57; Supplementary data, Figure S1A). Planned comparisons among the groups showed weaker rACC–AIC connectivity for AP and AR vs CON and AD, as well as AD vs CON, but not AR vs AP. Next, rACC connectivity with prefrontal and striatal regions was examined. Results for rostral caudate (22, 6, 14; peak z-score = 3.26; Supplementary data, Figure S1C) and pallidum (20, −2, −2; peak z-score = 3.26; Supplementary data, Figure S1D) replicated analyses using the functional defined ROI. Results for rostral putamen (−24, 8, 8; peak z-score = 3.91; Supplementary data, Figure S1B) showed a between-group difference for AP and AR vs CON and AD, as well as AP vs AR and a greater difference for AD vs CON.
Group differences in rACC connectivity for pleasant vs neutral words
Using ROI analyses, no group related differences were observed in functional coupling of rACC with AIC and amygdala during pleasant vs neutral words. Similarly, whole-brain analyses yielded no group-related differences in rACC functional connectivity for pleasant vs neutral words.
Discussion
Remaining task-focused in the presence of salient distractors involves effective integration of sensory, emotional, cognitive and motor processes. The rACC is anatomically well situated to perform this integrative function by actively communicating with limbic, prefrontal, and striatal regions, but its functional connectivity during tasks requiring affective and cognitive control is not well studied. The present study explored rACC connectivity during a task that required attention to task-relevant information in the presence of task-irrelevant emotional distractors. Behavioral results demonstrated the effectiveness of the experimental paradigm in eliciting interference from unpleasant words, and a trend toward the hypothesized pattern of interference for AP > AR > AD = CON. While ignoring task-irrelevant unpleasant information, there was greater functional coupling of rACC with AIC and striatal regions in control participants. However, there was a reduction in this coupling during unpleasant vs neutral distractors in participants with different types of anxiety, more so for AP than AR. Finally, this reduction in connectivity was not seen in AD, suggesting that this effect is specific to anxiety. These findings were confirmed using both functionally and anatomically defined rACC seed regions. Our findings clarify the functional connections via which rACC integrates emotional, cognitive and behavioral processes in the service of effective emotional and cognitive control, as well as the failure of this connectivity in anxiety. Furthermore, present findings were seen only for unpleasant words; no group-related behavioral or connectivity differences were observed for pleasant words. These results are consistent with the expectation that unpleasant stimuli would be more distracting than pleasant stimuli and serve to capture attention more effectively in the presence of anxiety.
Although there have been few studies examining task-based differences in functional connectivity between the ACC and the AIC, the two regions have often been shown to be co-activated at rest and across a range of tasks (Seeley et al., 2007; Medford and Critchley, 2010; Palaniyappan and Liddle, 2012). The AIC plays an important role in interoceptive and emotional awareness (Craig, 2009, 2010, 2011; Singer et al., 2009; Jones et al., 2010; Seth et al., 2011; Gu et al., 2013). Interoceptive awareness is the awareness of the physiological condition of the body (Craig, 2002, 2003), and emotional awareness refers to the ability to identify and label internal emotional experience (Penza-Clyve and Zeman, 2002). It has been proposed that AIC plays a critical role in interoceptive predictive coding, i.e. the inference of emotions from the physiological condition of the body (Seth et al., 2011; Gu et al., 2013). Since emotional awareness is an important contributor to emotion regulation (Subic-Wrana et al., 2014), rACC–AIC connectivity may play a significant role in emotion regulation. Interestingly, AIC and ACC are the two regions of the human brain that contain Von Economo neurons which are recently evolved cells that may be involved in assessment of emotional and social situations (Allman et al., 2005). Earlier functional imaging studies have shown stronger resting-state connectivity between AIC and pregenual ACC (Taylor et al., 2009) than between AIC and other parts of ACC. Present results bolster these findings by showing that this connectivity varies with task and individual differences in anxiety, with compromised connectivity when regulating emotion-related distraction in anxiety, especially in high AP.
Contrary to hypothesis, rACC-amygdala connectivity did not vary with task or individual differences in anxiety. The lack of rACC-amygdala connectivity differences may appear inconsistent with reports of a negative relationship between rACC and amygdala (Etkin et al., 2006); however, other studies involved different tasks, requiring resolution of emotional conflict. Although there is attentional competition between the unpleasant meaning of words and the color of words in the present stimuli, there is no direct conflict between these two dimensions (Algom et al., 2004). The absence of direct emotion-related conflict may have contributed to the negative finding in the present study. Furthermore, the use of pregenual rACC as opposed to subgenual rACC as the seed region as well as present imaging parameters were not well optimized for precise measurements of amygdala activation or connectivity.
Since striatal regions are typically involved in reward-related processing (Phan et al., 2004), it is intriguing here that task and group-related differences were found in rACC connectivity with caudate/putamen, ventral pallidum, and thalamus. The rACC, thalamus and striatal regions in the present study constitute parts of the corticobasal circuit (Giguere and Goldman-Rakic, 1988; Carmichael and Price, 1995; Haber et al., 1995; Haber et al., 2006), which along with other circuits, plays an important role in integrating sensory input with emotional/motivational processing to modulate learning and develop task-directed behaviors and action plans (Haber, 2011). The present study provides evidence supporting the role of rACC functional connectivity to striatal, pallidal and thalamic regions in situations requiring cognitive and motor control during motivationally salient distractors. These results are consistent with studies showing resting-state connectivity (Di Martino et al., 2008) and greater task-based co-activation (Postuma and Dagher, 2006) between vmPFC (including rACC), and the ventral striatum and pallidum. Present results of increased rACC-striatal connectivity in CON are in line with the view that frontostriatal circuitry plays an important role in emotional control (Wang et al., 2008; Marchand, 2010; Shafer et al., 2012) and with studies showing that individual differences in frontostriatal connectivity predict efficiency of cognitive control (Liston et al., 2006; Shannon et al., 2009). Finally, decreased rACC-striatal functional connectivity in anxiety is consistent with the involvement of this circuitry in anxiety disorders such as panic disorder (Marchand et al., 2010), and social phobia (Sareen et al., 2007; van der Wee et al., 2008).
Individual differences in state and trait anxiety have been shown to bias attention toward unpleasant stimuli and slow disengagement from unpleasant stimuli but not pleasant or neutral stimuli (Fox et al., 2001; Sass et al., 2010; Sharp et al., 2015). In the present study, both groups high in anxiety showed lower rACC connectivity to insula and striatal regions than did CON. AP is characterized by worry which impairs attentional control on other tasks with emotional distractors. According to the attentional control theory, worry impairs processing efficiency and not performance effectiveness via distraction and/or impaired inhibition (Eysenck et al., 2007). AP is strongly associated with an aberrantly high error-related negativity, hypothesized to reflect compensation for an initial failure of goal maintenance as worry consumes working memory resources (Moser et al., 2013). In line with this, AP is associated with increased activity in dorsolateral PFC (Warren et al., 2013) and dorsal aspects of ACC (Silton et al., 2011) during interference from task-irrelevant distractors. These frontocingulate increases are interpreted as evidence of recruitment of increased top-down control to mitigate the distracting effect of worry. It is possible that in the present study AP participants employed greater connectivity to compensate for effects of worry while staying task-focused during neutral distractors but were unable to do so in the presence of unpleasant distractors.
In studies in which participants are diagnosed using Diagnostic and Statistical Manual of Mental Disorders criteria, it is often difficult or impossible to attribute the participants’ attentional control problems to depression, anxiety or both, given the high comorbidity of depression and anxiety. Although present groups are not representative of the heterogeneous presentation typically seen in individuals with clinically diagnosed anxiety and depression, the aim of the study was not to study clinical phenomena but to examine pure constructs such as AP, AA and AD and their relationship with emotional-related interferences and corresponding rACC connectivity. To develop effective and targeted treatments for anxiety and depression, it is important to develop clinical assessment methods with high symptom sensitivity and specificity. The identification of how attentional impairment operates in anxiety and depression may allow the development of evidenced-based treatments involving training in attentional control methods such as cognitive control therapy (Siegle et al., 2007) or mindfulness-based cognitive behavioral therapy (Segal et al., 2002). Using a carefully selected sample in which levels of anxiety and depression were controlled, the present study sheds light on the specificity of attentional control impairments in the presence of emotional distractors, suggesting that these impairments are specific to anxiety and not present in depression. Furthermore, the scores in the present study for measures of different types of anxiety and depression are generalizable to an American sample. Findings from this study highlight the connectivity through which rACC plays a critical role not only in normal emotion regulation but in emotion dysregulation in anxiety.
Supplementary data
Supplementary data are available at SCAN online.
Funding
This research was supported by the National Institute of Drug Abuse (R21 DA14111), the National Institute of Mental Health (R01 MH61358, P50 MH079485, T32 MH14257, T32 MH19554), and the University of Illinois Beckman Institute and Intercampus Research Initiative in Biotechnology. The authors thank Nancy Dodge, Mike Niznikiewicz, Allie Letkiewicz, Sarah Sass, Brad Sutton, Holly Tracy, Andrew Webb and Tracey Wszalek for their contributions to this project.
Conflict of interest. None declared.
Supplementary Material
References
- Algom D., Chajut E., Lev S. (2004). A rational look at the emotional Stroop phenomenon: a generic slowdown, not a Stroop effect. Journal of Experimental Psychology: General, 133(3), 323.. [DOI] [PubMed] [Google Scholar]
- Allman J.M., Watson K.K., Tetreault N.A., Hakeem A.Y. (2005). Intuition and autism: a possible role for Von Economo neurons. Trends in Cognitive Sciences, 9(8), 367–73. [DOI] [PubMed] [Google Scholar]
- Banks S.J., Eddy K.T., Angstadt M., Nathan P.J., Phan K.L. (2007). Amygdala-frontal connectivity during emotion regulation. Social Cognitive Affective Neuroscience, 2(4), 303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y., Lamy D., Pergamin L., Bakermans-Kranenburg M.J., Van Ijzendoorn M.H. (2007). Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychological Bulletin, 133(1), 1.. [DOI] [PubMed] [Google Scholar]
- Barlow D.H. (1991). Disorders of emotion. Psychological Inquiry, 2(1), 58–71. [Google Scholar]
- Bijl R.V., de Graaf R., Hiripi E., et al. (2003). The prevalence of treated and untreated mental disorders in five countries. Health Affairs, 22(3), 122–33. [DOI] [PubMed] [Google Scholar]
- Bishop S., Duncan J., Brett M., Lawrence A.D. (2004). Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nature Neuroscience, 7(2), 184–8. [DOI] [PubMed] [Google Scholar]
- Bishop S.J. (2009). Trait anxiety and impoverished prefrontal control of attention. Nature Neuroscience, 12(1), 92–8. [DOI] [PubMed] [Google Scholar]
- Bradley B.P., Mogg K., Falla S.J., Hamilton L.R., (1998). Attentional bias for threatening facial expressions in anxiety: Manipulation of stimulus duration. Cognition & Emotion, 12(6), 737–53. [Google Scholar]
- Bradley B.P., Mogg K., White J., Groom C., Bono J., (1999). Attentional bias for emotional faces in generalized anxiety disorder. British Journal of Clinical Psychology, 33(3), 267–78. [DOI] [PubMed] [Google Scholar]
- Bradley M.M., Lang P.J., (1999). Affective norms for English words (ANEW): Instruction manual and affective ratings (pp. 1-45). Technical report C-1, the center for research in psychophysiology, University of Florida.
- Bush G., Luu P., Posner M.I. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences, 4(6), 215–22. [DOI] [PubMed] [Google Scholar]
- Carmichael S.T., Price J.L. (1995). Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. The Journal of Comparative Neurology, 363(4), 615–41. [DOI] [PubMed] [Google Scholar]
- Carmichael S.T., Price J.L. (1996). Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. Journal of Comparative Neurology, 371(2), 179–207. [DOI] [PubMed] [Google Scholar]
- Cisler J.M., James G.A., Tripathi S., et al. (2013). Differential functional connectivity within an emotion regulation neural network among individuals resilient and susceptible to the depressogenic effects of early life stress. Psychological Medicine, 43(03), 507–18. [DOI] [PubMed] [Google Scholar]
- Compton R.J., Heller W., Banich M.T., Palmieri P.A., Miller G.A. (2000). Responding to threat: hemispheric asymmetries and interhemispheric division of input. Neuropsychology, 14(2),254–64. [DOI] [PubMed] [Google Scholar]
- Cox R.W. (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29(3), 162–73. [DOI] [PubMed] [Google Scholar]
- Craig A.D. (2002). How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience, 3(8), 655–66. [DOI] [PubMed] [Google Scholar]
- Craig A.D. (2003). Interoception: the sense of the physiological condition of the body. Current Opinion in Neurobiology, 13(4), 500–5. [DOI] [PubMed] [Google Scholar]
- Craig A.D. (2009). How do you feel—now? The anterior insula and human awareness. Nature Reviews Neuroscience, 10(1), 59–70. [DOI] [PubMed] [Google Scholar]
- Craig A.D. (2010). The sentient self. Brain Structure and Function, 1–15. [DOI] [PubMed] [Google Scholar]
- Craig A.D. (2011). Significance of the insula for the evolution of human awareness of feelings from the body. Annals of the New York Academy of Sciences, 1225, 72–82. [DOI] [PubMed] [Google Scholar]
- Dalgleish T. (1995). Performance on the emotional Stroop task in groups of anxious, expert, and control subjects: a comparison of computer and card presentation formats. Cognition & Emotion, 9(4), 341–62. [Google Scholar]
- Deen B., Pitskel N.B., Pelphrey K.A. (2011). Three systems of insular functional connectivity identified with cluster analysis. Cerebral Cortex, 21(7), 1498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Vega A., Chang L.J., Banich M.T., Wager T.D., Yarkoni T. (2016). Large-scale meta-analysis of human medial frontal cortex reveals tripartite functional organization. Journal of Neuroscience, 36(24), 6553–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O., Morrell M.J., Vogt B.A. (1995). Contributions of anterior cingulate cortex to behaviour. Brain, 118 (Pt 1), 279–306. [DOI] [PubMed] [Google Scholar]
- Di Martino A., Scheres A., Margulies D.S., et al. (2008). Functional connectivity of human striatum: a resting state FMRI study. Cerebral Cortex, 18(12), 2735–47. [DOI] [PubMed] [Google Scholar]
- Egner T., Etkin A., Gale S., Hirsch J. (2008). Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cerebral Cortex, 18(6), 1475–84. [DOI] [PubMed] [Google Scholar]
- Elliott R., Rubinsztein J.S., Sahakian B.J., Dolan R.J. (2002). The neural basis of mood-congruent processing biases in depression. Archives of General Psychiatry, 59(7), 597–604. [DOI] [PubMed] [Google Scholar]
- Engels A.S., Heller W., Mohanty A., et al. (2007). Specificity of regional brain activity in anxiety types during emotion processing. Psychophysiology, 44(3), 352–63. [DOI] [PubMed] [Google Scholar]
- Engels A.S., Heller W., Spielberg J.M., et al. (2010). Co-occurring anxiety influences patterns of brain activity in depression. Cognitive, Affective, & Behavioral Neuroscience, 10(1), 141–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Egner T., Peraza D.M., Kandel E.R., Hirsch J. (2006). Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron, 51(6), 871–82. [DOI] [PubMed] [Google Scholar]
- Etkin A., Prater K.E., Schatzberg A.F., Menon V., Greicius M.D. (2009). Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Archives of General Psychiatry, 66(12), 1361–72. [DOI] [PubMed] [Google Scholar]
- Eugène F., Joormann J., Cooney R.E., Atlas L.Y., Gotlib I.H. (2010). Neural correlates of inhibitory deficits in depression. Psychiatry Research: Neuroimaging, 181(1), 30–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck M.W., Derakshan N., Santos R., Calvo M.G. (2007). Anxiety and cognitive performance: attentional control theory. Emotion, 7(2), 336–53. [DOI] [PubMed] [Google Scholar]
- Forman S.D., Cohen J.D., Fitzgerald M., Eddy W.F., Mintun M.A., Noll D.C. (1995). Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster‐size threshold. Magnetic Resonance in Medicine, 33(5), 636–47. [DOI] [PubMed] [Google Scholar]
- Fox E., Russo R., Bowles R., Dutton K. (2001). Do threatening stimuli draw or hold visual attention in subclinical anxiety? Journal of Experimental Psychology: General, 130(4), 681.. [PMC free article] [PubMed] [Google Scholar]
- Fox E., Russo R., Dutton K., (2002). Attentional bias for threat: Evidence for delayed disengagement from emotional faces. Cognition & Emotion, 16(3), 355–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman L.J., Insel T.R., Smith Y. (2000). Subcortical projections of area 25 (subgenual cortex) of the macaque monkey. Journal of Comparative Neurology, 421(2), 172–88. [PubMed] [Google Scholar]
- Friston K.J., Buechel C., Fink G.R., Morris J., Rolls E., Dolan R.J., (1997). Psychophysiological and modulatory interactions in neuroimaging. NeuroImage, 6(3), 218–29. [DOI] [PubMed] [Google Scholar]
- George M.S., Ketter T.A., Parekh P.I., et al. (1997). Blunted left cingulate activation in mood disorder subjects during a response interference task (the Stroop). Journal of Neuropsychiatry and Clinical Neurosciences. [DOI] [PubMed] [Google Scholar]
- Ghashghaei H.T., Barbas H. (2002). Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience, 115(4), 1261–79. [DOI] [PubMed] [Google Scholar]
- Gotlib I.H., Joormann J. (2010). Cognition and depression: current status and future directions. Annual Review of Clinical Psychology, 6, 285.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguere M., Goldman-Rakic P.S. (1988). Mediodorsal nucleus: areal, laminar, and tangential distribution of afferents and efferents in the frontal lobe of rhesus monkeys. Journal of Comparative Neurology, 277(2), 195–213. [DOI] [PubMed] [Google Scholar]
- Gitelman D.R., Penny W.D., Ashburner J., Friston K.J., (2003). Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. NeuroImage, 19(1), 200–7. [DOI] [PubMed] [Google Scholar]
- Gu X., Hof P.R., Friston K.J., Fan J. (2013). Anterior insular cortex and emotional awareness. Journal of Comparative Neurology, 521(15), 3371–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S.N. (2011). Neuroanatomy of Reward: A View from the Ventral Striatum In Gottfried J.A., editor. Neurobiology of sensation and reward, CRC Press; Boca Raton, FL. [PubMed] [Google Scholar]
- Haber S.N., Kim K.S., Mailly P., Calzavara R. (2006). Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. The Journal of Neuroscience, 26(32), 8368–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S.N., Kunishio K., Mizobuchi M., Lynd-Balta E. (1995). The orbital and medial prefrontal circuit through the primate basal ganglia. The Journal of Neuroscience, 15(7), 4851–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen C.H., Hansen R.D. (1988). Finding the face in the crowd: an anger superiority effect. Journal of Personality and Social Psychology, 54(6), 917–24. [DOI] [PubMed] [Google Scholar]
- Hardy S.G.P., Leichnetz G.R. (1981). Cortical projections to the periaqueductal gray in the monkey: a retrograde and orthograde horseradish peroxidase study. Neuroscience Letters, 22(2), 97–101. [DOI] [PubMed] [Google Scholar]
- Heatherton T.F., Wagner D.D. (2011). Cognitive neuroscience of self-regulation failure. Trends in Cognitive Sciences, 15(3), 132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller W., Nitschke J.B., Etienne M.A., Miller G.A. (1997). Patterns of regional brain activity differentiate types of anxiety. Journal of Abnormal Psychology, 106(3), 376–85. [DOI] [PubMed] [Google Scholar]
- Herrington J.D., Heller W., Mohanty A., et al. (2010). Localization of asymmetric brain function in emotion and depression. Psychophysiology, 47(3), 442–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holle C., Neely J.H., Heimberg R.G. (1997). The effects of blocked versus random presentation and semantic relatedness of stimulus words on response to a modified Stroop task among social phobics. Cognitive Therapy and Research, 21(6), 681–97. [Google Scholar]
- Hölzel B.K., Hoge E.A., Greve D.N., et al. (2013). Neural mechanisms of symptom improvements in generalized anxiety disorder following mindfulness training. NeuroImage: Clinical, 2, 448–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C.L., Ward J., Critchley H.D. (2010). The neuropsychological impact of insular cortex lesions. Journal of Neurology, Neurosurgery & Psychiatry, 81(6), 611–8. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H., Gutman D.A., Behrens T.E.J., et al. (2008). Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cerebral Cortex, 18(6), 1374–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns J.G., Cohen J.D., MacDonald A.W., Cho R.Y., Stenger V.A., Carter C.S. (2004). Anterior cingulate conflict monitoring and adjustments in control. Science, 303(5660), 1023–6. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Matthias A., Anthony J.C., et al. (2007). Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization s World Mental Health Survey Initiative. 6(3), 168–76. [PMC free article] [PubMed] [Google Scholar]
- Klein J.C., Behrens T.E.J., Robson M.D., Mackay C.E., Higham D.J., Johansen-Berg H. (2007). Connectivity-based parcellation of human cortex using diffusion MRI: establishing reproducibility, validity and observer independence in BA 44/45 and SMA/pre-SMA. Neuroimage, 34(1), 204–11. [DOI] [PubMed] [Google Scholar]
- Klumpp H., Angstadt M., Phan K.L. (2012). Shifting the focus of attention modulates amygdala and anterior cingulate cortex reactivity to emotional faces. Neuroscience Letters, 514(2), 210–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M.J., Cuthbert B.N. (2016). The NIMH research domain criteria initiative: background, issues, and pragmatics. Psychophysiology, 53, 286–97. [DOI] [PubMed] [Google Scholar]
- Kunishio K., Haber S.N. (1994). Primate cingulostriatal projection: limbic striatal versus sensorimotor striatal input. Journal of Comparative Neurology, 350(3), 337–56. [DOI] [PubMed] [Google Scholar]
- Larson C.L., Nitschke J.B., Davidson R.J., (2007). Common and distinct patterns of affective response in dimensions of anxiety and depression. Emotion, 7(1), 182. [DOI] [PubMed] [Google Scholar]
- Levin R.L., Heller W., Mohanty A., Herrington J.D., Miller G.A. (2007). Cognitive deficits in depression and functional specificity of regional brain activity. Cognitive Therapy and Research, 31(2), 211–33. [Google Scholar]
- Liston C., Watts R., Tottenham N., et al. (2006). Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cerebral Cortex, 16(4), 553–60. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Burdette J.H., (2004). Precentral gyrus discrepancy in electronic versions of the Talairach atlas. NeuroImage, 21(1), 450–455. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H., (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage, 19(3), 1233–1239. [DOI] [PubMed] [Google Scholar]
- Marchand W.R. (2010). Cortico-basal ganglia circuitry: a review of key research and implications for functional connectivity studies of mood and anxiety disorders. Brain Structure and Function, 215(2), 73–96. [DOI] [PubMed] [Google Scholar]
- Margulies D.S., Kelly A.M., Uddin L.Q., Biswal B.B., Castellanos F.X., Milham M.P. (2007). Mapping the functional connectivity of anterior cingulate cortex. NeuroImage, 37(2), 579–88. [DOI] [PubMed] [Google Scholar]
- Mayer A.R., Mannell M.V., Ling J., Gasparovic C., Yeo R.A. (2011). Functional connectivity in mild traumatic brain injury. Human Brain Mapping, 32(11), 1825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren D.G., Ries M.L., Xu G., Johnson S.C. (2012). A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage, 61(4), 1277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally R.J. (1998). Abnormalities in anxiety implications for cognitive neuroscience. Cognition & Emotion, 12(3), 479–95. [Google Scholar]
- Medford N., Critchley H.D. (2010). Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain Structure & Function, 214(5–6), 535–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M.M., Mufson E.J. (1982). Insula of the old world monkey. III: Efferent cortical output and comments on function. Journal of Comparative Neurology, 212(1), 38–52. [DOI] [PubMed] [Google Scholar]
- Meyer T.J., Miller M.L., Metzger R.L., Borkovec T.D. (1990). Development and validation of the Penn State Worry Questionnaire. Behaviour Research and Therapy, 28(6), 487–95. [DOI] [PubMed] [Google Scholar]
- Miller G.A., Rockstroh B., Hamilton H.K., Yee C.M. (2016). Psychophysiology as a core strategy in RDoC. Psychophysiology, 53, 410–4. [DOI] [PubMed] [Google Scholar]
- Mitterschiffthaler M.T., Williams S.C.R., Walsh N.D., et al. (2008). Neural basis of the emotional Stroop interference effect in major depression. Psychological Medicine, 38(2), 247–56. [DOI] [PubMed] [Google Scholar]
- Mogg K., Bradley B.P., Williams R. (1995). Attentional bias in anxiety and depression: the role of awareness. British Journal of Clinical Psychology, 34(1), 17–36. [DOI] [PubMed] [Google Scholar]
- Mogg K., Bradley B.P., Williams R.L., Mathews A. (1993). Subliminal processing of emotional information in anxiety and depression. Journal of Abnormal Psychology, 102(2), 304.. [DOI] [PubMed] [Google Scholar]
- Mohanty A., Engels A.S., Herrington J.D., et al. (2007). Differential engagement of anterior cingulate cortex subdivisions for cognitive and emotional function. Psychophysiology, 44(3), 343–51. [DOI] [PubMed] [Google Scholar]
- Molina S., Borkovec T.D. (1994). The Penn State Worry Questionnaire: Psychometric properties and associated characteristics. In G. C. L. D. F. Tallis (Ed.), Worrying: Perspectives on theory, assessment and treatment (pp. 265-283). Oxford, England: John Wiley & Sons.
- Morecraft R.J., Geula C., Mesulam M.M. (1992). Cytoarchitecture and neural afferents of orbitofrontal cortex in the brain of the monkey. Journal of Comparative Neurology, 323(3), 341–58. [DOI] [PubMed] [Google Scholar]
- Moser J.S., Moran T.P., Jendrusina A.A., (2012). Parsing relationships between dimensions of anxiety and action monitoring brain potentials in female undergraduates. Psychophysiology, 49(1), 3–10. [DOI] [PubMed] [Google Scholar]
- Moser J.S., Moran T.P., Schroder H.S., Donnellan M.B., Yeung N. (2013). On the relationship between anxiety and error monitoring: a meta-analysis and conceptual framework. Frontiers in Human Neuroscience, 7, 466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Preuss P., Jürgens U. (1976). Projections from the ‘cingular’ vocalization area in the squirrel monkey. Brain Research, 103(1), 29–43. [DOI] [PubMed] [Google Scholar]
- Nitschke J.B., Heller W. (2002). The neuropsychology of anxiety disorders: affect, cognition, and neural circuitry. Biological Psychiatry, 8, 975–88. [Google Scholar]
- Nitschke J.B., Heller W., Imig J.C., McDonald R.P., Miller G.A. (2001). Distinguishing dimensions of anxiety and depression. Cognitive Therapy and Research, 25(1), 1–22. [Google Scholar]
- Nitschke J.B., Heller W., Palmieri P.A., Miller G.A. (1999). Contrasting patterns of brain activity in anxious apprehension and anxious arousal. Psychophysiology, 36(5), 628–37. [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. (2005). The cognitive control of emotion. Trends in Cognitive Sciences, 9(5), 242–9. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Ray R.D., Cooper J.C., et al. (2004). For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage, 23(2), 483–99. [DOI] [PubMed] [Google Scholar]
- Palaniyappan L., Liddle P.F. (2012). Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. Journal of Psychiatry & Neuroscience, 37(1), 17.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomero-Gallagher N., Mohlberg H., Zilles K., Vogt B. (2008). Cytology and receptor architecture of human anterior cingulate cortex. The Journal of Comparative Neurology, 508(6), 906–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomero-Gallagher N., Vogt B.A., Schleicher A., Mayberg H.S., Zilles K. (2009). Receptor architecture of human cingulate cortex: evaluation of the four-region neurobiological model. Human Brain Mapping, 30(8), 2336–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya D.N., Van Hoesen G.W., Mesulam M.M. (1981). Efferent connections of the cingulate gyrus in the rhesus monkey. Experimental Brain Research, 42(3–4), 319–30. [DOI] [PubMed] [Google Scholar]
- Penny W.D., Ashburner J., Kiebel S., Henson R., Glaser D.E., Phillips C., Friston K., (2001). Statistical parametric mapping: An annotated bibliography. 2001-08-27). http://www.fil.ion.ucl.ac.uk/spm.
- Penza-Clyve S., Zeman J. (2002). Initial validation of the emotion expression scale for children (EESC). Journal of Clinical Child and Adolescent Psychology, 31(4), 540–7. [DOI] [PubMed] [Google Scholar]
- Petrovic P., Dietrich T., Fransson P., Andersson J., Carlsson K., Ingvar M. (2005). Placebo in emotional processing—induced expectations of anxiety relief activate a generalized modulatory network. Neuron, 46(6), 957–69. [DOI] [PubMed] [Google Scholar]
- Phan K.L., Wager T.D., Taylor S.F., Liberzon I. (2004). Functional neuroimaging studies of human emotions. CNS Spectrums, 9(04), 258–66. [DOI] [PubMed] [Google Scholar]
- Pollatos O., Gramann K., Schandry R. (2007). Neural systems connecting interoceptive awareness and feelings. Human Brain Mapping, 28(1), 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma R.B., Dagher A. (2006). Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cerebral Cortex, 16(10), 1508–21. [DOI] [PubMed] [Google Scholar]
- Sass S.M., Heller W., Stewart J.L., et al. (2010). Time course of attentional bias to threat in anxiety: emotion and gender specificity. Psychophysiology, 47, 247–59. NIHMSID: NIHMS276693. PMCID: PMC3073148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-van Maaren Y.W.M., Carlier I.V.E., Zitman F.G., van Hemert A.M., de Waal M.W.M., van Noorden M. S., Giltay E.J., (2012). Reference values for generic instruments used in routine outcome monitoring: the leiden routine outcome monitoring study. BMC Psychiatry, 12, 203. doi: 10.1186/1471-244x-12-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., et al. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience, 27(9), 2349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergerie K., Chochol C., Armony J.L. (2008). The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neuroscience & Biobehavioral Reviews, 32(4), 811–30. [DOI] [PubMed] [Google Scholar]
- Seth A.K., Suzuki K., Critchley H.D. (2011). An interoceptive predictive coding model of conscious presence. Frontiers in Psychology, 2, 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer A.T., Matveychuk D., Penney T., O'Hare A.J., Stokes J., Dolcos F. (2012). Processing of emotional distraction is both automatic and modulated by attention: evidence from an event-related fMRI investigation. Journal of Cognitive Neuroscience, 24(5), 1233–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon K.E., Sauder C., Beauchaine T.P., Gatzke-Kopp L.M. (2009). Disrupted effective connectivity between the medial frontal cortex and the caudate in adolescent boys with externalizing behavior disorders. Criminal Justice and Behavior, 36(11), 1141–57. [Google Scholar]
- Sharp P.B., Miller G.A., Heller W. (2015). Transdiagnostic dimensions of anxiety: neural mechanisms, executive functions, and new directions. International Journal of Psychophysiology, 98(2), 365-77. [DOI] [PubMed] [Google Scholar]
- Shin L.M., Whalen P.J., Pitman R.K., et al. (2001). An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biological Psychiatry, 50(12), 932–42.11750889 [Google Scholar]
- Shin L.M., Wright C.I., Cannistraro P.A., et al. (2005). A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archives of General Psychiatry, 62(3), 273–81. [DOI] [PubMed] [Google Scholar]
- Siegle G.J., Thompson W., Carter C.S., Steinhauer S.R., Thase M.E. (2007). Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biological Psychiatry, 61(2), 198–209. [DOI] [PubMed] [Google Scholar]
- Silton R.L., Heller W., Engels A.S., et al. (2011). Depression and anxious apprehension distinguish frontocingulate cortical activity during top-down attentional control. Journal of Abnormal Psychology, 120(2), 272.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T., Critchley H.D., Preuschoff K. (2009). A common role of insula in feelings, empathy and uncertainty. Trends in Cognitive Science, 13(8), 334–40. [DOI] [PubMed] [Google Scholar]
- Stein M.B., Goldin P.R., Sareen J., Zorrilla L.T., Brown G.G. (2002). Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Archives of General Psychiatry, 59(11), 1027–34. [DOI] [PubMed] [Google Scholar]
- Subic-Wrana C., Beutel M.E., Brähler E., et al. (2014). How is emotional awareness related to emotion regulation strategies and self-reported negative affect in the general population. PLoS One, 9(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor K.S., Seminowicz D.A., Davis K.D. (2009). Two systems of resting state connectivity between the insula and cingulate cortex. Human Brain Mapping, 30(9), 2731–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toglia M.P., Battig W.F., (1978). Handbook of semantic word norms. Lawrence Erlbaum.
- Tromp D.P., Grupe D.W., Oathes D.J., et al. (2012). Reduced structural connectivity of a major frontolimbic pathway in generalized anxiety disorder. Archives of General Psychiatry, 69(9), 925–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoesen G.W., Morecraft R.J., Vogt B.A., (1993). Connections of the monkey cingulate cortex Neurobiology of cingulate cortex and limbic thalamus (pp. 249-284): Springer.
- Vogt B.A., Pandya D.N. (1987). Cingulate cortex of the rhesus monkey: II. Cortical afferents. Journal of Comparative Neurology, 262(2), 271–89. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P., Armony J.L., Driver J., Dolan R.J. (2001). Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron, 30(3), 829–41. [DOI] [PubMed] [Google Scholar]
- Wang L., LaBar K.S., Smoski M., et al. ,, (2008). Prefrontal mechanisms for executive control over emotional distraction are altered in major depression. Psychiatry Research: Neuroimaging, 163(2), 143–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D., Clark L.A., Weber K., Assenheimer J.S., Strauss M.E., McCormick R.A. (1995a). Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. Journal of Abnormal Psychology, 104(1), 15–25. [DOI] [PubMed] [Google Scholar]
- Watson D., Weber K., Assenheimer J.S., Clark L.A., Strauss M.E., McCormick R.A. (1995b). Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. Journal of Abnormal Psychology, 104(1), 3–14. [DOI] [PubMed] [Google Scholar]
- Whalen P.J., Bush G., McNally R.J., et al. ,, (1998). The emotional counting Stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biological Psychiatry, 44(12), 1219–28. [DOI] [PubMed] [Google Scholar]
- Whalen P.J., Johnstone T., Somerville L.H., et al. (2008). A functional magnetic resonance imaging predictor of treatment response to venlafaxine in generalized anxiety disorder. Biological Psychiatry, 63(9), 858–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheaton M.G., Fitzgerald D.A., Phan K.L., Klumpp H. (2014). Perceptual load modulates anterior cingulate cortex response to threat distractors in generalized social anxiety disorder. Biological Psychology, 101, 13–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee C.M., Javitt D.C., Miller G.A. (2015). Replacing categorical with dimensional analyses in psychiatry research: the RDoC initiative. JAMA Psychiatry, 72, 1159–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.