ABSTRACT
Objective: The study aims to explore the relationship between expressions of HER2 and JAK/STAT3-SOCS3 signaling pathway and clinicopathological features and prognosis of ovarian cancer (OC).Methods: A total of 136 OC patients were collected. Immunohistochemistry was applied to measure the expressions of STAT3, p-STAT3, SOCS3, HER2 and p-HER2 in the tumor tissues and adjacent normal tissues. Quantitative real-time polymerase chain reaction (qRT-PCR) was used to detect the mRNA expressions of HER2, SOCS3 and STAT3 and western blotting was applied for protein expressions of HER2, p-HER2, SOCS3, STAT3 and p-STAT3 in the tumor tissues and adjacent normal tissues. Flow cytometry was used for the cell apoptosis in the blank, afatinib (A), ruxolitinib (R) and afatinib + ruxolitinib (A + R) groups. Follow-up was performed to explore relationship of HER2, SOCS3, and STAT3 expressions with survival time of OC patients.Results: HER2, p-HER2, STAT3, and p-STAT3 expressions were higher while SOCS3 expression was lower in the tumor tissues. The positive expressions of STAT3, HER2, p-HER2 and p-STAT3 were lower while the positive expression of SOCS3 was higher in the adjacent normal tissues. The expressions of HER2, SOCS3, and p-STAT3 were associated with clinical stage and lymph node metastasis (LNM), and STAT3 expression has correlation with histological grade and LNM. The mRNA and protein expressions of HER2, STAT3 and p-STAT3 in the tumor tissues were higher than those in the adjacent normal tissues, but SOCS3 expression was significantly decreased. The positive expressions of HER2, p-HER2 and STAT3, the negative expression of SOCS3 and pathological stages were important risk factors for the prognosis of patients with OC.Conclusion: Our study showed that the expressions of HER2, STAT3, and SOCS3 are associated with the progression of OC, and higher expressions of HER2 and STAT3 and lower expression of SOCS3 predict poor prognosis of OC.
KEYWORDS: Clinicopathological features, human epidermal growth factor receptor 2, ovarian cancer, prognosis, signal transducer and activator of transcription 3, signaling pathway, suppressors of cytokine signaling 3
Introduction
Ovarian cancer (OC) remains one of the first gynecological killers all over the world, which is the fifth leading cause of cancer death in women with high incidence rates.1,2 OC cannot be diagnosed because it accounted for 75% patients with advanced disease (stage III) or distant metastasis (stage IV), so that the survival rate is relatively low.3,4 The present treatment of patients with advanced OC are surgery and platinum-based chemotherapy, but strategies of OC recurrence are not standardized and clear.5-7 Tumor prognostic markers are useful in evaluating therapeutic response and monitoring relapse.8 Therefore, it is necessary to have a better understanding of molecular alterations in OC to identify novel targets for early detection and improved treatment.
As a member of the epidermal growth factor receptor (EGFR) family of receptor tyrosine kinases (RTKs), human epidermal growth factor receptor 2 (HER2) is located on chromosome 17q12∼21 and encodes a tyrosine kinase receptor of 185 kDa, the overexpression of which has been shown in 25% to 30% of OC.9,10 Over expression of HER2 protein is connected to OC poor prognosis as an independent prognostic factor, and patients with high expression of HER2 tends to get worse survival rates.9 Signal transducer and activator of transcription 3 (STAT3) belongs to 7 different transcription factors that are important in cytokine signaling as an acute-phase response factor, constitutive activation of which is found in a variety of tumor cells and human tumors including breast, haematopoietic and OC.11 As a member of the JAK/STAT signaling family, STAT3 signaling promotes initiation and progression of human cancers by inhibiting apoptosis or inducing cell proliferation, angiogenesis, invasion, and metastasis.12,13 Janus kinase (JAK) was tyrosin kinase which meditated signal pathway by STAT adjacent to cytoplasma.11 JAK/STAT3 is a major signaling pathway that is associated with aberrantly activation in OC, tumor progression and poor prognosis, which shows inhibition of JAK/STAT3 signaling pathways could significantly suppress dissemination of tumor cells into the peritoneal cavity and the production of ascites in vivo.14 Suppressors of cytokine signaling 3 (SOCS3) regulator expression is initially induced by STAT3 signaling, which could bind to the catalytic domain of JAK and prevent STAT3 phosphorylation by negative feedback regulation.15 In the breast of a lesser extent, SOCS gene hypermethylation and silencing could increase the responsiveness of cytokines in the tissues, which would facilitate the tumor occurrence.16 Therefore, our study aims to explore the relationship between HER2 and JAK/STAT3-SOCS3 signaling pathway and clinicopathological features and prognosis of OC.
Results
Expressions of HER2, p-HER2, SOCS3, STAT3 and p-STAT3
The expressions of HER2, p-HER2, SOCS3, STAT3 and p-STAT3 in the adjacent normal tissues were significantly different compared with those in the tumor tissues by immunohistochemistry (Fig. 1). HER2 and p-HER2 were mainly expressed in the cell membrane, and there were no obvious tan particles in the adjacent normal tissues, while in the tumor tissues, there were obvious tan particles in the membrane tissue. In contrast, the expression of SOCS3 was higher in the adjacent normal tissues than in the tumor tissues, and the yellow particles were not detected in the tumor tissues. The expressions of STAT3 and p-STAT3 indicated that there were dense particles in tumor tissues, while less particles in the adjacent normal tissues. The results of positive rate showed that SOCS3 was higher in the adjacent normal tissues than in the tumor tissues, while the positive rates of HER2, p-HER2, STAT3 and p-STAT3 were higher in the tumor tissues than that in adjacent normal tissues (all P < 0.05).
Figure 1.
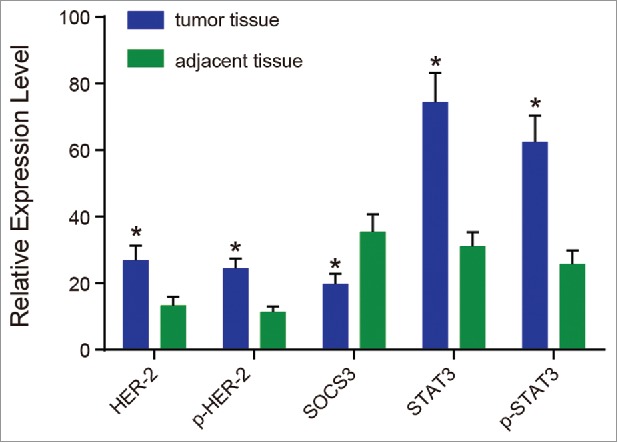
The expressions of HER2, p-HER2, SOCS3, STAT3 and p-STAT3 tested by immunohistochemistry.
Note: HER2, human epidermal growth factor receptor 2; p-HER2, phosphorylated HER2; SOCS3, suppressors of cytokine signaling 3; STAT3, signal transducer and activator of transcription 3; p-STAT3, phosphorylated STAT3; *, P < 0.05 compared with the adjacent normal tissues.
Correlation between expressions of HER2, p-HER2, SOCS3, STAT3 and p-STAT3 and clinicopathological features of OC
According to the staining results and the clinicopathological features (Table 2), the expressions of HER2, SOCS3 and STAT3 were correlated with clinicopathological features of OC. The expressions of SOCS3, HER2 and p-HER2 were associated with clinical stages and lymph node metastasis (LNM), the expression of STAT3 was related to histological grade and LNM, and p-STAT3 was correlated with clinical stages and LNM (all P < 0.05). No significant association of age and tumor types with the expressions of HER2, p-HER2, SOCS3, STAT3 and p-STAT3 was found (all P > 0.05). The results indicated that the expressions of HER2, p-HER2, SOCS3, STAT3 and p-STAT3 were directly related to the differentiation and development of tumor cells.
Table 2.
Expressions of HER-2, p-HER-2, SOCS3, STAT3 and p-STAT3 with clinicopathological features of ovarian cancer.
| HER2 |
p-HER2 |
SOCS3 |
STAT3 |
p-STAT3 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| clinicopathological features | Case | positive | negative | P | positive | negative | P | positive | negative | P | positive | negative | P | positive | negative | P |
| Age (years) | ||||||||||||||||
| ≤ 50 | 51 | 15 | 36 | 0.885 | 17 | 34 | 0.434 | 20 | 31 | 0.448 | 32 | 19 | 0.927 | 27 | 24 | 1.000 |
| > 50 | 85 | 26 | 59 | 22 | 63 | 39 | 46 | 54 | 31 | 45 | 40 | |||||
| Pathological type | ||||||||||||||||
| Serous carcinoma | 72 | 21 | 51 | 0.497 | 21 | 51 | 0.829 | 30 | 42 | 0.584 | 47 | 25 | 0.183 | 38 | 34 | 0.826 |
| Mucinous carcinoma | 36 | 14 | 22 | 14 | 22 | 15 | 21 | 18 | 18 | 21 | 15 | |||||
| Endometrioid carcinoma | 15 | 3 | 12 | 3 | 12 | 9 | 6 | 12 | 3 | 7 | 8 | |||||
| Clear-cell carcinoma | 13 | 3 | 10 | 3 | 10 | 5 | 8 | 9 | 4 | 6 | 7 | |||||
| Histological grade | ||||||||||||||||
| High-middle differentiation | 52 | 13 | 39 | 0.303 | 13 | 39 | 0.846 | 28 | 24 | 0.053 | 27 | 25 | 0.031 | 28 | 24 | 0.868 |
| Poor differentiation | 84 | 28 | 56 | 28 | 56 | 31 | 53 | 59 | 25 | 44 | 40 | |||||
| Clinical stage | ||||||||||||||||
| Early stage | 56 | 7 | 49 | < 0.001 | 7 | 49 | 0.002 | 33 | 23 | 0.002 | 33 | 23 | 0.384 | 20 | 36 | 0.001 |
| Terminal stage | 80 | 34 | 46 | 34 | 46 | 26 | 54 | 53 | 27 | 52 | 28 | |||||
| Lymph node metastasis | ||||||||||||||||
| With | 59 | 23 | 36 | 0.049 | 23 | 36 | < 0.001 | 18 | 41 | 0.008 | 46 | 13 | 0.002 | 40 | 19 | 0.002 |
| Without | 77 | 18 | 59 | 18 | 59 | 41 | 36 | 40 | 37 | 32 | 45 | |||||
Notes: HER-2, human epidermal growth factor receptor 2; p-HER2, phosphorylated HER2; SOCS3, suppressors of cytokine signaling 3; STAT3, signal transducer and activator of transcription 3; p-STAT3, phosphorylated STAT3.
The mRNA expressions of HER2, SOCS3 and STAT3 in OC
The results of qRT-PCR reflected that the mRNA expressions of HER2, SOCS3 and STAT3 in the adjacent normal tissues were significantly different from those in the tumor tissues. Compared with the adjacent normal tissues, the mRNA expressions of HER2 and STAT3 in the tumor tissues were significantly higher compared with the adjacent normal tissues(both P < 0.05). The mRNA expression of SOCS3 was significantly lower in the tumor tissues compared with that in the adjacent normal tissues (P < 0.05) (Fig. 2). According to the correlation analysis, HER2 was positively correlated with STAT3, SOCS3 was negatively correlated with STAT3 and HER2 (r = 0.830, −0.954 and −0.900, respectively, P < 0.01).
Figure 2.
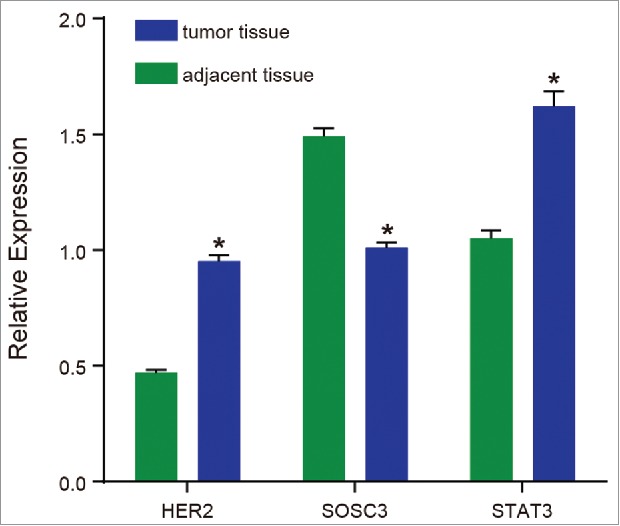
The mRNA expressions of HER2, SOCS3 and STAT3.
Note: HER2, human epidermal growth factor receptor 2; SOCS3, suppressors of cytokine signaling 3; STAT3, signal transducer and activator of transcription 3; *, P < 0.05 compared with the adjacent normal tissues.
Protein expressions of HER2, p-HER2, SOCS3, STAT3 and p-STAT3 in OC
The protein expressions of HER2, p-HER2, SOCS3, STAT3 and p-STAT3 detected by western blotting were consistent with the results of mRNA expressions by qRT-PCR (Fig. 3). Compared with the adjacent normal tissues, the expressions of HER2, p-HER2, STAT3 and p-STAT3 in the tumor tissues were significantly higher (all P < 0.05). The expression of SOCS3 was significantly lower in the tumor tissues compared with the adjacent normal tissues (P < 0.05). According to the correlation analysis, HER2 and STAT3 were positively correlated, SOCS3 was negatively correlated with HER2, p-HER2, STAT3 and p-STAT3 (r = 0.854, −0.937, −0.932, −0.943, respectively, P < 0.01). The results suggested that the increased expressions of HER2 and STAT3 could promote the formation of tumor cells, and SOCS3 might inhibit tumor differentiation and proliferation.
Figure 3.
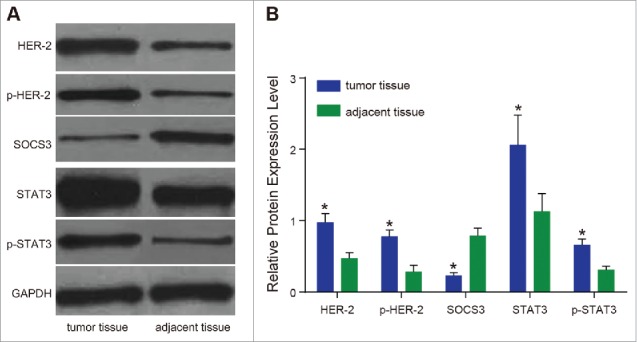
The protein expressions of HER2, p-HER2, SOCS3, STAT3 and p-STAT3.
Note: (A) Western blotting detected the protein expressions of HER2, p-HER2, SOCS3, STAT3 and p-STAT3; (B) relative protein expression levels of HER2, p-HER2, SOCS3, STAT3 and p-STAT3; HER2, human epidermal growth factor receptor 2; p-HER2, phosphorylated HER2; SOCS3, suppressors of cytokine signaling 3; STAT3, signal transducer and activator of transcription 3; p-STAT3, phosphorylated STAT3; *, P < 0.05 compared with the adjacent normal tissues.
Survival conditions and risk factors for prognosis of OC patients
The median survival time was 24 months in 136 patients. The survival rates of 6, 12, 24, and 48 months were 88.2%, 78.7%, 52.9%, and 22.8%, respectively. The expressions of HER2, SOCS3 and STAT3 were sorted out, and the effects on the survival rates were shown in Fig. 4. The median survival time of HER2-positive patients was 14 months, while it was 28 months for the HER2-negative patients (P < 0.05). The median survival time of p-HER2-positive patients was 14 months, while it was 30 months for the p-HER2-negative patients (P < 0.05). The median survival time was 24 months in SOCS3-negative patients while 40 months in SOCS3-positive patients (P < 0.05). The median survival time of STAT3-positive patients was lower than that of STAT3-negative patients, which were 19 months and 28 months, respectively. The median survival time of p-STAT3-positive patients was lower than that of p-STAT3-negative patients, which were 18 months and 35 months, respectively. The results of multivariate survival analysis (Table 3) showed that the positive expressions of HER2, p-HER2 and STAT3, the negative expression of SOCS3 and pathological stages were important risk factors for the prognosis of patients with OC (all P < 0.05). Therefore, the positive expressions of HER2, p-HER2 and STAT3 and the negative expression of SOCS3 would shorten the survival time of patients with OC.
Figure 4.
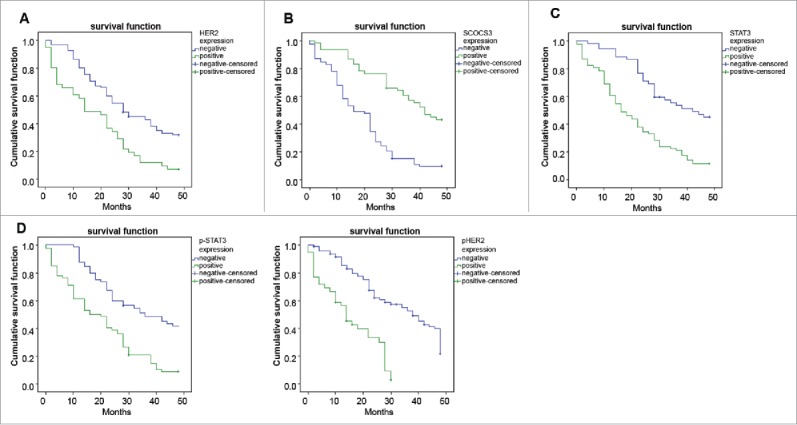
Correlations of the survival curves of patients within 24 months with HER2, p-HER2, SOCS3, STAT3 and p-STAT3.
Note: (A) Correlation between expression of HER2 and the survival time of patients; (B) correlation between expression of SOCS3 and the survival time of patients; (C) correlation between expression of STAT3 and the survival time of patients; (D) correlation between expression of p-STAT3 and the survival time of patients; (E) correlation between expression of p-HER2 and the survival time of patients; HER2, human epidermal growth factor receptor 2; p-HER2, phosphorylated HER2; SOCS3, suppressors of cytokine signaling 3; STAT3, signal transducer and activator of transcription 3; p-STAT3, phosphorylated STAT3.
Table 3.
Multivariate survival analysis of risk factors for prognosis in patients with ovarian cancer.
| B | SE | Wald | P | Exp (B) | 95%CI | |
|---|---|---|---|---|---|---|
| Clinical grade | 0.015 | 0.248 | 0.003 | 0.953 | 1.015 | 0.623–1.651 |
| Pathological stage | 0.675 | 0.262 | 6.669 | 0.010 | 1.965 | 1.177–3.281 |
| Lymph node metastasis | −0.052 | 0.272 | 0.036 | 0.850 | 0.950 | 0.577–1.619 |
| Positive expression of HER2 | 0.594 | 0.227 | 6.826 | 0.009 | 1.811 | 1.160–2.827 |
| Positive expression of p-HER2 | 1.023 | 0.263 | 15.094 | <0.001 | 2.781 | 1.660–4.658 |
| Positive expression of SOCS3 | −1.058 | 0.262 | 16.295 | <0.001 | 0.347 | 0.208–0.580 |
| Positive expression of STAT3 | 0.503 | 0.229 | 4.837 | 0.028 | 1.654 | 1.056–2.589 |
| Positive expression of p-STAT3 | 0.401 | 0.270 | 2.197 | 0.138 | 1.493 | 0.879–2.535 |
Notes: B, the partial regression coefficient; SE, the standard error of the partial regression coefficient; Wald, whether the difference was statistically significant when the overall partial regression coefficient was compared with 0; Exp (B), the relative risk degree; 95%CI, the 95% confidence interval; HER-2, human epidermal growth factor receptor 2; p-HER2, phosphorylated HER2; SOCS3, suppressors of cytokine signaling 3; STAT3, signal transducer and activator of transcription 3; p-STAT3, phosphorylated STAT3.
Cell apoptosis in the blank, A, R and A + R groups
As shown in Fig. 5, in comparison to the blank group, the apoptosis rates in the afatinib, ruxolitinib and afatinib + ruxolitinib groups were significantly increased (all P < 0.05). There were no significant differences between the afatinib group and ruxolitinib group (P > 0.05). The apoptosis rate in the afatinib + ruxolitinib group was significantly higher compared with the afatinib group and ruxolitinib group (P < 0.05), indicating that ruxolitinib combined with afatinib could kill ovarian cancer cells.
Figure 5.
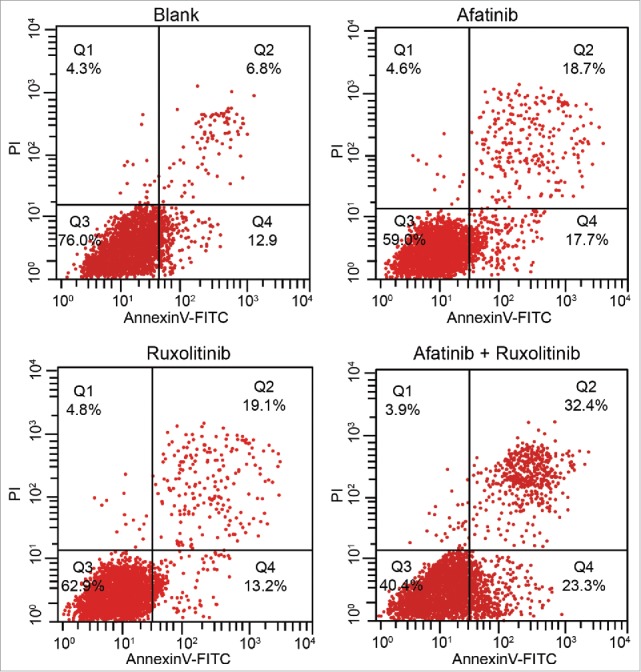
The apoptosis rates of human ovarian cancer cells in the blank, afatinib, ruxolitinib and afatinib + ruxolitinib groups were detected by flow cytometry with AnnexinV-FITC/PtdIns double staining.
Discussion
In the western world, OC has become the leading risk of death from the gynecologic tumors.17 For the past years, the standard therapies for patients with OC have been platinum-based chemotherapy and surgery,5 while treatment of OC remains challenging in spite of many advances in therapeutic choices, and the molecular-targeted therapy is applied for earlier stages of the treatment.18 This paper explored the effects of HER2, STAT3 and SOCS3 signaling pathway on OC via exploring the expressions of HER2, SOCS3 and STAT3 in tumor tissues and adjacent normal tissues.
From this study, we could see that the mRNA and protein expressions of HER2, STAT3 and p-STAT3 in the tumor tissues were significantly higher, while the expression of SOCS3 was relatively lower. The results suggested that the increased expressions of HER2 and STAT3 could promote the formation of tumor cells, and SOCS3 might inhibit tumor differentiation and proliferation. In accordance with the results in our study, a previous study has revealed that STAT3 is an important transcription factor which involves in apoptosis and cell cycle progression.19 The overexpression of HER2/neu oncogene appeared in about 15∼30% of OC.20 And it has been demonstrated that HER2 has effects on biologic targeting therapy of metastatic disease and is the most promising predictive factor for breast cancer.21 Some relevant researches showed that when STAT3 was continually activated, the expression of anti-apoptotic Bcl−2 protein, cyclinD1, vascular endothelial growth factor VEGF would be induced to upregulate and matrix metalloproteinase (MMP) was induced to downregulate, and these factors could promote cell proliferation, inhibit cell apoptosis and promote cell invasion and metastasis in OC.22-25 However, the study indicated that the expression of STAT3 was negatively correlated with the expression of SOCS3 in gastric cancer.26 And STAT3 plays a role in inhibiting the growth of OC.27 All of these studies have demonstrated the above results in this paper.
Furthermore, our study indicated that patients with positive expressions of HER2, STAT3 and p-STAT3 or with negative expressions of SOCS3 had shorter survival time. It is suggested that expressions of these 4 factors and pathologic stages were important risk factors for the prognosis of patients. It is also suggested that HER2 and STAT3 may be important index for the formation, metastasis and prognosis of gynecological tumors and provide a new way for clinical diagnosis and targeted therapy of gynecologic tumors. HER2 expression was correlated with the poor prognosis of OC.9 Some relevant researches have revealed that STAT3 plays an important role in the generation and development of human cancer, the positive expression of STAT3 is significantly correlated with increasing tumor grade.28-29 It is reported that knockdown of SOCS3 expression led to reduced let-7-mediated effects in cancer cells, which can inhibit tumor differentiation and proliferation.15 And the suppression of SOCS3 can prevent cancer metastasis.30 In this study, it was also found that the expressions of SOCS3, HER2, p-HER2 and p-STAT3 were correlated with clinical stages and LNM. In the study reported by Deng et al., SOCS3 can suppress LNM in gastric cancer.31 Consistent with this result, it is also studied that STAT3 and pSTAT3 were found to be associated with LNM and cell survival in cancer.32 HER2 expression is essential for the treatment of gastric cancer with extensive LNM.33
In conclusion, these results have demonstrated that expressions of HER2, SOCS3 and STAT3 are related to the progression of OC. High expressions of HER2 and STAT3 and low expression of SOCS3 are associated with poor prognosis in patients with OC. Therefore, the expressions of HER2, STAT3 and SOCS3 may be a prognostic indicator for the prognosis of OC, and will provide a theoretical basis for the biologic treatment of OC.
Materials and methods
Ethics statement
All research subjects in this study met the inclusion and exclusion criteria. And this study was approved by the Ethics Committee of The First People's Hospital of Yancheng City and The Sixth People's Hospital of Yancheng, and all patients were informed and signed the informed consent.
Clinical sample collection
From February 2009 to January 2012, a total of 136 OC patients in the Department of Gynecology of The First People's Hospital of Yancheng City and The Sixth People's Hospital of Yancheng were collected, aged 21 ∼83 y old, and the median age was 54 y. According to World Health Organization (WHO) standard for preliminary histological classification and grading,34 there were 72 cases of serous cystadenocarcinoma, 15 cases of endometrial carcinoma, 13 cases of clear cell carcinoma, and 36 cases of mucinous cystadenocarcinoma. Patients included 52 cases of high-middle differentiation and 84 cases of poor differentiation, and 77 cases without lymph node metastasis (LNM) and 59 cases with LNM. The surgical staging of international Federation of Gynecology and Obstetrics (FIGO) was adopted in 2000,35 which included 56 cases of early stage (stage I and stage II) and 80 cases of advanced stage (stage III and IV). All samples were collected by surgeries. Tumor tissues and adjacent normal tissues (5 cm from the tumor site) were collected, part of which was stored in liquid nitrogen and fixed with 10% formalin. All samples were confirmed by histopathology, excluding gynecological diseases (by medical history, gynecological examination and color ultrasound examination), and patients had no radiotherapy and chemotherapy before operation.
Immunohistochemistry
Fixed by 10% formalin, the tumor tissues and adjacent normal tissues were collected, cut into 4 μm and paraffin-embedded. Then samples were baked for 30 min at 65°C and washed 3 times with double distilled water with dewaxing and hydration. Tissues were soaked for 15 min by using 3% aquae hydrogenii dioxidi, washed 3 times with 0.01 mol/L Phosphate Buffered Saline (PBS) buffer, soaked by citrate buffer, incubated for 15 min in boiled water and cooled sufficiently. PBS buffer was used to flush tissues 3 times and primary antibody was added into samples with incubation in a refrigerator at 4°C overnight. After flushing with PBS 3 times, tissues were added with secondary antibody and incubated for 20 min at constant temperature of 37°C. Using PBS buffer to flush 3 times, tissues were supplemented with streptavidin-perosidase (SP) liquid for incubating 20 min at 37°C. PBS buffer was used again to wash 3 times. Diallyl maleate (DAM) was used for staining and hematoxylin was used for re-dyeing. After dehydration and mounting, Olympus BX51 (Olympus, JP) was applied for observation and photograph. The primary antibodies rabbit anti-mouse HER2, SOCS3, STAT3 and p-STAT3 were diluted for 1:50 with Tris Buffered saline (TBS), and the secondary antibody IgG-CY3 were diluted for 1:200 with TBS, and all were purchased from the America uptake company (Chicago, USA). Stained cells were generally tan particles and the score was evaluated according to the percentage of positive cells and staining intensity. Detailed criteria were as follows: 0 score for without color or with unobvious color, 1 score for pale yellow, 2 scores for tan and 3 scores for puce. The standard for the percentage of positive cells: < 5% recorded 0 score, 5%∼25% recorded 1 score, 26%∼50% recorded 2 scores and > 50% recorded 3 scores. Two scores were added up to final score: 0∼1 score was negative (−); 2∼3 scores indicated weakly positive (+), 4∼6 scores were positive (++). In this study, “−”and “+” were regarded as negative, and “++”was positive.
Quantitative real time polymerase chain reaction (qRT-PCR)
RNeasy micro kit (QIAGEN, GmbH, Germany) was used to extract RNA from tumor tissues and adjacent normal tissues. Total RNA reverse transcription was performed using TaqMan MicroRNA Reverse Transcription (Applied Biosystem, CA, USA). The total reaction system was 15 μL, and reaction conditions were at 16°C for 30 min, at 42°C for 30 min and at 85°C for 5 min. The qRT-PCR detection was conducted by TaqMan Universal PCR kit (Applied Biosystem, CA, USA). The reaction condition was at 95°C with a warm start for 10 min, and then at 95°C for 15 s and at 60°C for 1 min, with 40 cycles. And qRT-PCR was conducted by ABI 7500 (Applied Biosystem, CA, USA). The experiment was repeated 3 times with glyceraldehyde phosphate dehydrogenase (GAPDH) as a reference gene. Table 1 shows the primer sequences. And 2−ΔΔC method was used for relative quantification.
Table 1.
Primer sequences used in the study.
| Gene | Primer sequence |
|---|---|
| HER-2 | F: 5′-CATCAACTGCACCCACTCCTGT-3′ |
| R: 5′-ACCAGCAGAATGCCAACCACC-3′ | |
| SOCS3 | F: 5′-ATGGTCACCCACAGCAAGTT-3′ |
| F: 5′-CTGTCGCGGATAAGAAAGGT-3′ | |
| STAT3 | F: 5′-AACGACCTGCAGCAATACCA-3′ |
| R: 5′-TCCATGTCAAACGTGAGCGA-3′ | |
| GAPDH | F: 5′-GCGAGATCCCGCTAACATCA-3′ |
| R: 5′- CTCGTGGTTCACACCCATCA-3′ |
Notes: HER-2, human epidermal growth factor receptor 2; SOCS3, suppressors of cytokine signaling 3; STAT3, signal transducer and activator of transcription 3; GAPDH, glyceraldehyde phosphate dehydrogenase; F, forward; R, reverse.
Western blotting
After being washed by PBS, tumor tissues and adjacent normal tissues were added with cell lysis solution containing a moderate amount of protease inhibitor. Samples were shaken for 5 min at 4°C and centrifuged at 12000 × g for 10 min at 4°C. The supernatant was collected and protein was extracted by proteome Mammalian Protein Prer kit (QIAGEN, GmbH, Germany). After measuring the corresponding protein concentration, balancing was performed, and the protein was added with 6 × buffer samples and heated to 100°C, and then stored at −20°C for use. A total of 50 μg protein was obtained for sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes by siphon action. After blocking by the skim milk, HER2, rabbit anti-human phosphorylated HER2 IgG, SOCS3, STAT3 and STAT3 phosphorylated antibodies (sc-7993) were diluted with TBS (1:50) for incubation overnight. Tris-buffered Saline with Tween 20 (TBST) washed membranes 4 times, with 10 min each time. Then IgG marked by IRDye™800DX (1:200) was added into membranes to incubate for 1 h, and membranes were washed with TBST 4 times and developed with developing substrate. The antibodies were purchased from the America uptake company (Chicago, USA) and phosphorylated antibodies were from Santa Cruz (Argentina). Quantitative analysis of the protein bands was conducted by Lab Works Image Acquisition and Analysis Software (UVP, Inc., Upland, CA, USA) for the relevant protein concentration.
Ovarian cancer cell extraction and culture
Tissues from advanced ovarian cancer were cut into pieces (1–2 mm) and placed into the centrifuge tube (50 mL). After addition of 0.25% trypsin (20 mL) contaning ethylenediamine tetraacetic acid (EDTA), tissues were digested in a 37°C water bath for 30–40 min, then added with 20 mL McCoy's 5A medium (PYG0057, Boster Biological Technology Company, Wuhan, China) to neutralize the trypsin and transferred into the centrifuge tube (15 mL) after filtration. The supernatant was removed after centrifugation at 1000 × g for 5 min and 4 mL McCoy's 5A medium was added for cell suspension. PBS was used to make 90% and 45% cell separation medium. Two centrifuge tubes (15 mL) were obtained for addition with 90% cell separation medium (4 mL), 45% cell separation medium (4 mL) and cell suspension (2 mL) on the separation medium, and then centrifuged at 1500 × g for 30 min. After centrifugation, there was cell medium, ovarian cancer cells, fibroblasts and erythrocytes from top to bottom. Ovarian cancer cells were extracted and transferred into the new centrifuge tube (15 mL). Cells were added with 3 times volume of McCoy's 5A medium, blown and centrifuged at 1000 × g for 5 min, with repetition 3 times. The obtained cells were placed into the new culture dish with addition of McCoy's 5A medium for culture. The medium was replaced every 2 or 3 d and the growth of cells was observed.
Flow cytometry with AnnexinV-FITC/PI double staining
Extracted ovarian cancer cells were assigned into the blank, afatinib, ruxolitinib and afatinib + ruxolitinib groups. Afatinib (No.20120406, purity > 99%) was purchased from Dalian Meilun Biology Technology Co., Ltd. (Liaoning, China) and ruxolitinib (No.Axon 1598) was from Beijing Shengkeboyuan Biology Technology Co., Ltd. (Beijing, China). About 1 × 106 cells were collected in each tube. Washed with pre-cooled PBS 3 times, cells were centrifuged for removal of the supernatant. Cells were resuspended with 500 μL blinding buffer, reacted with 5 μL annexin V-FITC and 5 μL PtdIns at room temperature for 15 min in the dark, and detected by flow cytometry.
Follow-up
The survival time of OC patients was followed up to analyze correlation between the expressions of HER2, SOCS3, and STAT3 and the survival period of OC patients. Follow-up began from the time after operation to December 2015 by telephone or review for 48 months with 2 times every 1 month. A total of 127 cases had completed follow-up among 136 patients, in which 9 patients were lost to follow-up.
Statistical analysis
SPSS 19.0 statistical software (SPSS Inc., Chicago, IL, USA) was applied for statistical analysis and measurement data was indicated by mean ± standard deviation (SD) (x¯ ± s). The comparison between 2 samples was analyzed by t test, while the comparison among multiple groups was analyzed by One-Way analysis of variance (ANOVA) (homogeneity test of variance was conducted before analysis). LSD-t test was used for pairwise comparisons among multiple groups, in which bilateral P < 0.05 showed statistical significance. Pearson correlation analysis was used for bivariate correlation analysis and Kaplan-Meier log-rank test was applied to test survival analysis. Cox proportional hazard model was used for multivariate survival analysis.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgment
We are grateful for the contributions and useful suggestions of all the reviewers on our article.
Funding
This study was supported by 2015 Graduate Education Innovation Program (No. NXYC201511) and the function and mechanism of Calcineurin signaling in the quorum sensing system of Candida albicans (No. YK2016074).
Author contributions
Y.Y.L., A.Q.S., J.W., F.B., Y.J.Z, L.R.X., L.L.L., F.F.C., W.W.W., J.J.Z. designed the study. A.Q.S., J.W., F.B., Y.J.Z, L.R.X. collated the data, designed and developed the database, performed data analyses. L.L.L., F.F.C. produced the initial draft of the manuscript. W.W.W., J.J.Z. prepared figures. All authors have read and approved the final submitted manuscript.
References
- 1.Du XL, Jiang T, Sheng XG, Li QS, Wang C, Yu H. PET/CT scanning guided intensity-modulated radiotherapy in treatment of recurrent ovarian cancer. Eur J Radiol 2012; 81(11):3551-6; PMID:22521528; https://doi.org/ 10.1016/j.ejrad.2012.03.016 [DOI] [PubMed] [Google Scholar]
- 2.Liu B, Nash J, Runowicz C, Swede H, Stevens R, Li Z. Ovarian cancer immunotherapy: Opportunities, progresses and challenges. J Hematol Oncol 2010; 3:7; PMID:20146807; https://doi.org/ 10.1186/1756-8722-3-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vergote I, Trope CG, Amant F, Kristensen GB, Ehlen T, Johnson N, Verheijen RH, van der Burg ME, Lacave AJ, Panici PB, et al.. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 2010; 363(10):943-53; PMID:20818904; https://doi.org/ 10.1056/NEJMoa0908806 [DOI] [PubMed] [Google Scholar]
- 4.Demir L, Yigit S, Sadullahoglu C, Akyol M, Cokmert S, Kucukzeybek Y, Alacacioglu A, Cakalagaoglu F, Tarhan MO. Hormone receptor, HER2/NEU and EGFR expression in ovarian carcinoma–is here a prognostic phenotype? Asian Pac J Cancer Prev 2014; 15(22):9739-45; PMID:25520097; https://doi.org/ 10.7314/APJCP.2014.15.22.9739 [DOI] [PubMed] [Google Scholar]
- 5.Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, Carey MS, Beale P, Cervantes A, Kurzeder C, et al.. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 2011; 365(26):2484-96; PMID:22204725; https://doi.org/ 10.1056/NEJMoa1103799 [DOI] [PubMed] [Google Scholar]
- 6.Lee H, Park CS, Deftereos G, Morihara J, Stern JE, Hawes SE, Swisher E, Kiviat NB, Feng Q. MicroRNA expression in ovarian carcinoma and its correlation with clinicopathological features. World J Surg Oncol 2012; 10:174; PMID:22925189; https://doi.org/ 10.1186/1477-7819-10-174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plotti F, Capriglione S, Terranova C, Montera R, Aloisi A, Damiani P, Muzii L, Scaletta G, Benedetti-Panici P, Angioli R. Does HE4 have a role as biomarker in the recurrence of ovarian cancer? Tumour Biol 2012; 33(6):2117-23; PMID:22875782; https://doi.org/ 10.1007/s13277-012-0471-7 [DOI] [PubMed] [Google Scholar]
- 8.Huang HN, Chiang YC, Cheng WF, Chen CA, Lin MC, Kuo KT. Molecular alterations in endometrial and ovarian clear cell carcinomas: Clinical impacts of telomerase reverse transcriptase promoter mutation. Mod Pathol 2015; 28(2):303-11; PMID:25081752; https://doi.org/ 10.1038/modpathol.2014.93 [DOI] [PubMed] [Google Scholar]
- 9.Cai Y, Wang J, Zhang L, Wu D, Yu D, Tian X, Liu J, Jiang X, Shen Y, Zhang L, et al.. Expressions of fatty acid synthase and HER2 are correlated with poor prognosis of ovarian cancer. Med Oncol 2015; 32(1):391; PMID:25433947; https://doi.org/ 10.1007/s12032-014-0391-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schluter B, Gerhards R, Strumberg D, Voigtmann R. Combined detection of Her2/neu gene amplification and protein overexpression in effusions from patients with breast and ovarian cancer. J Cancer Res Clin Oncol 2010; 136(9):1389-400; PMID:20217132; https://doi.org/ 10.1007/s00432-010-0790-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joung YH, Na YM, Yoo YB, Darvin P, Sp N, Kang DY, Kim SY, Kim HS, Choi YH, Lee HK, et al.. Combination of AG490, a Jak2 inhibitor, and methylsulfonylmethane synergistically suppresses bladder tumor growth via the Jak2/STAT3 pathway. Int J Oncol 2014; 44(3):883-95; PMID:24402583; https://doi.org/ 10.3892/ijo.2014.2250 [DOI] [PubMed] [Google Scholar]
- 12.Dominguez E, Mauborgne A, Mallet J, Desclaux M, Pohl M. SOCS3-mediated blockade of JAK/STAT3 signaling pathway reveals its major contribution to spinal cord neuroinflammation and mechanical allodynia after peripheral nerve injury. J Neurosci 2010; 30(16):5754-66; PMID:20410127; https://doi.org/ 10.1523/JNEUROSCI.5007-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siveen KS, Sikka S, Surana R, Dai X, Zhang J, Kumar AP, Tan BK, Sethi G, Bishayee A. Targeting the STAT3 signaling pathway in cancer: Role of synthetic and natural inhibitors. Biochim Biophys Acta 2014; 1845(2):136-54; PMID:24388873; https://doi.org/ 10.1016/j.bbcan.2013.12.005 [DOI] [PubMed] [Google Scholar]
- 14.Wen W, Liang W, Wu J, Kowolik CM, Buettner R, Scuto A, Hsieh MY, Hong H, Brown CE, Forman SJ, et al.. Targeting JAK1/STAT3 signaling suppresses tumor progression and metastasis in a peritoneal model of human ovarian cancer. Mol Cancer Ther 2014; 13(12):3037-48; PMID:25319391; https://doi.org/ 10.1158/1535-7163.MCT-14-0077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel K, Kollory A, Takashima A, Sarkar S, Faller DV, Ghosh SK. MicroRNA let-7 downregulates STAT3 phosphorylation in pancreatic cancer cells by increasing SOCS3 expression. Cancer Lett 2014; 347(1):54-64; PMID:24491408; https://doi.org/ 10.1016/j.canlet.2014.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sutherland KD, Lindeman GJ, Choong DY, Wittlin S, Brentzell L, Phillips W, Campbell IG, Visvader JE. Differential hypermethylation of SOCS genes in ovarian and breast carcinomas. Oncogene 2004; 23(46):7726-33; PMID:15361843; https://doi.org/ 10.1038/sj.onc.1207787 [DOI] [PubMed] [Google Scholar]
- 17.Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, Scott C, Meier W, Shapira-Frommer R, Safra T, et al.. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med 2012; 366(15):1382-92; PMID:22452356; https://doi.org/ 10.1056/NEJMoa1105535 [DOI] [PubMed] [Google Scholar]
- 18.Raja FA, Chopra N, Ledermann JA. Optimal first-line treatment in ovarian cancer. Ann Oncol 2012; 23(Suppl 10):x118-27; PMID:22987945; https://doi.org/ 10.1093/annonc/mds315 [DOI] [PubMed] [Google Scholar]
- 19.Rosen DG, Mercado-Uribe I, Yang G, Bast RC Jr., Amin HM, Lai R, Liu J. The role of constitutively active signal transducer and activator of transcription 3 in ovarian tumorigenesis and prognosis. Cancer 2006; 107(11):2730-40; PMID:17063503; https://doi.org/ 10.1002/cncr.22293 [DOI] [PubMed] [Google Scholar]
- 20.Verri E, Guglielmini P, Puntoni M, Perdelli L, Papadia A, Lorenzi P, Rubagotti A, Ragni N, Boccardo F. HER2/neu oncoprotein overexpression in epithelial ovarian cancer: Evaluation of its prevalence and prognostic significance. Clinical study. Oncology 2005; 68(2–3):154-61; PMID:16020953; https://doi.org/ 10.1159/000086958 [DOI] [PubMed] [Google Scholar]
- 21.Zidan J, Dashkovsky I, Stayerman C, Basher W, Cozacov C, Hadary A. Comparison of HER-2 overexpression in primary breast cancer and metastatic sites and its effect on biological targeting therapy of metastatic disease. Br J Cancer 2005; 93(5):552-6; PMID:16106267; https://doi.org/ 10.1038/sj.bjc.6602738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baspinar S, Bircan S, Yavuz G, Kapucuoglu N. Beclin 1 and bcl-2 expressions in bladder urothelial tumors and their association with clinicopathological parameters. Pathol Res Pract 2013; 209(7):418-23; PMID:23722017; https://doi.org/ 10.1016/j.prp.2013.04.006 [DOI] [PubMed] [Google Scholar]
- 23.Dai J, Zhang PH, Liu PS, Qu HH. [Expressions and significance of cyclinD1 in epithelial ovarian cancer cell 3AO]. Zhonghua Yi Xue Za Zhi 2012; 92(5):351-3; PMID:22490843. [PubMed] [Google Scholar]
- 24.McCann GA, Naidu S, Rath KS, Bid HK, Tierney BJ, Suarez A, Varadharaj S, Zhang J, Hideg K, Houghton P, et al.. Targeting constitutively-activated STAT3 in hypoxic ovarian cancer, using a novel STAT3 inhibitor. Oncoscience 2014; 1(3):216-28; PMID:25594014; https://doi.org/ 10.18632/oncoscience.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zou M, Zhang X, Xu C. IL6-induced metastasis modulators p-STAT3, MMP-2 and MMP-9 are targets of 3,3′-diindolylmethane in ovarian cancer cells. Cell Oncol (Dordr) 2016; 39(1):47-57; PMID:26510945; https://doi.org/ 10.1007/s13402-015-0251-7 [DOI] [PubMed] [Google Scholar]
- 26.Setsu N, Kohashi K, Endo M, Yamamoto H, Tamiya S, Takahashi Y, Yamada Y, Ishii T, Matsuda S, Yokoyama R, et al.. Phosphorylation of signal transducer and activator of transcription 3 in soft tissue leiomyosarcoma is associated with a better prognosis. Int J Cancer 2013; 132(1):109-15; PMID:22644781; https://doi.org/ 10.1002/ijc.27655 [DOI] [PubMed] [Google Scholar]
- 27.Gritsina G, Xiao F, O'Brien SW, Gabbasov R, Maglaty MA, Xu RH, Thapa RJ, Zhou Y, Nicolas E, Litwin S, et al.. Targeted Blockade of JAK/STAT3 Signaling Inhibits Ovarian Carcinoma Growth. Mol Cancer Ther 2015; 14(4):1035-47; PMID:25646015; https://doi.org/ 10.1158/1535-7163.MCT-14-0800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang MX, Zhao X, Wang ZG, Zhao WM, Wang YS. Constitutive activation of signal transducer and activator of transcription 3 regulates expression of vascular endothelial growth factor in human meningioma differentiation. J Cancer Res Clin Oncol 2010; 136(7):981-8; PMID:20052595; https://doi.org/ 10.1007/s00432-009-0743-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garner JM, Fan M, Yang CH, Du Z, Sims M, Davidoff AM, Pfeffer LM. Constitutive activation of signal transducer and activator of transcription 3 (STAT3) and nuclear factor kappaB signaling in glioblastoma cancer stem cells regulates the Notch pathway. J Biol Chem 2013; 288(36):26167-76; PMID:23902772; https://doi.org/ 10.1074/jbc.M113.477950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hiwatashi K, Tamiya T, Hasegawa E, Fukaya T, Hashimoto M, Kakoi K, Kashiwagi I, Kimura A, Inoue N, Morita R, et al.. Suppression of SOCS3 in macrophages prevents cancer metastasis by modifying macrophage phase and MCP2/CCL8 induction. Cancer Lett 2011; 308(2):172-80; PMID:21624767; https://doi.org/ 10.1016/j.canlet.2011.04.024 [DOI] [PubMed] [Google Scholar]
- 31.Deng J, Jiao X, Liu H, Wu L, Zhang R, Wang B, Pan Y, Hao X, Liang H. Lymph node metastasis is mediated by suppressor of cytokine signaling-3 in gastric cancer. Tumour Biol 2013; 34(6):3627-36; PMID:23824571; https://doi.org/ 10.1007/s13277-013-0944-3 [DOI] [PubMed] [Google Scholar]
- 32.Deng JY, Sun D, Liu XY, Pan Y, Liang H. STAT-3 correlates with lymph node metastasis and cell survival in gastric cancer. World J Gastroenterol 2010; 16(42):5380-7; PMID:21072904; https://doi.org/ 10.3748/wjg.v16.i42.5380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsumoto T, Sasako M, Mizusawa J, Hirota S, Ochiai A, Kushima R, Katai H, Tanaka Y, Fukushima N, Nashimoto A, et al.. HER2 expression in locally advanced gastric cancer with extensive lymph node (bulky N2 or paraaortic) metastasis (JCOG1005-A trial). Gastric Cancer 2015; 18(3):467-75; PMID:24993498; https://doi.org/ 10.1007/s10120-014-0398-3 [DOI] [PubMed] [Google Scholar]
- 34.K PM. WHO handbook for reporting results of cancer treatment. Br J Cancer 1982; 38(3):484-5. [Google Scholar]
- 35.Nowak M, Szpakowski M, Malinowski A, Wieczorek A, Szpakowski A, Raczkowska Z, Maciolek-Blewniewska G, Laskowski J, Budzko W, Kolasa D. [Ovarian cancer. I. Epidemiology, symptoms, FIGO staging]. Ginekol Pol 2000; 71(9):1179-83; PMID:11082999 [PubMed] [Google Scholar]


