Abstract
Background
The quantitative relationship between antimicrobial agent consumption and rise or fall of antibiotic resistance has rarely been studied. We began all admission surveillance testing for methicillin-resistant Staphylococcus aureus (MRSA) in August 2005 with subsequent contact isolation and decolonization using nasally applied mupirocin ointment for those colonized. In October 2012, we discontinued decolonization of medical (nonsurgical service) patients.
Methods
We conducted a retrospective study from 2007 through 2014 of 445680 patients; 35235 were assessed because of mupirocin therapy and positive test results for MRSA. We collected data on those patients receiving 2% mupirocin ointment for decolonization to determine the defined daily doses (DDDs). A nonparametric regression technique was used to quantitate the effect of mupirocin consumption on drug resistance in MRSA.
Results
Using regressive modeling, we found that, when consumption was consistently >25 DDD/1000 patient-days, there was a statistically significant increase in mupirocin resistance with a correlating positive rate of change. When consumption was ≤25 DDD/1000 patient-days, there was a statistically significant decrease in mupirocin resistance with a correlating negative rate of change. The scatter plot of fitted versus observed mupirocin resistance values showed an R2 value of 0.89—a high correlation between mupirocin use and resistance.
Conclusions
Use of the antimicrobial agent mupirocin for decolonization had a threshold of approximately 25 DDD/1000 patient-days that separated a rise and fall of resistance within the acute-care setting. This has implications for how widely mupirocin can be used for decolonization, as well as for setting consumption thresholds when prescribing antimicrobials as part of stewardship programs.
Keywords: antimicrobial resistance, antimicrobial stewardship, decolonization, methicillin-resistant Staphylococcus aureus (MRSA), mupirocin
Summary
We studied the impact of mupirocin use over 7.5 years at a 4-hospital organization. With use >25 defined daily doses/1000 patient-days, resistance increased. Universal decolonization would cause resistance; antimicrobial stewardship programs should track consumption of individual agents in acute-care facilities.
Antimicrobial resistance remains an increasing plague to modern medicine associated with increased mortality, economic loss, and hospital admission. Estimates are that by 2050 antimicrobial resistance will result in 10 million deaths worldwide, with an economic burden approaching $100 trillion annually [1]. Methicillin-resistant Staphylococcus aureus (MRSA) continues to be one of the most challenging organisms [2, 3]. The US Centers for Disease Control and Prevention (CDC) estimates >2 million infections per year are caused by antimicrobial-resistant organisms, resulting in 23000 deaths in the United States alone [4]. Although no simple formula exists to predict resistance, the concept is straightforward—increasing consumption of an antibiotic drives antimicrobial resistance, and reducing use should reverse the trend [5, 6].
Methicillin-resistant S. aureus is one of the critical pathogens causing antibiotic-resistant infections [7–10]. In the effort to improve patient safety, hospitals and legislatures have implemented programs to reduce the risk of MRSA infection [2, 11–13]. The REDUCE MRSA trial suggested universal decolonization may lead to decreased MRSA clinical disease in the acute-care setting [12]. One concern raised by the authors was that targeted decolonization could cause resistance to mupirocin and/or chlorhexidine. We found resistance for mupirocin in MRSA increased from 3.9% in 2005 to 10.9% in 2012 when targeted decolonization was used [14]. Recently we reported that decolonization did not add benefit, and we ceased inpatient decolonization of medical patients (eg, those cared for on nonsurgical service wards) [15]. Our hypothesis is that the data from our experience could define a quantitative relationship between the consumption of mupirocin and the increase (or decrease) of resistance to this agent. We also believe that this information can add to knowledge for improving the approach to antimicrobial stewardship.
METHODS
Study Design
We conducted a retrospective, observational, cohort study of 445680 inpatients at the 4 NorthShore University HealthSystem (NorthShore) hospitals in the northern suburbs of Chicago from May 2007 through December 2014. The goal was to assess the relationship between nasal administration of mupirocin and the development of resistance in MRSA. Of these 445680 inpatients, 35235 patients were in the population of those given mupirocin therapy and harboring MRSA. There were no exclusions from the data set.
Mupirocin Intervention
Patient’s who had a nasal swab positive for MRSA were placed into contact precautions, and topical mupirocin 2% ointment was given for 5 days twice daily. This was accompanied by a bath or shower using chlorhexidine gluconate solution 4.0% W/V (CHG) as a liquid soap on days 1, 3, and 5 of therapy [16]. The use of mupirocin plus CHG in medical patients was halted in September 2012 after demonstration that decolonization did not add to lowering of MRSA transmission from patients in contact precautions isolation [15]. The hospital that was the basis for this study is 1 of 4 inpatient facilities in the NorthShore system. All of these facilities are located within 10 miles of the central hospital (Evanston Hospital) and share similar demographics with each other. The impact of this change has not negatively impacted the rate of MRSA disease at NorthShore [2].
Microbiologic Methods
Surveillance testing was performed by swabbing both nares with a premoistened double swab. Real-time quantitative polymerase chain reaction (qPCR) was performed on 1 of the nasal swabs. The second swab was cultured using a direct plus broth enrichment method on positive samples to recover MRSA. The isolates were tested for high-level mupirocin resistance by detecting the specific gene (mupA) encoding this trait using qPCR validated as a laboratory-developed test [17].
Data Collection
Since 2003 we have used Epic Systems’ Electronic Health Record across the full range of inpatient and outpatient care. Part of this exclusively electronic documentation is a locally developed, comprehensive Enterprise Data Warehouse with searchable content, including all physiological data, laboratory results, orders, medication prescriptions, administration events, laboratory and pathology data, and the full text of provider notes. We used NorthShore’s Enterprise Data Warehouse to obtain data regarding each patient’s characteristics as well as the results of active surveillance testing for MRSA and any decolonization therapy during the inpatient stay [18]. The majority of isolates were from unique patients, but because we were investigating the development of resistance over time some patients contributed >1 isolate. No more than 1 unique isolate was included per patient per month. In the analysis, we used time-series modeling (ie, error terms being correlated by an autoregressive process) to account for this approach.
Statistical Analysis
A nonparametric regression technique (GAM) was used to assess how the covariate (mupirocin use) relates to the response variable (mupirocin resistance). We modeled the nonlinear behavior of the percentage resistance over time using a generalized additive model [19]. This approach first allows the model for percentage resistance to account for both within-year and between-year (ie, trend) variation with 2 separate smoothing functions. While performing the analysis, we found that there was no seasonal (within-year) variability (ie, not statistically significant) and therefore removed any seasonal smoothing function from the fitted model. The final fitted model for percentage resistance includes an intercept term, a trend smooth function, and an error term that is assumed to be normally distributed with a mean of zero and constant variance, with the error terms correlated by a continuous time autoregressive AR(1) process that accounts for serial temporal within-year dependence in the data. The generalized additive model is fitted to the data using the function “gamm” of the library “mgcv” in the software package R [20], where the function “gamm” allows for fitting the model with a correlation structure in the residuals.
The data on the total number of mupirocin doses prescribed and percentage resistance were recorded per month from May 2007 through December 2014. We use the defined daily doses (DDDs) per 1000 patient-days as a measure of antibiotic consumption that estimates the proportion of people taking antibiotics. Because there is only 1 standard dosage for mupirocin, the DDD is equivalent to a days of therapy (DOT) calculation [21]. The DDD at a specific time point t is defined as
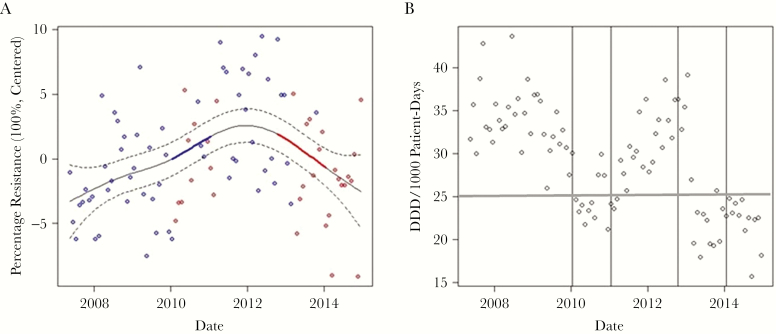
In our study, the time point t represents a specific month in a given year. Figure 1A is a time-series plot of the centered percentage resistance (in 100%), and Figure 1B is a time-series plot of DDD (per 1000 patient-days). The nonlinear trend of percentage resistance is statistically significant; however, it may not be significantly increasing or decreasing everywhere. Therefore, we identified the periods of time at which the percentage resistance was changing significantly using the method of finite differences that estimates the rate of change in the fitted spline function, and we used the information recorded to identify the periods of statistically significant change in the time series of percentage resistance.
Figure 1.
Time series plots of resistance and mupirocin consumption. A, Time series plot of the percentage resistance centered around its mean (units in 100%). The fitted trend for the centered percentage resistance is the solid black line, and its approximate 95% confidence intervals are displayed using the dashed lines. The fitted trend shows periods of statistically significant increase (in blue) between January 2010 and January 2011 and periods of statistically significant decrease (in red) between October 2012 and January 2014. The original data are shown as gray points. The red circles correspond to consumption of ≤25 defined daily doses (DDD)/1000 patient-days and the blue circles correspond to >25 DDD/1000 patient-days, where 25 DDD/1000 patient-days is the first quartile of the monthly DDD data. B, Time-series plot of DDD per 1000 patient-days. The vertical lines mark the 2 following significant time periods during which the percentage resistance changes and either significantly increases or decreases. The horizontal line indicates the 25 DDD/1000 patient-days threshold. Of note is that, even with fluctuations near the threshold, resistance was maintained until use consistently remained below that defined level. Abbreviation: DDD, defined daily dose.
For our literature review, we used PubMed.gov (MEDLINE) and Google with the search terms “MRSA,” “Staphylococcus aureus,” “mupirocin,” “resistance,” “susceptibility,” “quantitative,” “defined daily dose,” and “antimicrobial” to search from the beginning of citations through November 2016. The research was approved by NorthShore’s Institutional Review Board (Protocol EH15-125).
RESULTS
The demographics for the patient population are shown in Table 1. Figure 1A shows the fitted smooth spline function of the centered percentage resistance (centered around its “0” mean) and an approximate 95% pointwise confidence interval for the trend spline. The correlation parameter of the autoregressive AR(1) process of the error terms is estimated to be 0.1 with a 95% confidence interval (0.01–0.6) in the fitted model.
Table 1.
Characteristics of All Patients and Mupirocin-Treated Patients
| Characteristics | Mupirocin Recipients (n = 35235) | All Patient Records (n = 445680) |
|---|---|---|
| Female, no. (%) | 19340 (54.9) | 285235 (64) |
| Male, no. (%) | 15894 (45.1) | 160445 (36.0) |
| Mean age ± 1 SD, y | 75.4 ± 17.3 | 61.0 ± 14.0 |
| Non-Hispanic, no. (%) | 34900 (99.1) | 435875 (97.8) |
| ICU patients,a no. (%) | 6342 (18.0) | 49916 (11.2) |
| Surgical patients, no. (%) | 4545 (12.9) | 88245 (19.8) |
| Medical patients, no. (%) | 30690 (87.1) | 357435 (80.2) |
| Mean length of stay ± 1 SD, d | 5.7 ± 5.5 | 4.1 ± 4.5 |
Abbreviations: ICU, intensive care unit; SD, standard deviation.
aICU patients were both surgical and medical patients.
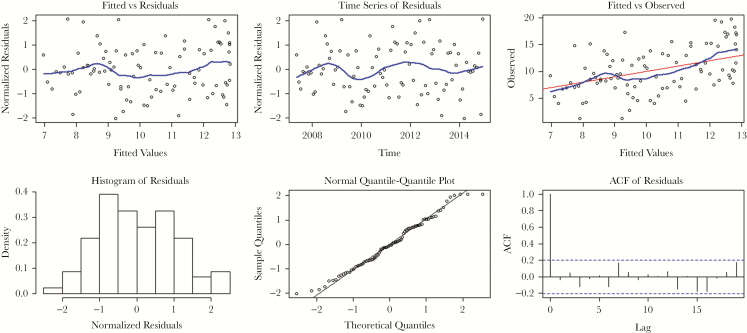
The plots of the normalized residuals, which take into account the covariance matrix of the residuals (Figure 2), show that the residuals are normally distributed, do not reveal any heteroscedastic pattern, and do not show significant residual autocorrelation. The fitted model for the percentage resistance is therefore used to explain its dynamic behavior over time. In the graph of fitted versus observed values of percentage resistance of mupirocin, the R2 is equal to 0.89, indicating high correlation.
Figure 2.
Plots of the normalized residuals take into account the covariance matrix of the residuals. Left Upper Panel: Plot of normalized residuals versus fitted values of mupirocin percentage resistance. Middle Upper Panel: Time-series plot of normalized residuals in the fitted model for mupirocin percentage resistance. Right Upper Panel: Observed values versus fitted values of the mupirocin percentage resistance. The blue curves in the upper panels are the Locally Weighted Scatter-plot Smoother function curves fitted using locally weighted regression. Left Lower Panel: Histogram of normalized residuals in the fitted model for percentage resistance. Middle Lower Panel: Normal quantile-quantile plot of normalized residuals in the fitted model for percentage resistance. Right Lower Panel: Autocorrelation function of the normalized residuals in the fitted model for percentage resistance. Abbreviation: ACF, autocorrelation function.
In retrospect, looking at the behavior of DDD per 1000 patient-days, we see that the time point at which this significant increase in percentage resistance (from January 2010 to January 2011) occurs is subsequent to >2 years of consistent use >25 DDD/1000 patient-days. The time point at which the significant decrease in percentage resistance (from October 2012 to January 2014) occurs rapidly is subsequent to a hospital intervention where routine decolonization of medical patients was discontinued [15].
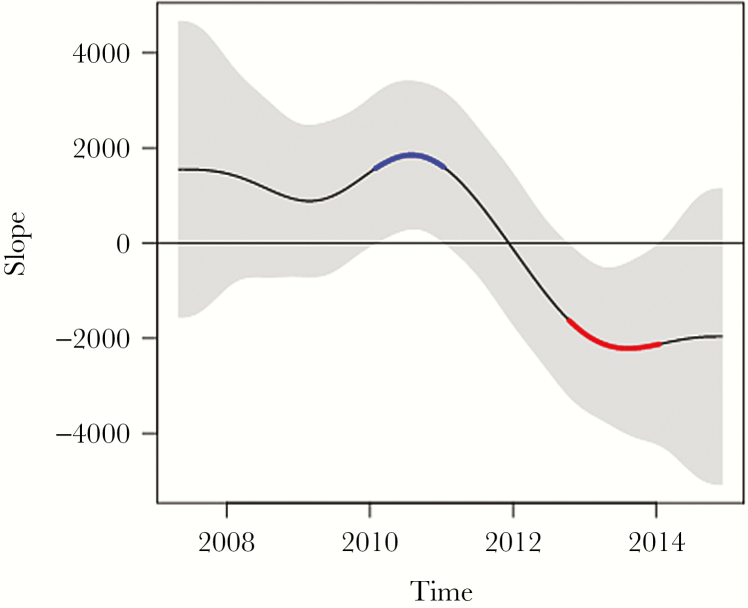
Figure 3 demonstrates that at the times of statistically significant change in mupirocin consumption, there is also a statistically significant rate of change (slope) in resistance with a 95% confidence interval above the baseline of zero, corresponding to the same timeframe shown in Figure 1B. The time period before January 2010 when use was consistently >25 DDD/1000 patient-days (Figure 1B) is followed by a statistically significant rate of change in the positive direction corresponding to increased mupirocin resistance from January 2010 to January 2011 (Figure 1A). In the subsequent October 2012 to January 2014 time period when use was ≤25 DDD/1000 patient-days, there is a rapid, statistically significant rate of change in the negative direction corresponding to less mupirocin resistance, as noted in Figure 1A.
Figure 3.
The rate of change (slope) in susceptibility/resistance during the times of significant change. First derivative (ie, slope) of the fitted spline. The gray band is a 95% simultaneous confidence interval. Sections of the slope where the (gray-shaded) confidence interval does not include zero are 2 periods of statistically significant change in percentage resistance. The curve in blue corresponds to the derivative line being above the zero horizontal line and, hence, marks a significant increase in (centered, × 100) resistance, and the curve in red corresponds to the derivative line being below the zero line and, therefore, indicates a period of significant decrease in resistance.
Thus, for the use of mupirocin therapy, our data show that 25 DDD/1000 patient-days is the critical, quantitative level for inpatient prescribing that determines whether resistance in MRSA is likely to increase or decrease. For our healthcare system, with a mean length of inpatient stay of approximately 5 days, every 5 patients generate 25 DDDs, and 1000 patient-days represents 200 patients, so that 25 DDD/1000 patient-days approximates 2.5% (n = 5/200) of patients in the population receiving mupirocin as where a change in susceptibility occurred.
DISCUSSION
Our findings suggest a strong link to mupirocin resistance and its use for MRSA nasal decolonization (eg, the association R2 of 0.89). Of particular interest is Figure 1B, which shows a statistically significant rise in resistance occurred only after 2 years of elevated use but that a significant lowering of resistance occurred quickly after prescribing was consistently sustained below a specified threshold. This suggests that resistance trends can be impacted fairly rapidly if the correct intervention is implemented. The clinical implications for this are at least 3-fold. First, even targeted nasal decolonization of inpatients carries a significant risk of mupirocin resistance development that could eliminate the use of this agent in MRSA control programs. Second, widespread development of mupirocin resistance would remove this drug as a useful tool in the prevention of postoperative infections from S. aureus—a practice with significant benefit in a large prospective, randomized, multicenter clinical trial [22]. Finally, our findings demonstrating a quantitative level of consumption associated with the rise and fall of resistance have broad implications for overall antibiotic stewardship. Thus, if a goal of optimizing antimicrobial use is to reduce antibiotic resistance, then careful attention needs to be paid to the actual amount of antibiotic use of each agent consumed—implying that prescribing diversity should be a component of optimizing therapy, a concept that has supporting evidence but has not yet been widely tested [23].
Austin and colleagues studied the quantitative association between antibiotic consumption and development of resistance in Iceland and Finland [6]. Their modeling concluded that resistance to β-lactam drugs began when use reached 9 DDD/1000 children in the population, was sustainable at 15 DDD/1000 children, and with prescribing of 60 DDD/1000 children the susceptible bacterial population could not be maintained. Because our mean length of stay is 5 days and 25 DDD is generated by 5 patients, the threshold we found of 25 DDD/1000 patient-days is roughly equivalent to 2.5% of the population receiving mupirocin therapy, which is remarkably close to what Austin and colleagues found (<5% of children) as a use level where resistance was detected, thus supporting our findings. We were unable to find other reports assessing the quantitative relationship between antimicrobial use and resistance development. However, semiquantitative reports do support our findings. In New Zealand, Upton and colleagues found that mupirocin sales increased when the medication became publicly available [24]. As sales of mupirocin increased from $50000 to nearly $250000 New Zealand dollars over 8 years, resistance increased from <2% to 28% [24]. Additionally, Bathoorn et al showed an increase in mupirocin-resistant coagulase-negative staphylococci within a healthcare center from 8% to 22% as mupirocin consumption increased from 3.6 kg in 2006 to 13.3 kg in 2010 [25]. Therefore, it is very reasonable to conclude that mupirocin resistance increases once a certain threshold of mupirocin use is reached within a given population.
The first area where our findings have impact is directly related to the report by Huang and colleagues [12]. This investigation did not further lower MRSA clinical disease or blood stream infection when targeted decolonization (eg, of MRSA carriers only) was compared with universal decolonization in the intensive care unit (ICU), but one of their conclusions was that universal decolonization should be considered as a hospital-wide practice [12]. High-level mupirocin resistance fell from 3.8 to 2.8 in ICU-attributable MRSA isolates per 1000 trial participants (odds ratio [OR] = 0.7; 95% confidence interval [CI] = 0.09–0.56) for the targeted decolonization group but rose from 1.6 to 2.0 (OR = 1.4; 95% CI = 0.13–15.63) in the universal decolonization arm [26]. It also rose from 2.5 to 3.4 in ICU-attributable MRSA isolates per 1000 trial participants (OR = 1.5; 95% CI = 0.25–9.02) in the control group where approximately 1% of participants received intranasal mupirocin [12, 26]. Our data from the NorthShore targeted decolonization practice strongly suggests that hospital-wide universal decolonization would most certainly lead to development of mupirocin resistance in a high percentage of MRSA and could eliminate all mupirocin-susceptible strains, according to the Austin et al model [6]. Compounding the risk from universal decolonization is the potential for widespread chlorhexidine use leading to colistin resistance in Gram-negative bacteria, as the linkage between chlorhexidine exposure and colistin resistance in Klebsiella pneumonia was recently demonstrated by Wand and colleagues [27].
The second important area for the implications of this research is in the prevention of postoperative infection from S. aureus. Preoperative testing coupled with decolonization of those harboring any S. aureus is demonstrated to reduce the risk of significant postoperative infection from this pathogens by 3–5-fold [22, 28, 29]. For this practice, the availability of an active decolonizing agent is imperative. Should mupirocin be overused for hospitalized patients and meaningful resistance develop in all S. aureus, this would have a significant impact on those healthcare practices using mupirocin as a decolonizing agent to prevent postoperative infection.
The final, and perhaps most important, area of impact for our study is in the critical discipline of antibiotic stewardship. The implication of our findings and the modeling from Austin et al is that, to avoid the development of antibiotic resistance, close monitoring of the level of consumption of each antimicrobial (or antimicrobial class) used in the acute healthcare setting is needed—and that thresholds for use of antimicrobial agents must be low. China has deployed a national plan for antimicrobial use and has set a threshold of 40 DDD per 100 patient-days as the maximum total antimicrobial use within any acute-care hospital [30]. Assuming the data from this study and that from the Austin et al modeling apply across the broad range of available antimicrobial agents (eg, no more than 25 DDD/1000 patient-days can be used for any agent or class), then prescribing diversity that includes monitoring use of multiple antimicrobial agents/classes should be considered as part of any stewardship plan. A very helpful report is from Polk and colleagues who assessed 130 US hospitals during 2002–2003 and included data on >175000 patients [21]. They found that 60% of inpatients received at least 1 antibiotic dose and the use of various agents ranged 18–80 DDD/1000 patient-days. Six agents exceeded 30 DDD/1000 patient-days use, and 2 exceeded 50 DDD/1000 patient-days use. This suggests that a hypothesis to be tested in preventing the development of bacterial resistance for an antimicrobial stewardship program would be to set a threshold for any given drug at consumption of 25–30 DDD/1000 patient-days and then suggest alternative agents if this level is breached.
The limitations in this study are inherent to those seen with retrospective studies. However, our methodology that included all admitted patients from 4 relatively diverse hospitals allowed investigation of a population encompassing a large geographic area and one that is representative of US healthcare. The isolates used in this data were collected from the anterior nares; therefore, we are unable to determine whether the MRSA isolates from other body sites were highly resistant to mupirocin. However, most MRSA infections are endogenous, so we believe the sample is representative of all MRSA in our patient population. Finally, we were unable to specifically determine what contribution medical and surgical patients made to the mupirocin-resistant MRSA burden. In our program, most medical patients were decolonized in the hospital, whereas surgical patients were given mupirocin before admission. We only had full data separating medical and surgical patients from 2009 through 2014, and in that major portion of the data, the percentage of surgical patients harboring MRSA who had a mupirocin-resistant strain was 1.43% versus 9.47% for the medical patients who were MRSA colonized. Thus, it appeared that inpatient prescription to medical patients was the major contributor of mupirocin use and driver of resistance development. The strengths of this study include a large sample size, a standardized protocol for MRSA surveillance and decolonization, use of genotypic methods to assess mupirocin resistance, and the extensive follow-up period to accurately trend the development of mupirocin resistance.
Our study sets the foundation for other research. One area is to investigate other approaches to decolonization that might avoid the development of resistance to mupirocin. Specifically evaluating the contribution of community- and hospital-onset MRSA strains would also be useful because we did not collect that data in our analysis. Perhaps most important, a prospective study to ascertain whether limiting the consumption of individual antimicrobials/antimicrobial classes, each to a low threshold (such as 25–30 DDD/1000 patient-days), will reverse an antimicrobial resistance trend and prevent any development of new antibacterial resistance.
In conclusion, our large data set analysis supports that use of mupirocin in MRSA decolonization above a certain threshold (25 DDD/1000 patient-days) results in an increase in mupirocin resistance. The scant research and publications in the area of a quantitative association between drug use and resistance development indicate that more work on this focus is needed. We suggest that antimicrobial stewardship programs pay close attention to the specific consumption of agents used in their acute-care facilities to succeed in preventing or reversing antimicrobial resistance trends.
Acknowledgments
We greatly appreciate the support of all NorthShore University HealthSystem personnel in their cooperation with the Infection Control Program to prevent infection and enhance patient safety.
Finanical support. This work was supported by NorthShore University HealthSystem, Evanston, Illinois. There was no external funding support.
Potential conflicts of interest. L. R. P. has received speaking honoraria from Becton Dickinson, Cepheid, Roche, and CareFusion and has received research funding from Becton Dickinson, Cepheid, NanoSphere, 3M, GeneWEAVE and Roche. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. O’Neill J. Tackling drug-resistant infections globally: Final report and recommendations http://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf Accessed 14 December 2016.
- 2. Peterson LR, Schora DM. Methicillin-resistant Staphylococcus aureus in the 21st century: laboratory involvement affecting disease impact and economic benefit from large population studies. J Clin Microbiol. 2016;54:2647–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention. Methicillin-resistant Staphylococcus aureus (MRSA). MRSA tracking http://www.cdc.gov/mrsa/tracking/ Accessed 10 December 2016.
- 4. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013 http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf Accessed 30 December 2016.
- 5. Aryee A, Price N. Antimicrobial stewardship—can we afford to do without it? Br J Clin Pharmacol. 2015;79:173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Austin DJ, Kristinsson KG, Anderson RM. The relationship between the volume of antimicrobial consumption in human communities and the frequency of resistance. Proc Natl Acad Sci U S A. 1999;96:1152–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jarvis WR, Jarvis AA, Chinn RY. National prevalence of methicillin-resistant Staphylococcus aureus in inpatients at United States health care facilities, 2010. Am J Infect Control. 2012;40:194–200. [DOI] [PubMed] [Google Scholar]
- 8. Klevens RM, Morrison MA, Nadle J et al. ; Active Bacterial Core surveillance (ABCs) MRSA Investigators Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–71. [DOI] [PubMed] [Google Scholar]
- 9. Klevens RM, Edwards JR, Richards CL Jr et al. Estimating health care-associated infections and deaths in U.S. Hospitals, 2002. Public Health Rep. 2007;122:160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sievert DM, Ricks P, Edwards JR et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol. 2013;34:1–14. [DOI] [PubMed] [Google Scholar]
- 11. National Conference of State Legislatures. Methicillin-resistant Staphylococcus aureus (MRSA) and other healthcare-associated infections http://www.ncsl.org/research/health/healthcare-associated-infections-homepage.aspx Accessed 10 November 2016.
- 12. Huang SS, Septimus E, Kleinman K et al. ; CDC Prevention Epicenters Program; AHRQ DECIDE Network and Healthcare-Associated Infections Program Targeted versus universal decolonization to prevent ICU infection. N Engl J Med. 2013;368:2255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Robicsek A, Beaumont JL, Paule SM et al. Universal surveillance for methicillin-resistant Staphylococcus aureus in 3 affiliated hospitals. Ann Intern Med. 2008;148:409–18. [DOI] [PubMed] [Google Scholar]
- 14. Dusich IK, Schora D, Peterson LR, Thomson RB Jr. Decreasing mupirocin susceptibility over 7 years in a hospital system using mupirocin for nasal decolonization of hospitalized patients with MRSA colonization and pre-surgical patients with S. aureus colonization. In Program and abstracts of the 113th annual meeting of the American Society for Microbiology, May 18–21, 2013, Denver, CO. Abstract C-118. [Google Scholar]
- 15. Peterson LR, Wright MO, Beaumont JL et al. Nonimpact of decolonization as an adjunctive measure to contact precautions for the control of methicillin-resistant staphylococcus aureus transmission in acute care. Antimicrob Agents Chemother. 2015;60:99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peterson LR, Hacek DM, Robicsek A. 5 Million Lives Campaign. Case study: an MRSA intervention at Evanston Northwestern Healthcare. Jt Comm J Qual Patient Saf. 2007;33:732–8. [DOI] [PubMed] [Google Scholar]
- 17. Paule SM, Robicsek A, Thomson R Jr, Peterson LR. Three years of universal surveillance and decolonization: The effect on mupirocin resistance. In Program and Abstracts, 48th Interscience Conference on Antimicrobial Agents and Chemotherapy, October 25–28, 2008, Washington, DC,. Abstract C2-1069. [Google Scholar]
- 18. Hebert C, Beaumont J, Schwartz G, Robicsek A. The influence of context on antimicrobial prescribing for febrile respiratory illness: a cohort study. Ann Intern Med. 2012;157:160–9. [DOI] [PubMed] [Google Scholar]
- 19. Wood SN. Generalized Additive Models: An Introduction with R. Boca Raton, FL: Chapman and Hall/CRC Press; 2006. [Google Scholar]
- 20. R Core Team (2016). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: http://www.R-project.org/ Accessed 15 November 2016. [Google Scholar]
- 21. Polk RE, Fox C, Mahoney A et al. Measurement of adult antibacterial drug use in 130 US hospitals: comparison of defined daily dose and days of therapy. Clin Infect Dis. 2007;44:664–70. [DOI] [PubMed] [Google Scholar]
- 22. Bode LG, Kluytmans JA, Wertheim HF et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med. 2010;362:9–17. [DOI] [PubMed] [Google Scholar]
- 23. Howard P, Pulcini C, Levy Hara G et al. ; ESCMID Study Group for Antimicrobial Policies (ESGAP); ISC Group on Antimicrobial Stewardship. An international cross-sectional survey of antimicrobial stewardship programmes in hospitals. J Antimicrob Chemother. 2015;70:1245–55. [DOI] [PubMed] [Google Scholar]
- 24. Upton A, Lang S, Heffernan H. Mupirocin and Staphylococcus aureus: a recent paradigm of emerging antibiotic resistance. J Antimicrob Chemother. 2003;51: 613–7. [DOI] [PubMed] [Google Scholar]
- 25. Bathoorn E, Hetem DJ, Alphenaar J et al. Emergence of high-level mupirocin resistance in coagulase-negative staphylococci associated with increased short-term mupirocin use. J Clin Microbiol. 2012;50:2947–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hayden MK, Lolans K, Haffenreffer K et al. Chlorhexidine and mupirocin susceptibility of methicillin-resistant Staphylococcus aureus isolates in the REDUCE-MRSA trial. J Clin Microbiol. 2016;54:2735–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wand ME, Bock LJ, Bonney LC, Sutton JM. Mechanisms of increased resistance to chlorhexidine and cross-resistance to colistin following exposure of Klebsiella pneumoniae clinical isolates to chlorhexidine. Antimicrob Agents Chemother. 2017;61:e01162–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hacek DM, Robb WJ, Paule SM et al. Staphylococcus aureus nasal decolonization in joint replacement surgery reduces infection. Clin Orthop Relat Res. 2008;466:1349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Humphreys H, Becker K, Dohmen PM et al. Staphylococcus aureus and surgical site infections: benefits of screening and decolonization before surgery. J Hosp Infect. 2016;94:295–304. [DOI] [PubMed] [Google Scholar]
- 30. Xiao Y, Zhang J, Zheng B, Zhao L, Li S, Li L. Changes in Chinese policies to promote the rational use of antibiotics. PLoS Med. 2013;10:e1001556. [DOI] [PMC free article] [PubMed] [Google Scholar]