Abstract
Despite the widespread outbreak, few cases of Zika virus associated with cardiac manifestations have been described. We present a case of pericarditis in the setting of an acute, symptomatic Zika virus infection in a traveler returning from St. Thomas. Clinicians should be alert for this potential complication of Zika virus infection.
Keywords: pericarditis, Zika virus
Zika virus (ZIKV) is a member of the Flaviviridae family and is primarily transmitted to humans by Aedes mosquitoes [1]. The World Health Organization declared ZIKV as a Public Health Emergency of International Concern in February 2016, and, currently, there are ZIKV outbreaks in the Americas as well as islands of the Caribbean Sea and Pacific Ocean. In the United States, most cases have occurred among travelers returning from locations in the Western Hemisphere. Symptomatic ZIKV infections in adults are typically described as a mild illness with some combination of fever, rash, conjunctivitis, arthralgia, myalgia, and/or headache. However, ZIKV infections have also been associated with severe complications typically affecting the nervous system [2–4]. In this study, we describe a case of acute ZIKV infection presenting with pericarditis.
CASE PRESENTATION
In October 2016, a 45-year-old woman presented to our clinic with 4 days of subjective fever, chest pain, rash, and joint pain after returning from St. Thomas in the United States Virgin Islands. She had no prior flavivirus exposure based on immunization history and previous travel. She visited the island for 11 days, and on the day of her return, she noted a small, pruritic rash on her left arm. During the flight home, she noted that the rash was spreading, and her eyes felt swollen. The patient also experienced an episode of stabbing, substernal chest pain that radiated around her right side to her scapula. The pain improved after she stood and walked in the aisle of the plane. On the day before her presentation in clinic, she had 2 additional episodes of transient chest pain, both occurred while sitting and improved with walking.
On St. Thomas, the patient stayed in a friend’s guest house, which had no air conditioning or running water. There were screens on the windows but visible holes around the sides of the screens. The patient reported having many mosquito bites despite using insect repellent. Her friend’s son had also recently been diagnosed with Zika. The patient was not sexually active during her trip.
Her past medical history included mitral valve prolapse, irregular menses, and a congenital single kidney. Her family history was significant for cardiovascular disease. The patient had smoked cigarettes for over 20 years and smoked half a pack a day at the time of her visit. She walked approximately 1 mile a day but had not been able to exercise after her return. She was not on any prescription medications but took cranberry extract, glucosamine-chondroitin, fish oil, and a multivitamin daily.
On physical exam, 4 days after symptom onset, the patient had a temperature of 36.6°C. Her blood pressure was 155/95, but other vital signs were within normal limits. Heart rate and rhythm were regular with normal heart sounds, no rubs, murmurs or gallops, and she had a non-displaced point of maximum impulse. Her jugular venous pressure was flat. The patient had 2+ distal pulses and nonpitting edema of her ankles. There was no evidence of synovitis, but skin exam revealed a diffuse, predominantly papular rash over her upper and lower extremities (Figure 1A), back, and chest. Results of routine laboratory tests were within normal limits (Table 1). Notably, the levels of creatine kinase-MB fraction (1 ng/mL; normal ≤6 ng/mL) and troponin I (<0.03 ng/mL; normal ≤0.04 ng/mL) were normal. Screening tests for human immunodeficiency virus and hepatitis B and C were negative. Reverse-transcription polymerase chain reactions (RT-PCRs) performed on serum were negative for dengue virus (DENV) and chikungunya virus.
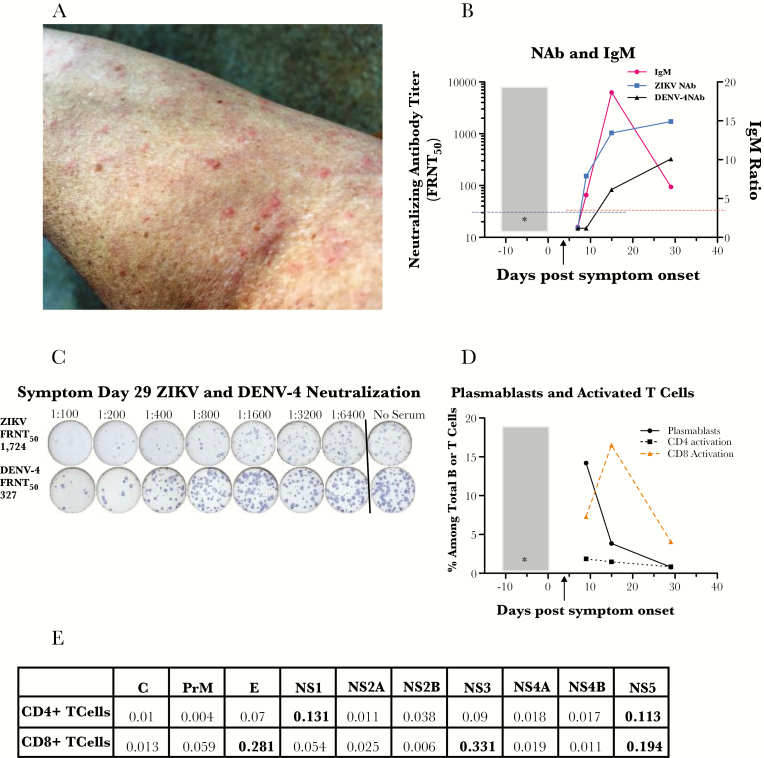
Figure 1.
(A) Rash on patient’s left arm on symptom day 9. (B) Antibody responses against Zika virus (ZIKV) and dengue virus (DENV) on symptom days 7, 9, 15, and 29. Gray box, 11-day exposure risk period during travel; *, estimated day of infection in the middle of the exposure period. Black arrow indicates symptom day 4 when serum and urine polymerase chain reactions were positive for ZIKV and serum was negative for DENV and chikungunya virus. Immunoglobulin (Ig)M (in red) was not detectable on day 7 but was positive on days 9, 15, and 29. Horizontal red dashed line indicates IgM ratio positive cutoff of ≥3. Focus reduction neutralization test (FRNT) for ZIKV (in blue) was <30 on day 7 but became positive (≥titer of 30; cutoff shown with dashed blue horizontal line); DENV-4 titer was <30 on days 7 and 9 but was positive on days 15 and 29. (C) Focus reduction neutralization test. Day 29 serum neutralized both ZIKV and DENV-4 but with a higher titer against ZIKV (1724) than DENV-4 (327), which is consistent with this ZIKV infection being an initial flavivirus infection in this patient. (D) Plasmablasts and activated CD4+ and CD8+ T cells on symptom days 9, 15, and 29. CD27+CD38+ plasmablasts (antibody-secreting B cells; solid black line) had a robust detected peak at the first time-point studied (symptom day 9) then decreased. A very strong peak in human leukocyte antigen (HLA)-DR+CD38+ activated CD8+ T cells (dashed tan line) occurred later (symptom day 15), and they were still elevated on day 29, suggesting ongoing antigenic stimulation. The HLA-DR+CD38+ CD4+ T cells (dotted black lines) had a much more modest detected peak on day 9. (E) ZIKV-specific CD4+ or CD8+ T-cell responses in peripheral blood mononuclear cells from day post symptom onset (DPO) 27. The percentages of total cytokine-producing cells among all CD4+ or CD8+ T cells were determined by intracellular cytokine staining and flow cytometry; results are shown for peptide pools spanning all 10 ZIKV proteins. Results for production of 5 cytokines (interferon-γ, interleukin-2, tumor necrosis factor-α, CD107, and macrophage inflammatory protein-1β) were summed in a Boolean analysis. Percentages >0.1% (bolded in the table) were considered significantly elevated relative to healthy human controls (data not shown). The patient’s CD4+ T cells mounted their strongest DPO 27 response against the nonstructural proteins NS1 and NS5. The patient’s CD8+ T cells mounted their strongest response against the structural protein E and the nonstructural proteins NS3 and NS5.
Table 1.
Results of Routine Laboratory Testing
| Laboratory Test | Result | Normal Range |
|---|---|---|
| White blood cell count | 5000/µL | 4000–10000/µL |
| Hemoglobin | 14.4 g/dL | 11.4–14.4 g/dL |
| Platelet count | 230000/µL | 150000–400000/µL |
| Sodium | 135 mmol/L | 136–144 mmol/L |
| Potassium | 3.9 mmol/L | 3–6 mmol/L |
| Chloride | 102 mmol/L | 101–111 mmol/L |
| Carbon dioxide | 24 mmol/L | 22–32 mmol/L |
| Blood urea nitrogen | 9 mg/dL | 8–25 mg/dL |
| Creatinine | 0.85 mg/dL | 0.4–1.0 mg/dL |
| Glucose | 88 mg/dL | 65–110 mg/dL |
| Bilirubin | 0.4 mg/dL | <1.3 mg/dL |
| Alanine aminotransferase | 34 U/L | 5–35 U/L |
| Asparagine aminotransferase | 10 U/L | 5–35 U/L |
| Alkaline phosphatase | 62 U/L | 40–120 U/L |
| Troponin I | <0.03 ng/mL | ≤0.04 ng/mL |
| Creatine kinase-MB fraction | 1 ng/mL | ≤6 ng/mL |
Serum and urine collected at her initial visit were positive for ZIKV ribonucleic acid (RNA) using a real-time RT-PCR protocol based on assays developed by Lanciotti et al [5]. Results of serologic and cellular immunology testing for ZIKV are shown in Figure 1B–E. Serologic testing for anti-ZIKV immunoglobulin (Ig)M was negative on day 7 post-illness onset, but it was positive on day 9 and remained detectable through day 29, which was the last time point tested. ZIK virus focus reduction neutralization test (FRNT) titers similarly increased from <30 on day 7 post-illness onset to 153, 1036, and 1724 on days 9, 15, and 29, respectively. Anti-DENV IgG was not detected by enzyme-linked immunosorbent assay at any time point during her clinical course. Dengue virus-1, DENV-3, and DENV-4 FRNT titers showed minimal rises to day 29 titers of 76, 104, and 327, respectively, and DENV-2 remained undetectable. Fluorescence-activated cell sorting phenotyping of fresh whole blood demonstrated very robust activation of CD8+ T cells (peaking day 15) and plasmablasts (detected peak at day 9) along with modest CD4+ T-cell activation. Cytokine-producing antiviral CD4+ or CD8+ cells were detected against peptides from the ZIKV E, NS1, NS3, and NS5 proteins (Figure 1E).
Two weeks after symptom onset, the patient was seen in the Cardiology Clinic due to persistent chest pain. The pain worsened with inspiration, which caused her to take short, shallow breaths, and it was worse when lying down, such that the patient had devised a way to cushion herself while sleeping so as to not recline. The pain did not worsen on exertion. She denied palpitations, orthopnea, and nausea. Her electrocardiogram showed normal sinus rhythm at rate of 76 beats per minute, no ST segment abnormalities, T wave abnormalities, or PR segment elevations or depressions. Transthoracic echocardiogram revealed a trivial pericardial effusion and 2 areas of hyperechogenicity in the right ventricular free wall and anteroapical septum with preserved wall motion. Although nonspecific, these could represent areas of myocardial inflammation. Based on her symptoms, a clinical diagnosis of pericarditis was made. The patient was initiated on colchicine 0.6 mg by mouth twice daily. Her chest pain resolved after 4 days on colchicine, further supporting the diagnosis of pericarditis, and her pain did not recur after completing a 28-day course of the medication.
DISCUSSION
In this report, we describe a case of pericarditis that occurred during an acute, symptomatic ZIKV infection. The neurological complications of ZIKV infection have been well described [4, 6]. Cardiac manifestations have not been commonly observed in the setting of Congenital Zika Syndrome [7]. However, ZIKV infection has reportedly been associated with cardiac complications such as dysrhythmias and heart failure in Venezuelan adults [8], and a case report of myocarditis associated with Zika in a traveler who returned to France from La Martinique has recently been published [9]. In our patient, clinical history and antiviral antibody testing were consistent with an acute ZIKV infection being a primary flavivirus infection. Humoral and cell-mediated immunity assays demonstrated robust responses in the setting of this infection. The immunologic milieu in our patient differs from that of adult patients in whom prior flavivirus infection and/or vaccination are common, and it also differs from the case report of myocarditis, in which the individual had evidence of a previous DENV infection [9]. Our case indicates that cardiac manifestations may occur in flavivirus-naive individuals as well as those with previous flavivirus exposure.
The diagnosis of pericarditis in this case was based largely on the characteristic clinical presentation and her response to treatment [10]. In terms of other common causes of pericarditis [11], the patient was in good health, had no risk factors for tuberculosis, did not have evidence of a connective tissue disease, and had no recent trauma. She was up to date on cancer screening, had normal thyroid and kidney function testing, and was not taking medications typically associated with pericarditis. Although causation cannot be proven, ZIKV is the most likely etiologic agent in this case due to the concurrent symptoms of pericarditis and acute Zika, along with detection of ZIKV RNA in serum and urine samples. Diagnostic confirmation requires testing of pericardial fluid and/or tissue, but because of her uncomplicated clinical course and lack of a significant pericardial effusion, there was no indication for pericardiocentesis. Magnetic resonance imaging could further characterize myopericarditis, but it was not performed due to her clinical improvement.
Pericarditis has been reported in the setting of other flavivirus infections, most commonly in acute dengue [12, 13]. Although the total number of cases is small, the majority of patients have presented between days 3 and 6 post-illness onset with ongoing symptoms of dengue fever [12–14]. Cases include both primary and secondary DENV infections [12], and when RT-PCR has been performed, DENV RNA is detectable at the time of presentation [12, 14]. The clinical history in our patient is consistent with reports of pericarditis in the DENV literature as well as the case of myocarditis associated with ZIKV [9]. Although we did observe a rise in DENV FRNT titers, this is consistent with known cross-reactions between anti-ZIKV antibodies and DENV serologic assays [1]. Dengue virus RNA was not detected in acute-phase serum, and her anti-ZIKV FRNT titers were >4-fold higher than the DENV titers, which is consistent with an acute ZIKV infection.
Treatment of acute viral pericarditis is supportive and consists of nonsteroidal antiinflammatory drugs (NSAIDs) and colchicine. The addition of colchicine to conventional anti-inflammatory therapy reduces symptom persistence and risk of recurrence [15]. Our patient responded to colchicine without the addition of NSAIDs. Currently, there is no antiviral or vaccine for ZIKV, and prevention of infection is accomplished principally through avoidance of mosquito bites in endemic areas and barrier precautions during sexual intercourse after infection or exposure [16].
CONCLUSIONS
In conclusion, we present a case of pericarditis in a ZIKV-infected patient. Clinicians should be aware of this presentation during the current outbreak, because it requires close follow-up and may improve quickly with medical management.
Acknowledgments
We thank the clinical staff at The Hope Clinic (Mary Bower, Sara Jo Johnson, Allison Beck, Dean Kleinhenz, and Jo Ann Sadowski) and the laboratory staff at The Hope Clinic Laboratory (Pamela Lankford-Turner, Sree Aramgam, Dongli Wang, and Vinit Karmali).
Disclaimer. The patient described in this case report provided written informed consent to participate in a natural history study of Zika virus infection (DMID 16-0017). The immunologic testing presented herein was performed as part of this ongoing study.
Financial support. This case report details a research study participant in a Zika virus natural history study funded by a National Institutes of Health/National Institute of Allergy and Infectious Diseases/Division of Microbiology and Infectious Diseases (DMID) Vaccine and Treatment Evaluation Unit (VTEU) award to Emory University (Contract No. HHSN272201300018I funding the DMID 16-0017 study). Baylor College of Medicine support was provided by a VTEU award (Contract No. HHSN27220130015I). Additional support was provided by the Georgia Research Alliance.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Waggoner JJ, Pinsky BA. Zika virus: diagnostics for an emerging pandemic threat. J Clin Microbiol 2016; 54:860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cao-Lormeau VM, Blake A, Mons S et al. Guillain-Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet 2016; 387:1531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Araújo TV, Rodrigues LC, de Alencar Ximenes RA et al. Association between Zika virus infection and microcephaly in Brazil, January to May, 2016: preliminary report of a case-control study. Lancet Infect Dis 2016; 16:1356–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dos Santos T, Rodriguez A, Almiron M et al. Zika virus and the Guillain-Barré syndrome—case series from seven countries. N Engl J Med 2016; 375:1598–601. [DOI] [PubMed] [Google Scholar]
- 5. Lanciotti RS, Kosoy OL, Laven JJ et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 2008; 14:1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mlakar J, Korva M, Tul N et al. Zika virus associated with microcephaly. N Engl J Med 2016; 374:951–8. [DOI] [PubMed] [Google Scholar]
- 7. Moore CA, Staples JE, Dobyns WB et al. Characterizing the pattern of anomalies in congenital Zika syndrome for pediatric clinicians. JAMA Pediatr 2017; 171:288–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gonzalez Carta KA, Mendoza I, Morr I et al. Myocarditis, heart failure, and arrhythmias in patients with Zika. J Am Coll Cardiol 2017; 69:906.28209231 [Google Scholar]
- 9. Aletti M, Lecoules S, Kanczuga V et al. Transient myocarditis associated with acute Zika virus infection. Clin Infect Dis 2016; pii: ciw802. [DOI] [PubMed] [Google Scholar]
- 10. Troughton RW, Asher CR, Klein AL. Pericarditis. Lancet 2004; 363:717–27. [DOI] [PubMed] [Google Scholar]
- 11. Levy PY, Corey R, Berger P et al. Etiologic diagnosis of 204 pericardial effusions. Medicine (Baltimore) 2003; 82:385–91. [DOI] [PubMed] [Google Scholar]
- 12. Miranda CH, Borges Mde C, Matsuno AK et al. Evaluation of cardiac involvement during dengue viral infection. Clin Infect Dis 2013; 57:812–9. [DOI] [PubMed] [Google Scholar]
- 13. Sam SS, Omar SF, Teoh BT et al. Review of dengue hemorrhagic fever fatal cases seen among adults: a retrospective study. PLoS Negl Trop Dis 2013; 7:e2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mercado M, Acosta-Reyes J, Parra E et al. Clinical and histopathological features of fatal cases with dengue and chikungunya virus co-infection in Colombia, 2014 to 2015. Euro Surveill 2016; 21: doi: 10.2807/1560-7917.ES.2016.21.22.30244. [DOI] [PubMed] [Google Scholar]
- 15. Imazio M, Brucato A, Cemin R et al. A randomized trial of colchicine for acute pericarditis. N Engl J Med 2013; 369:1522–8. [DOI] [PubMed] [Google Scholar]
- 16. Petersen EE, Meaney-Delman D, Neblett-Fanfair R et al. Update: Interim guidance for preconception counseling and prevention of sexual transmission of Zika virus for persons with possible Zika virus exposure—United States, September 2016. MMWR Morb Mortal Wkly Rep 2016; 65:1077–81. [DOI] [PubMed] [Google Scholar]