Abstract
Worldwide crop losses due to plant diseases exceed $60 billion annually. Next to fungi, viruses represent the greatest contributor to those losses, and these are transmitted in nature primarily by insects. Mexican bean beetles (Epilachna varivestis) are formidable pests of soybean, as well as efficient vectors of several soybean-infecting viruses, including Bean pod mottle virus (BPMV). Beetle-borne viruses have a unique mode of transmission, though their interactions with host plants and vectors remain poorly understood. In these studies, we implemented targeted metabolite profiling and high throughput RNA sequencing approaches to explore metabolic and molecular changes in soybean leaves infected with BPMV. The virus-infected plants showed altered defence signaling and amino acid concentrations—and most strikingly—had dramatically higher sucrose levels. Based on the results, we performed a series of E. varivestis behavioral bioassays using near-isogenic soybean lines of differing foliar sucrose levels in an attempt to more directly associate sucrose content and E. varivestis feeding preferences. Choice assays revealed E. varivestis is more attracted to BPMV-infected soybean than to healthy plants. Moreover, no-choice assays indicated that beetles consume less foliage per plant but ultimately feed on more plants in a given time period if they are higher in sucrose. Importantly, these virus-driven changes to beetle feeding preferences are likely to increase BPMV spread in natural environments.
Keywords: beetles, viruses, metabolomics, transcriptomics, feeding behavior
Introduction
The interplays between pathogenic microbes, phytophagous insects, and their host plants are remarkably complex. The intricacies of these relationships are epitomized both for the underlying molecular and physiological plant defence mechanisms and for the much broader evolutionary and ecological implications (Pieterse and Dicke 2007; Guti�rrez et�al. 2013). In most cases, plant-infecting viruses must compete with their insect vectors for plant resources, but they also rely on them to cross the plant cell wall boundary and spread from host to host (Irwin et�al. 2007). Virus acquisition and inoculation typically occurs during vector feeding, thus the ability of the virus to modulate the vector’s feeding behavior in a manner that increases their transmission efficiencies and dispersal capacities could be particularly adaptive (Hurd 2003; Lef�vre and Thomas 2008; Stafford et�al. 2011). Indeed, virus-induced alterations to vector feeding behavior have been increasingly reported in a variety of plant pathosystems (Ingwell et�al. 2012; Moreno-Delafuente et�al. 2013; Lei et�al. 2016).
Often overlooked is the importance of beetles (Order Coleoptera) as vectors of plant-infecting viruses. Second only to aphids and leafhoppers, beetles transmit 11% of the approximately 550 reported insect-borne viruses, including several which infect economically important vegetable and grain crops (Astier et�al. 2001). Bean pod mottle virus (BPMV), a positive-sense single-stranded RNA comovirus, is one of the most prevalent and destructive viruses of soybean (Glycine max (L.) Merr.) in North America, resulting in significant yield losses and reduced seed quality. The historical range of BPMV is not well studied, but it was first found in Eastern and Midwestern North America (Zaumeyer and Thomas 1948; Ghabrial et�al. 1977; Mabry et�al. 2003) and may have originated on beans (Phaseolus spp.). BPMV is vectored by at least eight beetle species in the family Chrysomelidae at different efficiencies, including Mexican bean beetles, Epilachna varivestis Mulsant (Fulton and Scott 1974). Native to the plateau region of southern Mexico, E. varivestis’ range now extends from Guatemala to southern Canada (Auclair 1959; Fan et�al. 1992). Since its establishment in United States in 1942, the beetle has become a serious economic pest of Phaseolus spp., including soybean (Fan et�al. 1992; Nakamura 2007). In addition to BPMV, E. varivestis efficiently vectors Cowpea severe mosaic comovirus (Fulton and Scott 1974), Southern bean mosaic sobemovirus (Musser et�al. 2003), and Black gram mottle carmovirus (Scott and Phatak 1979).
The mechanisms of virus transmission by beetles remain poorly understood and little research has been done over the past 20 years. Beetle-borne viruses have a highly specific relationship with their vectors, as well as a mode of transmission unique among characterized insect transmission systems (Fulton et�al. 1987). While there is no evidence of virus propagation in beetles, some viruses are circulative, moving into the insect hemolymph immediately after ingestion (Sanderlin 1973; Scott and Fulton 1978). Virus retention in the beetle is affected by properties and characteristics of both the virus (Walters 1969; Selman 1973) and the insect (Wang et�al. 1992). Inoculative beetles deposit the active virus in regurgitant on the surface of the wounded leaf during feeding, and these virus particles are readily translocated in the xylem and can infect unwounded plant cells (Gergerich and Scott 1988), though little is known about the infection process after translocation occurs.
Investigations into virus-associated modifications to insect feeding behavior that facilitate disease transmission and spread have been steadily accumulating for plant pathosystems (Bosque-P�rez and Eigenbrode 2011; Ingwell et�al. 2012). Nearly all of this work has focused on ‘sap-sucking’ hemipterans, which include aphids, whiteflies, and hoppers. Plant viruses may modify their vector’s behavior in several different ways. They may directly manipulate vector settling, probing, and feeding after ingestion and circulation through their body (Hodge and Powell 2008; Stafford et�al. 2011; Ingwell et�al. 2012; Luan et�al. 2013; Moreno-Delafuente et�al. 2013). Further, they may indirectly alter vector feeding by inducing changes to the infected plant (e.g., nutrient composition, toxins, volatile attractants), which influence plant attractiveness to the vector (Eigenbrode et�al. 2002; Jim�nez-Mart�nez et�al. 2004; Belliure et�al. 2005; Fereres and Moreno 2009; Mauck et�al. 2010). In economically important crop systems such as soybean, a more complete understanding of virus-induced changes to insect vector or pest feeding behavior could help improve agricultural management tactics, which currently rely mostly on vector population suppression via insecticides (Perring et�al. 1999).
To date, little is known about how beetle-borne viruses may modulate host plant chemistry or feeding behavior preferences of their ‘chewing’ coleopteran vectors. To begin to characterize this, we carried out targeted metabolomic and global transcriptomic approaches to identify changes in BPMV-infected soybean leaves at the metabolic and molecular levels. While plant defence signaling and amino acid content were altered by viral infection, the most extraordinary finding was a substantial increase in foliar sucrose content. By carrying out a priori choice and no-choice E. varivestis behavioral bioassays using near-isogenic soybean lines, we better implicated these virus-induced changes in sucrose concentrations in mediating E. varivestis feeding preferences. Ultimately, the altered beetle feeding preferences are likely to increase BPMV incidence in natural environments.
Materials and Methods
Beetle Colony and Virus Maintenance
Experiments were carried out using a laboratory colony of Epilachna varivestis established in 2014 from multiple, ongoing field collections in Ohio, USA and reared in growth chambers under controlled conditions of 25 � 3 �C, 65% RH with a 14-h:10-h light-dark cycle that included 1.5 h dawn and dusk transitions. Beetles were maintained on ‘Sloan’ seedlings placed in 47.5 cm�47.5 cm�47.5 cm cages.
The BPMV isolate was maintained in ‘Sloan’ cultivar soybean through serial leaf-rub inoculation with inoculum made from leaves of infected plants (Louie et�al. 2000). To generate BPM-infected experimental plants, inoculum was made by grinding infected leaf tissue into 10 mM KHPO4, pH 7 (1:4 w/v) with carborundum to induce wounding. The inoculum was mechanically inoculated onto 10-day-old soybean plants (20 �l per plant). After visual assessment of symptom development, virus infection was confirmed using enzyme-linked immunosorbent assays (Todd et�al. 2010).
Transcriptional Accumulation in BPMV-Infected Soybean Leaves
Sample Collections
The youngest trifoliate leaf of both the experimental V2-stage (20day old) BPMV-infected and healthy (control) ‘Sloan’ soybean plants were collected at the same time (2pm EDT) to circumvent potential diurnal changes in mRNA accumulation profiles. Both treatments were replicated 4 times using seedlings from different stocks planted at different times (eight samples total).
RNA Isolation and cDNA Library Synthesis
For each replicate, total RNA was extracted from pools of five leaves sampled from different plants using the RNeasy Plant Mini Kit (QIAGEN, Germantown, MD). RNA quality was assessed using the Nanophotometer NP80 (Implen Inc., Westlake Village, CA), and quantity was calculated on the Qubit 3.0 fluorometer using the RNA HS assay kit (Thermo Fisher Scientific, Waltman, MA). RNA (1�g per sample) was used to generate adaptor-ligated double-stranded cDNA libraries for RNA sequencing using the TruSeq Sample Prep Kit V1 (Illumina, San Diego, CA) following the manufacturer’s protocol. Quantity and quality of each cDNA library was evaluated using the BioAnalyzer 2100 (Agilent Technologies, Santa Clara, CA). Samples were diluted to 50fmoles and pooled to generate the multiplexed cDNA library.
Illumina Sequencing and Read Preprocessing
The multiplexed cDNA library was sequenced in 100-bp paired-end fashion on a partial flow cell lane using the Illumina HiSeq 2500 platform at the G�nome Qu�bec Innovation Centre. Illumina Analysis Package CASAVA 1.8.2 was used to perform bcl conversion and demultiplexing. Image deconvolution and quality value calculations were carried out using the Illumina GA pipeline v1.6. Raw reads were imported into CLC Genomics Workbench (v7.5; CLC bio, Aarhus, Denmark) where adapter indexes and poly (A) tails were trimmed (Ambiguous limit = 2, quality limit = 0.05). Quality assessment of the data file included hierarchical clustering of samples (Measure: Euclidean distance, Clusters: Average linkage) and principle component analyses in CLC bio. The raw sequence reads can be retrieved from the NCBI short sequence read archive under the accession numbers SRR4676898 to SRR4676905 (study SRP092286).
Differential Gene Expression Analysis
The most recent Glycine max [Glyma2.0; 56,044 genes] reference cDNA database was retrieved using the BioMart tool in Phytozome (Goodstein et�al. 2012). Preprocessed reads were aligned to the cDNA database using the map to reference function in CLC bio Genomics Workbench and the following parameters: Similarity Fraction = 0.96; Length Fraction = 0.65; default settings herein. Reads of a given sample were only counted if they were unique (i.e., mapped to only one cDNA in the Glycine max cDNA database).
Genes differentially expressed between BPMV-infected and healthy (control) soybean were identified using the DESeq2 package (Anders and Huber 2010) in Bioconductor (Gentleman et�al. 2004), which calculates changes in transcript accumulation based on mapped reads counts using a negative binomial distribution model. Significance was defined at FDR <0.05 (Benjamini and Hochberg 2005), and only genes with a minimum of 10 reads across the 8 samples were included in the analysis (i.e., expressed). Functional annotation of differentially expressed genes was achieved manually by assessing putative functions derived from the Gene Ontogeny (GO), Panther, and PFAM databases.
Validation of Differential Expression Using qRT-PCR
Reactions (15 �l) were performed in duplicate using IQTM 2X SYBR Green Supermix (Bio-Rad, Hercules, CA) and 300 nm of each primer on a CFX96 Real-Time PCR Detection System according to the manufacturer’s recommendations. Cycling conditions were: 50 �C for 2 min; 95 �C for 10 min; 41 cycles of denaturation at 95 �C for 15 s, and annealing/extension at 60 �C for 1 min; and, 95 �C for 15 s. PCR efficiency (E) was evaluated by performing a dilution series experiment using a target assay and the equation E = 10(−1/slope) (Pfaffl 2001). Expression levels were measured separately for reference and target genes, using three biological replicates for each treatment/time point combination. Relative transcript abundance was calculated using the 2-ΔΔCT method (Livak and Schmittgen 2001). Reported threshold cycle (CT) values were normalized to the endogenous control (TATA-box binding protein, Bansal et�al. 2015) and converted to relative log-fold differences between the healthy and virus infected treatments. One-tailed independent t-tests (P <0.05) were used for statistical determination of differential expression between treatments (four replicates per).
Targeted Metabolomics Analyses of Soybean Leaves
Metabolite Extraction
Collection of experimental leaves was done identical to the transcriptional analysis (see above). Leaves were lyophilized, weighed (between 23 and 45 mg DW), and ground in 2 ml tubes containing a 5-mm tungsten bead for 3 min at 30 Hz in a mixer mill. Internal standards were added into each sample tube consisting of 500, 500, and 1,000 nmol of U-13C-labeled glucose, glycine, and fumarate, respectively. Water-soluble metabolites (sugars, sugar alcohols, amino acids, organic acids, and phosphorylated compounds) were extracted using 1 ml of boiling water and incubated for 10 min. Extracts were centrifuged at 14,000�g for 5 min at 4 �C and the supernatants were filtered through a 0.22-mm filter using a 5-ml syringe. Precipitates were then washed with 1 ml of cold water and filtered. The water-soluble metabolite filtrates were freeze-dried overnight.
Liquid Chromatography–Tandem Mass Spectrometry Quantification
Prior to quantification, freeze-dried extracts were resuspended in 300 �l of distilled water, loaded on a 0.2-�m nanosep MF centrifugal device and centrifuged at 14,000�g for 10 min. The LC utilized an ultra-high pressure liquid chromatography 1290 column (Agilent Technologies, Santa Clara, CA). The MS/MS analyses were carried out with a hybrid triple-quadruple/ion trap mass spectrometer QTRAP 5500 (AB Sciex, Framingham, MA). Liquid chromatography–tandem mass spectrometry (LC-MS/MS) data were acquired and processed using Analyst 1.6.1 software. LC-MS/MS quantification of sugars, sugar alcohols, amino acids, organic acids, and phosphorylated compounds were carried out at The Ohio State University Targeted Metabolomics Laboratory (OSU-TML) using previously outlined protocols (Alonso et�al. 2010; Cocuron and Alonso 2014; Cocuron et�al. 2014). Processed water-soluble metabolites were quantified (nmol/mg) relative to the internal standards (supplementary table S1, Supplementary Material online) and post hoc Student’s t-tests (P <0.05) with Bonferroni–Holm (1979) correction were performed to explore pairwise differences in metabolite quantities among the BPMV-infected and healthy (control) treatments.
Beetle-BPMV Transmission Assays
To quantify BPMV transmission rates by E. varivestis, newly adult beetles were fed on BPMV-infected soybean for a 5-day acquisition access period (AAP) and then transferred to individual uninfected seedlings for a 2-day inoculation access period (IAP). Beetles were then removed from the seedlings and the experimental plants were transferred to a growth chamber for 10 days to observe virus symptom development. After 10 days, BPMV presence in the upper trifoliate was assessed using RT–PCR (Cassone et�al. 2014a). Over 100 individuals were examined and the assays were replicated 3 times.
Beetle Behavioral Assays
No-Choice Assays
To assess beetle feeding behavior on soybean leaves differing in sucrose concentration, a minimum of 20 newly adult E. varivestis were starved overnight (∼12 h) and then placed into individual cages that contained either 8 Wildtype sucrose ‘V99-5089’ [WT], 8 high sucrose ‘MIPS mutant’ V99-5089 [HS], or 8 BPMV-infected ‘Sloan’ seedlings [BPMV]. Beetles were allowed to freely roam and feed on any of the plants within their respective cage over an 8-h period. The number of plants in each cage showing appreciable defoliation (i.e., >10 mm2) was recorded and total defoliation was determined using a desk-top scanner and public domain software. Independent sample t-tests (two tailed, P <0.05) were carried out to examine differences in the number of seedlings showing defoliation and total defoliation between treatments. Each treatment was replicated 10 times using adults from different cohorts and multiple maintenance cages. For this and the choice assays, only beetles that caused appreciable defoliation to at least one plant leaf were included in the analysis. Moreover, each replicate for this and the choice experiments was carried out using new seedlings of their respective treatment.
Choice Assays
To examine beetle preferences to healthy and BPMV-infected soybean, a minimum of 30 newly adult E. varivestis were starved overnight and then placed into individual cages that contained 6 healthy and 6 BPMV-infected wildtype seedlings from the V99-5089 soybean cultivar. The experiments were repeated using a near-isogenic ‘MIPS mutant’ line, which had been selected for high sucrose content. Seedlings were randomly distributed in each cage to ensure preferences were not due to spatial dispersion. Beetles were allowed to freely roam and feed any of the plants within their respective cage over a 2-h period. The number of plants in each cage showing appreciable (>10 mm2) foliar defoliation was recorded. Independent sample t-tests (two tailed, P <0.05) were used to assess differences in the number of defoliated seedlings. Each treatment was replicated 5 times using adults from different cohorts and multiple maintenance cages.
Results
Responses of Soybean to BPMV Infection
Transcriptomic Responses
To explore the changes to BPMV-infected soybean at the molecular level, large-scale changes in mRNA abundance were evaluated between infected and healthy leaves. A cDNA library composed of 8 pooled samples (4 per treatment) was sequenced, which generated 286,227,464 paired-end reads of 100 bp. After trimming for quality and removal of unpaired reads, between 27.7 and 43.3 million reads per sample were obtained (supplementary table S2, Supplementary Material online). On an average, 79.4% and 32.7% of preprocessed reads mapped uniquely to the Glycine max cDNA database for the healthy and BPMV-infected samples, respectively. The large discrepancy in mapping efficiency is attributed to the BPMV mRNA in the latter samples, indicating the virus has a massive presence in infected leaves.
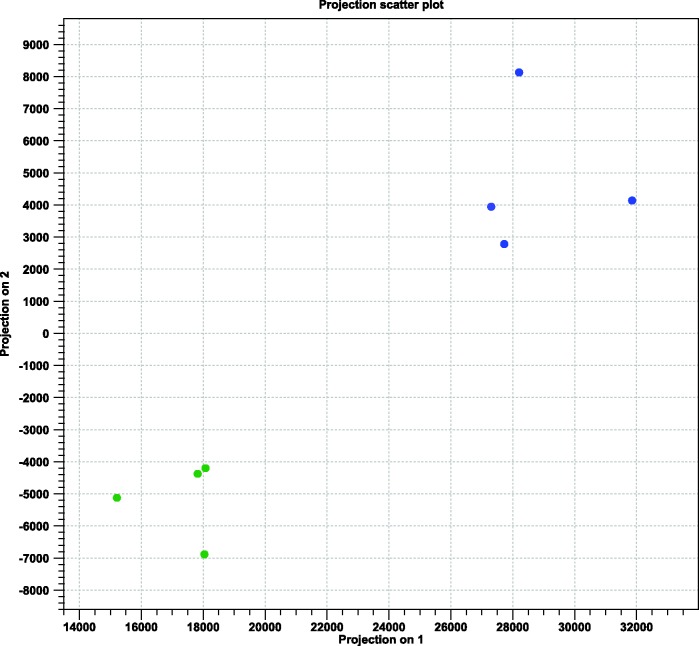
A 2D principal component analysis (PCA) was then constructed to visualize the relationships among the cDNA libraries (fig. 1). The healthy and BPMV-infected libraries clustered discretely from one another, confirming that viral infection influences the global expression of transcripts in soybean leaves.
Fig. 1.—
Principal component analysis (PCA) of the BPMV-infected and healthy soybean. Each circle represents an individual cDNA library from the indicated treatment. Green, healthy; Blue, BPMV-infected.
BPMV-Infected Soybean Show Elevated Defense Response
Over two-thirds of genes in the soybean cDNA database were determined to be expressed and thus included in the differential expression analysis (n = 38,488). DESeq analysis of the cDNA libraries revealed that BPMV-infection significantly altered the expression of nearly 1,000 soybean genes (FDR <0.05), with the majority upregulated in the virus-infected leaves relative to the healthy control (BPMV: n = 680; Healthy: n = 282).
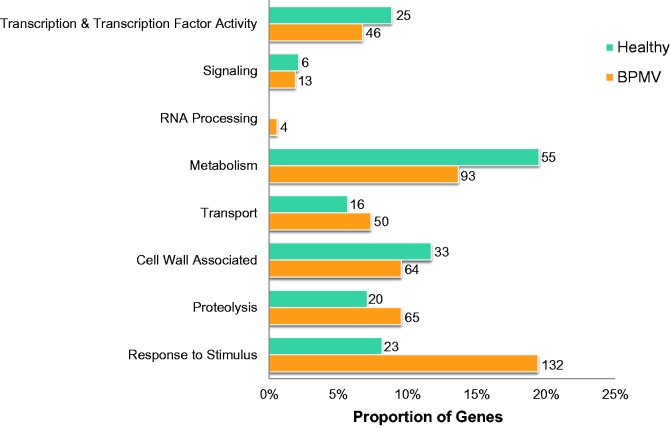
To gain insights into the functional pathways altered due to viral infection, we annotated each differentially expressed gene (where possible) using functions derived from the GO, Panther, and Pfam databases. Genes were first partitioned into two lists (up- or downregulated in BPMV-infected soybean relative to the control) and then manually placed into various categories and sub-categories based on consensus function (supplementary fig. S1 and table S3, Supplementary Material online). This was not possible for ∼29% of genes (n = 282), as they could not be assigned any putative function. The proportion of differentially expressed genes in eight functional categories (other categories not shown) is given in figure 2. Among the most populous categories for both lists was ‘Response to Stimulus’, ‘Metabolism’, ‘Proteolysis’, and ‘Cell Wall Associated’.
Fig. 2.—
Distribution of differentially expressed genes among functional categories of virus-infected and healthy soybean. Bars indicate the proportion of genes in each category: orange, BPMV-infected plants; light blue, healthy plants. Number of genes in each category is given beside each bar. Percentages do not total to 100 as not all categories are shown.
Most notable was the considerable number of genes upregulated in BPMV-infected soybean leaves that were sub-categorized as biotic stress-related (i.e., Response to Stimulus). This was the highest frequency sub-category among the lists by a wide margin. Within the category, most of the genes were annotated as encoding leucine-rich repeat containing proteins (LRRs; n = 75) or leucine-rich repeat receptor-like protein kinases (LRRPKs; n = 26). A chi-squared test (χ2) with Yates correction revealed that the number of LRR and LRRPK genes in the dataset were significantly overrepresented relative to the total number expressed in the Panther database (LRR: χ2 = 28.6 [1] P <0.0001; LRRPK: χ2 = 11.6 [1], P = 0.0003). Other sub-categories well represented in the BPMV treatment (n ≥15) include oxidation–reduction and amino acid metabolism (Metabolism), glycosyl hydrolase and transferase (Cell Wall Associated) and detoxification (Proteolysis). Also of interest are 10 genes implicated in sugar transport transcriptionally enhanced in BPMV-infected leaves—the most represented transport genes. Moreover, a number of genes encoded putative enzymes involved in sucrose biosynthesis (e.g., fructose-1,6-bisphosphatase, sucrose phosphate synthase, glyceraldehyde 3-phosphate dehydrogenase, glucose-6-phosphate isomerase). Four of these genes were subjected to qRT-PCR validation using primers designed in Primer3 (Rozen 2000). In all cases differential expression was confirmed, with similar magnitudes of expression between the different quantification methods (supplementary table S4, Supplementary Material online). Few findings of functional consequence could be disentangled for the subset of genes downregulated in BPMV-infected soybean, though there were a relatively high amount of carbohydrate metabolism genes (Metabolism).
Metabolomic Responses
To explore metabolite changes of soybean infected with BPMV, 85 water-soluble metabolites and derivatives (i.e., sugars, sugar alcohols, amino acids, organic acids, and phosphorylated compounds) were extracted and separated from infected and healthy soybean leaves using LC-tandem MS. Among the most abundant metabolites identified in healthy soybean leaves were the amino acids glutamine (76.49 �2 nmol/mg) and asparagine (52.64 �1.6 nmol/mg), and the sugars fructose (41.11 �2.1 nmol/mg) and malate (40.69 �3.4 nmol/mg).
BPMV-Infected Soybean Leaves Show Dramatically Elevated Sucrose Content
Student’s t-tests with Bonferroni–Holm (1979) correction were used to examine changes in soybean leaf metabolite profiles due to BPMV-infection. Our results revealed that the concentration of five metabolites differed among treatments (P <0.05), which included four amino acids and a sugar. The amino acids—alanine, arginine, asparagine, and citrulline—all exhibited significantly reduced concentration in BPMV-infected soybean leaves. Moreover, most other amino acids (e.g., serine, glycine, asparagine, tryptophan, histidine) showed considerable reduction in infected leaves but did not meet our threshold for significance. The relative concentrations of all amino acids profiled in BPMV-infected and healthy leaves are displayed in supplementary figure S2, Supplementary Material online.
The only other significant metabolite was sucrose, with a nearly 24-fold increase in concentration in virus-infected leaves (BPMV: = 14.42 nmol/mg, Healthy: = 0.607; P <0.0001). Not only did this metabolite show the most substantial change in magnitude by a wide margin, it was the only metabolite whose concentration significantly increased in infected plants.
To better gauge the robustness of our finding of elevated sucrose in BPMV-infected leaves, we carried out three independent a priori analyses, consisting of four replicates each. First, we wanted to deduce whether the elevated sucrose was a generalized response to virus-infection in soybean. Thus, foliar sucrose content was quantified for soybean infected with another virus, Soybean mosaic virus (SMV), at the same developmental stage. No significant differences were found between SMV-infected ( = 0.825 nmol/mg) and healthy plants. Next, to determine if increased sucrose concentration was associated with developmental stage, sucrose concentration was quantified in BPMV-infected leaves at two additional time points: 7- and 14-day postinoculation. Sucrose levels at both time points were also significantly elevated compared with healthy control plants, with a similar magnitude of difference (7 days: = 14.81; 14 days: = 13.29). Finally, to assess whether the increased sucrose is soybean cultivar dependent, we examined foliar sucrose content in BPMV-infected and healthy ‘24-10RY’ seedlings at 10-day postinoculation. The virus-infected leaves ( = 13.32 nmol/mg) had significantly higher sucrose than the control ( = 0.721 nmol/mg). Thus, these findings such that the elevated sucrose content is a product of BPMV infection rather than an artifact related to soybean cultivar, developmental stage, or common viral response.
Responses of Mexican Bean Beetles to Feeding on BPMV-Infected Soybean
Beetle-BPMV Transmission Rates
Mexican bean beetles, E. varivestis, are considered efficient vectors of BPMV (Scott and Fulton 1978). To confirm this in our laboratory colony, assays were carried out to determine BPMV transmission rates in E. varivestis. The proportions of beetles that acquired the virus and successfully inoculated a new host plant across three replicates were 35.2% (37 of 105), 37.9% (41 of 108), and 33.6% (39 of 116). Thus, over one-third of E. varivestis individuals are capable of transmitting BPMV, indicating the beetle is an efficient vector of the virus. Moreover, the consistency in BPMV transmission efficiency among replicates suggests that E. varivestis vector competence has a strong genetic component (Cassone et�al. 2014b).
No-Choice Behavioral Assays
The most interesting finding of the metabolic analysis was a dramatic ∼24-fold increase in sucrose concentration in BPMV-infected leaves. At the genetic level, this response was accompanied by increased transcript accumulation of genes implicated in sucrose transport and biosynthesis. Thus, we wanted to explore the role of sucrose in shaping vector–virus relationships; specifically, whether changes in sucrose levels altered E. varivestis leaf-feeding behavior. To do this, we conducted ‘no-choice bioassays’ to examine the feeding behavior of E. varivestis on soybean possessing high or normal sucrose content in their leaves. We utilized near-isogenic soybean lines (NILs), whose genetic makeup presumably differs only in genes related to sucrose production. Thus, our approach allows us to better implicate the role (if any) of foliar sucrose in beetle leaf-feeding behavior. The two NILs selected had comparable sucrose levels to those found in our healthy and BPMV-infected ‘Sloan’ leaves (see above): the regular sucrose ‘Wildtype’ V99-5089 soybean cultivar ‘WT’ (3.25 �0.9 nmol/mg) and the high sucrose ‘MIPS mutant’ V99-5089 cultivar ‘HS’ (11.73 �1.3 nmol/mg). To complement the study, a BPMV-infected (and thus high sucrose) Sloan treatment was also included in the analysis.
Beetles Feed on More Plants but for Less Time If They Are High in Sucrose
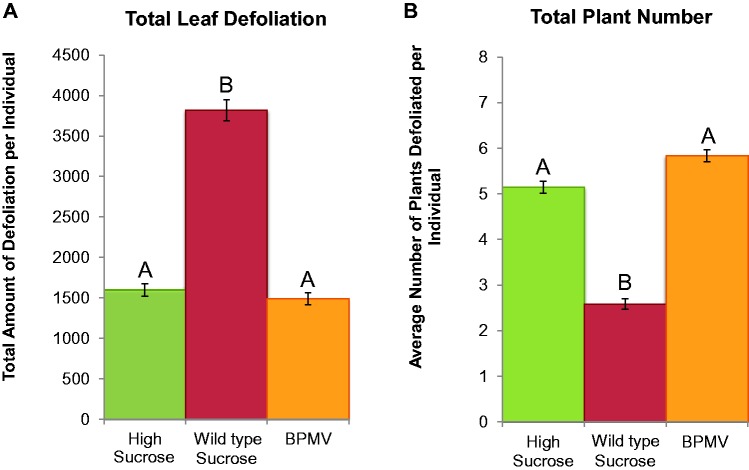
A total of 234, 248, and 212 beetles were assayed across 10 biological replicates for the WT, HS, and BPMV-infected Sloan treatments, respectively (fig. 3A). Relative to the normal sucrose NIL, the total amount of defoliation (i.e., all seedlings combined per experimental cage) was significantly lower for beetles fed on both the high sucrose and BPMV-infected seedlings (both comparisons, P <0.0001). Across all experimental cages and replicates, the moderate sucrose NIL had roughly 1.5-fold greater total defoliation than the higher sucrose treatments. Total defoliation was not significantly different between the high sucrose and BPMV treatments (P >0.05).
Fig. 3.—
No-choice leaf feeding assays of Epilachna varivestis on Wildtype (i.e., regular) and high sucrose V99-5089 NILs, as well as BPMV-infect plants. (A) The total defoliation (mm2) and (B) total number of plants showing appreciate defoliation (>10 mm2) was quantified across all plants in a given treatment. The bar graphs represent averages across treatments and replicates (standard errors bars are included).
In terms of number of seedlings exhibiting appreciable defoliation (i.e., between 0 and 8 per experimental cage), the high sucrose treatments both showed significantly greater quantities of defoliated seedlings than the moderate sucrose treatment (both comparisons, P <0.0001) (fig. 3B). Across all replicates, ‘WT’ averaged 2.58 defoliated plants per experimental cage, whereas the ‘HS’ and BPMV-infected cages averaged 5.15 and 5.84, respectively.
Choice Behavioral Assays
Virus-induced changes to host plants have been well documented to alter vector feeding behavior (Ingwell et�al. 2012; Guti�rrez et�al. 2013; Moreno-Delafuente et�al. 2013). Thus, we wanted to determine whether clean (i.e., uninfected) E. varivestis were more attracted to BPMV-infection soybean than to healthy plants. To do this, we performed ‘choice bioassays’ to explore preferential plant feeding by individual beetles. By employing a shorter feeding duration of 2 h and larger number of plants per experimental cage than the no-choice assays (see above), we are better able to gauge the beetles’ initial lure to the various treatments. In these experiments individual E. varivestis were given the option of feeding on healthy and BPMV-infected seedlings of the ‘HS’ NIL. The experiments were repeated using healthy and virus-infected ‘WT’ NIL soybean.
Beetles Are More Attracted to BPMV-Infected Plants
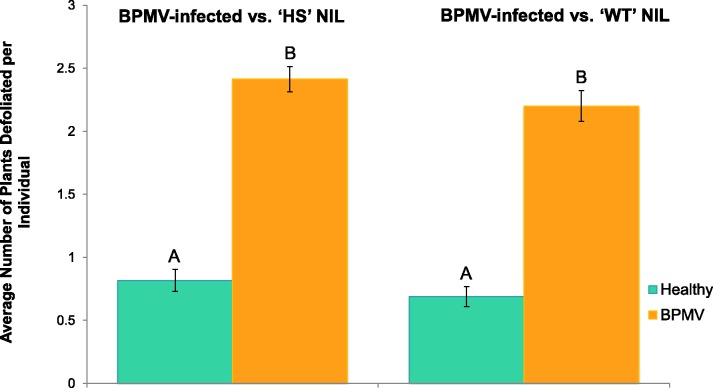
We examined 169 and 154 beetles from the ‘HS’ and ‘WT’ experiments, respectively (fig. 4). In both cases, E. varivestis fed on significantly more plants if they were BPMV-infected (both comparisons, P <0.0001). Across all experimental cages and replicates, beetles fed on BPMV-infected plants defoliated 2-fold more leaf tissue on an average than the beetles feeding on the healthy NILs. Epilachnavarivestis defoliated a greater total number of plants on an average during the ‘HS’ assays, presumably because of the higher foliar sucrose content.
Fig. 4.—
Choice leaf feeding assays of Epilachna varivestis on BPMV-infected and healthy (control) soybean of either the Wildtype ‘WT’ or high sucrose ‘HS’ V99-5089 NIL. The bar graphs represent averages across treatments and replicates (standard errors bars are included).
Discussion
Pathogenic microbes are known to elicit a richness of underlying physiological and molecular defence responses in host plants, and these relationships can often be extended into much broader evolutionary and ecological context. In these experiments, we used complementary targeted metabolite profiling and global transcriptomic approaches to explore the metabolic and molecular responses of soybean to BPMV infection. The soybean—BPMV—E. varivestis pathosystem was chosen for these studies because of its economic significance and tractability for laboratory assays. Intriguingly, virus-infected leaves had dramatically higher sucrose content than did healthy leaves. Underlying this change was heightened expression of many genes involved in sucrose biosynthesis and transport. These abrupt changes to plant host chemistry prompted the hypothesis that modifications to sucrose levels could be a tactic employed by the virus to indirectly promote its spread via competent beetle vectors—such as the efficient E. varivestis. Leaf-feeding bioassays indicated that E. varivestis is more attracted to BPMV-infected soybean than to healthy plants. Supplementary choice and no-choice experiments using soybean NILs of differing foliar sucrose content revealed that the beetles consumed less foliage per plant but grazed on a greater number of plants in a given time period if they were infected with BPMV, presumably due to the higher foliar sucrose content. Ultimately, this altered beetle feeding pattern is likely to increase BPMV prevalence in natural environments. Beetle-borne viruses are often acquired within minutes of feeding and sometimes even on first bite (Fulton et�al. 1980, 1987), and can then be transmitted to a new host plant with no associated latency period (Fulton et�al. 1980; Wang et�al. 1994; Hull 2002). Thus, from the beetle-borne virus’ perspective, the most effective transmission and dispersal strategy is having its inoculative vectors feeding for shorter durations on a greater number of plants.
While the idea of a plant-infecting virus indirectly manipulating the feeding behavior of their insect vectors may seem remarkable, several recent studies have demonstrated this may occur rather frequently in nature. For instance, bird cherry-oat aphids (Rhopalosiphum padi) infected with Barley yellow dwarf virus preferred noninfected wheat plants, whereas clean aphids preferred virus-infected plants. Similarly, plants infected with Tomato spotted wilt virus were more attractive than uninfected plants (i.e., increased feeding and oviposition) to their clean western flower thrip (Frankliniella occidentalis) vectors (Bautista et�al. 1995; Maris et�al. 2004). Cucumber mosaic virus induced changes in host plant chemistry that altered patterns of recruitment and dispersal of its aphid vectors (Myzus persicae and Aphis gossypii). Moreover, host plant virus infection has even been found to modulate the feeding behavior of nonvector insects (Hu et�al. 2013). Virtually all of the previous research has focused on piercing-sucking insects; to our knowledge this is the first study to begin to characterize plant virus-induced changes to feeding preferences of a ‘chewing’ insect vector. Further, while host plant sucrose levels have been shown to alter insect feeding behavior, this is the first report of virus-induced manipulations to sucrose content.
The underlying mechanism of MBB attraction to BPMV-infected plants or repulsion to higher levels of sucrose is not immediately known. Sucrose is a well-known feeding stimulant for phyophagous insects (Matsuda 1981). Indeed, sap-sucking insects feed directly on phloem sap whose main component is sucrose. These insects possess adaptive osmoregulatory mechanisms that allow for the ingestion of remarkably high levels of sucrose without leading to dehydration (Wilkinson et�al. 1997; Ashford et�al. 2000; Karley et�al. 2005). On the other hand, beetles feed on whole leaf tissue, which has comparatively lower sucrose levels than phloem. Lacking the sophisticated osmoregulatory system of sap-sucking insects, beetles are likely more susceptible to huge increases in sucrose content and thus, compensate by feeding shorter durations on these plants. Indeed, both hump beetles (Gibbium psylloides) and flour beetles (Tribolium confusum) were found to be sensitive to different concentrations of foliar sucrose (Loschiavo 1965; El-Sawaf and El-Sayes 1978). Beetle attraction to BPMV-infected plants is also not immediately understood but is presumably related to virus-induced modifications of the volatile profiles of infected plants. Previous work found volatiles from Potato leafroll virus-infected potato plants attract the virus vector Myzus persicae (Eigenbrode et�al. 2002). Similarly, volatile blends emitted by Cucumber mosaic virus and Tomato chlorosis virus infected plants increases their attractiveness to aphid and whitefly vectors, respectively (Mauck et�al. 2010; Fereres et�al. 2016).
In addition to elevated sucrose content, BPMV infection brought about other metabolomic and molecular modifications in infected leaves. Notably, the concentration of most amino acids and derivatives were considerably lower in the virus-infected leaves. This included a 6-fold decrease in glutamine, which is the predominant amino acid in healthy soybean and in many other plant species (Weibull et�al. 1990; Valle et�al. 1998; Lohaus and Moellers 2000; Sandstrom et�al. 2000; Amiard et�al. 2004). Citrulline and arginine, while less abundant in healthy leaves, were also significantly depleted in BPMV-infected leaves. While there is some discrepancy regarding the impact of BPMV infection on foliar amino acid content (Tu and Ford 1970), our finding was not completely unexpected. Amino acids are the primary source of nitrogen for plants; viruses are known to alter plant nutritional quality by diverting resources towards virus replication and shifting the allocation of nutrients among host tissues (Shalitin and Wolf 2000; Shalitin et�al. 2002; Mauck et�al. 2014). Thus, the decreased amino acid content in BPMV-infected leaves likely represents a by-product of the infection process rather than a strategy employed by the virus to promote its vector-mediated spread.
The transcriptional responses of soybean to BPMV-infection were mostly characterized by a massive upregulation of genes encoding leucine-rich repeat (LRR)-containing proteins and LRR-containing receptor-like protein kinases. Plants, despite lacking an explicit immune system protection against pathogen attack, are equipped with alternative mechanisms in the form of defence genes (Shanmugam 2005). LRR-containing proteins are widely associated with pathogen resistance in plants, providing an early warning system for the presence of potential pathogens and activating protective immune signaling (Jones and Jones 1997; Tor et�al. 2009; Jaillais et�al. 2011). Accordingly, our finding of substantial LRR gene activation in a BPMV susceptible soybean line at 10-day postinoculation may seem counterintuitive. However, these genes are perpetually present and expressed in plants under pathogen attack, irrespective of whether the plant is resistant or susceptible to a given pathogen (Shanmugam 2005). Rather, it is time of induction of these defence genes that differs among resistant and susceptible lines, with induction usually occurring later during pathogenesis in susceptible reactions (Newman et�al. 1994). Thus, if our soybean cultivar was resistant to BPMV, it is likely the LRR response would have subsided long before 10-day postinoculation. Several other biotic stress-related gene groups, as well as some proteolysis and cell wall-associated gene groups were also upregulated in BPMV-infected soybean and in relatively high frequency (e.g., cytochrome P450s, pathogenesis-related proteins Bet v l, glycosyl hydrolases). It is probable that many of these are associated with the LRR response and were activated downstream.
In conclusion, our experiments combined cutting-edge metabolomic and transcriptomic approaches with traditional behavioral bioassays to explore tritrophic interactions between an economically important crop (soybean), a prevalent soybean-infecting virus (BPMV), and an efficient beetle vector of the virus (E. varivestis). Remarkably, the dramatically elevated sucrose content in BPMV-infected soybean leaves appears to alter E. varivestis leaf-feeding behavior in a way that likely facilitates virus transmission and dispersal in natural settings. Future studies are needed to explore the underlying mechanisms of beetle attraction to BPMV-infected plants and to determine how the virus modulates foliar sucrose concentration. Interestingly, retention time of BPMV in E. varivestis is considerably shorter than in another efficient vector of virus, the bean leaf beetle, Ceratoma trifurcata (Fulton and Scott 1974; Fulton et�al. 1980). It would be intriguing to determine whether C. trifurcata also shows altered feeding behavior on sucrose-rich soybean, or if the behavior is specific to E. varivestis. Ultimately, we hope this work will help rejuvenate interest and stimulate future research into beetle-borne disease transmission dynamics.
Supplementary Material
Supplementary data are available at Genome Biology and Evolution online).
Author Contributions
B.J.C. conceived and designed the study. B.J.C., C.M.S. and C.R.G. conducted the experiments. B.J.C., C.M.S. and K.F.W. did the computation analysis. B.J.C. wrote and revised the article. All authors read and approved the final article.
Supplementary Material
Acknowledgments
We want to thank MA Saghai-Maroof (Virginia Tech) for graciously providing the soybean sucrose NILs. Funding for the project was provided by Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grants Program awarded to Bryan Cassone (RGPIN-2016-04335), and by the Center for Applied Plant Sciences—The Ohio State University.
Literature Cited
- Alonso AP, Piasecki RJ, Wang Y, LaClair RW, Shachar-Hill Y. 2010. Quantifying the labeling and the levels of plant cell wall precursors using ion chromatography tandem mass spectrometry. Plant Physiol. 153:915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiard W, et al. 2004. Carbohydrate and amino acid composition in phloem sap of Lolium perenne L. before and after defoliation. Can J Bot. 82:1594–1601. [Google Scholar]
- Anders S, Huber W. 2010. Differential expression analysis for sequence count data. Genome Biol. 11:R106.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashford DA, Smith WA, Douglas AE. 2000. Living on a high sugar diet: the fate of sucrose ingested by a phloem-feeding insect, the pea aphid Acyrthosiphon pisum. J Insect Physiol. 46(3):335–341. [DOI] [PubMed] [Google Scholar]
- Astier S, Albouy J, Maury Y, Lecoq H. 2001. Principes de Virologie V�g�tale. Paris: INRA. [Google Scholar]
- Auclair JL. 1959. Life-history, effects of temperature and relative humidity, and distribution of the Mexican bean beetle, Epilachna varivestis Mulsant (Coleoptera: Coccinellidae) in Quebec, with a review of the pertinent literature in North America. Ann Soc Entomol Quebec. 5:18–43. [Google Scholar]
- Bansal R, et al. 2015. Recommended reference genes for quantitative PCR analysis in soybean have variable stabilities during diverse biotic stresses. PLoS One 10(8):e0134890.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista RC, Mau RFL, Cho JJ, Custer DM. 1995. Potential of tomato spotted wilt tospovirus plant hosts in Hawaii as virus reservoirs for transmission by Frankliniella occidentalis (Thysanoptera: Thripidae). Phytopathology 85:953–958. [Google Scholar]
- Belliure B, Janssen A, Maris PC, Peters D, Sabelis MW. 2005. Herbivore arthropods benefit from vectoring plant viruses. Ecol Lett. 8:70–79. [Google Scholar]
- Benjamini Y, Hochberg Y. 2005. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B Stat Methodol. 57(1):289–300. [Google Scholar]
- Bosque-P�rez NA, Eigenbrode SD. 2011. The influence of virus-induced changes in plants on aphid vectors: insights from luteovirus pathosystems. Virus Res. 159(2):201–205. [DOI] [PubMed] [Google Scholar]
- Cassone BJ, Cisneros Carter FM, Michel AP, Stewart LR, Redinbaugh MG. 2014b. Genetic insights into Graminella nigrifrons competence for Maize fine streak virus infection and transmission. PLoS One 9(11):e113529.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassone BJ, et al. 2014a. Reduction in fecundity and shifts in cellular processes by a native virus on an invasive insect. Genome Biol Evol. 6(4):873–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocuron JC, Alonso AP. 2014. Liquid chromatography tandem mass spectrometry for measuring 13C-labeling in intermediates of the glycolysis and pentose phosphate pathway. Methods Mol Biol. 1090:131–142. [DOI] [PubMed] [Google Scholar]
- Cocuron JC, Anderson B, Boyd A, Alonso AP. 2014. Targeted metabolomics of Physaria fendleri, an industrial crop producing hydroxy fatty acids. Plant Cell Physiol. 55:620–633. [DOI] [PubMed] [Google Scholar]
- Eigenbrode SD, Ding H, Shiel P, Berger PH. 2002. Volatiles from potato plants infected with potato leafroll virus attract and arrest the virus vector, Myzus persicae (Homoptera: Aphididae). Proc Biol Sci. 269(1490):455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sawaf BM, El-Sayes SM. 1978. Feeding behaviour of the hump beetle, Gibbium psylloides Czemp. (Col., Ptinidae). J Appl Entomol. 86:46–52. [Google Scholar]
- Fan Y, Groden E, Drummond FA. 1992. Temperature-dependent development of Mexican bean beetle (Coleoptera: Coccinellidae) under constant and variable temperatures. J Econ Entomol. 85:1762–1770. [Google Scholar]
- Fereres A, et al. 2016. Tomato infection by whitefly-transmitted circulative and non-circulative viruses induce contrasting changes in plant volatiles and vector behaviour. Viruses 8:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fereres A, Moreno A. 2009. Behavioural aspects influencing plant virus transmission by homopteran insects. Virus Res. 141(2):158–168. [DOI] [PubMed] [Google Scholar]
- Fulton JP, Gergerich RC, Scott HA. 1980. Beetles In: Harris KF, Maramorosch K, editors. Vectors of plant pathogens. San Diego: Academic Press; p. 115–132. [Google Scholar]
- Fulton JP, Gergerich RC, Scott HA. 1987. Beetle transmission of plant viruses. Annu Rev Phytopathol. 25:111–123. [Google Scholar]
- Fulton JP, Scott HA. 1974. Virus-vectoring efficiencies of two species of leaf-feeding beetles. Proc Am Phytopathal Soc. 1:159. [Google Scholar]
- Gentleman R, et al. 2004. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 5:R80.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergerich RC, Scott HA. 1988. Evidence that virus translocation and virus infection of non-wounded cells are associated with transmissibility by leaf-feeding beetles. J Gen Virol. 69:2935–2938. [Google Scholar]
- Ghabrial SA, Pickard CM, Stuckey RE. 1977. Identification and distribution of virus diseases of soybean in Kentucky. Plant Dis Rep. 61:690–694. [Google Scholar]
- Goodstein DM, Shu S, Howson R, Neupane R, Hayes RD, Fazo J, Mitros T, Dirks W, Hellsten U, Putnam N. et al. 2012. Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res. 40(Database issue):D1178–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guti�rrez S, Michalakis Y, Van Munster M, Blanc S. 2013. Plant feeding by insect vectors can affect life cycle, population genetics and evolution of plant viruses. Funct Ecol. 27:610–622. [Google Scholar]
- Hodge S, Powell G. 2008. Do plant viruses facilitate their aphid vectors by inducing symptoms that alter behavior and performance. Environ Entomol. 37(6):1573–1581. [DOI] [PubMed] [Google Scholar]
- Holm S. 1979. A simple sequentially rejective multiple test procedure. Scand J Stat. 6:65–70. [Google Scholar]
- Hu Z, Zhao H, Thieme T. 2013. Modification of non-vector aphid feeding behavior on virus-infected host plant. J Insect Sci. 13:28.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull H. 2002. Transmission 1: by invertebrates, nematodes and fungi In: Roger Hull, editor. Matthews' plant virology. San Diego: Elseevier Academic Press; p. 485–531. [Google Scholar]
- Hurd H. 2003. Manipulation of medically important insect vectors by their parasites. Annu Rev Entomol. 48:141–161. [DOI] [PubMed] [Google Scholar]
- Ingwell LL, Eigenbrode SD, Bosque-P�rez NA. 2012. Plant viruses alter insect behavior to enhance their spread. Sci Rep. 2:578.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin ME, Kampmeier GE, Weisser WW. 2007. Aphid movement: process and consequences In: van Emden HF, Harrington R, editors. Aphids as crop pests. Wallingford, Oxfordshire, UK: CAB International; p. 153–186. [Google Scholar]
- Jaillais Y, Belkhadir Y, Balsemao-Pires E, Dangl JL, Chory J. 2011. Extracellular leucine-rich repeats as a platform for receptor/coreceptor complex formation. Proc Natl Acad Sci U S A. 108:8503–8507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jim�nez-Mart�nez ES, Bosque-P�rez NA, Berger PH, Zemetra RS. 2004. Life history of the bird cherry-oat aphid, Rhopalosiphum padi (Homoptera: Aphididae), on transgenic and untransformed wheat challenged with Barley yellow dwarf virus. J Econ Entomol. 97:203–212. [DOI] [PubMed] [Google Scholar]
- Jones D, Jones J. 1997. The role of leucine-rich repeat proteins in plant defenses. Adv Bot Res. 24:89–167. [Google Scholar]
- Karley AJ, Ashford DA, Minto LM, Pritchard J, Douglas AE. 2005. The significance of gut sucrase activity for osmoregulation in the pea aphid, Acyrthosiphon pisum. J Insect Physiol. 51(12):1313–1319. [DOI] [PubMed] [Google Scholar]
- Lef�vre T, Thomas F. 2008. Behind the scene, something else is pulling the strings: emphasizing parasitic manipulation in vector-borne diseases. Infect Genet Evol. 8:504–519. [DOI] [PubMed] [Google Scholar]
- Lei W, et al. 2016. EPG recordings reveal differential feeding behaviors in Sogatella furcifera in response to plant virus infection and transmission success. Sci Rep. 6:30240.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 25(4):402–408. [DOI] [PubMed] [Google Scholar]
- Lohaus G, Moellers C. 2000. Phloem transport of amino acids in two Brassica napus L. genotypes and one B. carinata genotype in relation to their seed protein content. Planta 211:833–840. [DOI] [PubMed] [Google Scholar]
- Loschiavo SR. 1965. Methods for studying aggregation and feeding behavior of the confused flour beetle, Tribolium confusum (Coleoptera: Tenebrionidae). Ann Entomol Soc Am. 58:383–388. [Google Scholar]
- Louie R, Redinbaugh MG, Gordon DT, Abt JJ, Anderson RJ. 2000. Maize necrotic streak virus, a new maize virus with similarity to species of the family Tombusviridae. Plant Dis. 84:1133–1139. [DOI] [PubMed] [Google Scholar]
- Luan JB, et al. 2013. Suppression of terpenoid synthesis in plants by a virus promotes its mutualism with vectors. Ecol Lett. 16:390–398. [DOI] [PubMed] [Google Scholar]
- Mabry TR, et al. 2003. Distribution of leaf-feeding beetles and Bean pod mottle virus (BPMV) in Illinois and transmission of BPMV in soybean. Plant Dis. 87:1221–1225. [DOI] [PubMed] [Google Scholar]
- Maris PC, Joosten NN, Goldbach RW, Peters D. 2004. Tomato spotted wilt virus infection improves host suitability for its vector Frankliniella occidentalis. Phytopathology 94:706–711. [DOI] [PubMed] [Google Scholar]
- Matsuda K. 1981. Feeding stimulation of nutrient chemical’s in Gastphysa atrocanea (Coleoptera: Chrysomelidae). Jap J App Ent Zool. 25:84–88. [Google Scholar]
- Mauck KE, De Moraes CM, Mescher MC. 2010. Deceptive chemical signals induced by a plant virus attract insect vectors to inferior hosts. Proc Natl Acad Sci U S A. 107(8):3600–3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauck KE, De Moraes CM, Mescher MC. 2014. Biochemical and physiological mechanisms underlying effects of Cucumber mosaic virus on host-plant traits that mediate transmission by aphid vectors. Plant Cell Environ. 37:1427–1439. [DOI] [PubMed] [Google Scholar]
- Moreno-Delafuente A, Garzo E, Moreno A, Fereres A. 2013. A plant virus manipulates the behavior of its whitefly vector to enhance its transmission efficiency and spread. PLoS One 8(4):e61543.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser RO, Hum-Musser SM, Felton GW, Gergerich RC. 2003. Increased larval growth and preference for virus-infected leaves by the Mexican bean beetle, a plant virus vector. J Insect Behav. 16:247–256. [Google Scholar]
- Nakamura H. 2007. Report of the investigation on Epilachna varivestis defoliation in Guatemala high land. Available from: http://agris.fao.org/agris-search/search.do?recordID=JP2007005104.
- Newman MA, Conrads-Strauch J, Scofield G, Daniels MJ, Dow JM. 1994. Defense related gene induction in Brassica campestris in response to defined mutants of Xanthomonas campestris with altered pathogenicity. Mol Plant Microbe Interact. 7:553–563. [DOI] [PubMed] [Google Scholar]
- Perring TM, Gruenhagen NM, Farrar CA. 1999. Management of plant viral diseases through chemical control of insect vectors. Annu Rev Entomol. 44:457–481. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29(9):e45.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse CM, Dicke M. 2007. Plant interactions with microbes and insects: from molecular mechanisms to ecology. Trends Plant Sci. 12(12):564–569. [DOI] [PubMed] [Google Scholar]
- Rozen S. 2000. Primer3 on the WWW for general users and for biologist programmers In: Rozen S, Skaletsky H, editors. Bioinformatics methods and protocols: methods in molecular biology. Totowa (NJ: ): Humana Press Inc; p. 365–386. [DOI] [PubMed] [Google Scholar]
- Sanderlin RS. 1973. Survival of bean pod mottle and cowpea mosaic virus in beetles following intrahemocoelic injections. Phytopathology 63:259–261. [Google Scholar]
- Sandstrom J, Telang A, Moran NA. 2000. Nutritional enhancement of host plants by aphids – a comparison of three aphid species on grasses. J Insect Physiol. 46:33–40. [DOI] [PubMed] [Google Scholar]
- Scott HA, Fulton JP. 1978. Comparison of the relationships of Southern bean mosaic virus and the cowpea strain of tobacco mosaic virus with the bean leaf beetle. Virology 84:207–209. [DOI] [PubMed] [Google Scholar]
- Scott HA, Phatak HC. 1979. Properties of blackgram mottle virus. Phytopathology 69:346–348. [Google Scholar]
- Selman BJ. 1973. Beetles-phytophagous Coleoptera In: Gibbs AJ, editor. Viruses and invertebrates. North-Holland, Amsterdam, The Netherlands: North-Holland Publishing Company. p. 158–177. [Google Scholar]
- Shalitin D, Wang Y, Omid A, Wolf S. 2002. Cucumber mosaic virus movement protein affects sugar metabolism and transport in tobacco and melon plants. Plant Cell Environ. 25:989–997. [Google Scholar]
- Shalitin D, Wolf S. 2000. Cucumber mosaic virus infection affects sugar transport in melon plants. Plant Physiol. 123:597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam V. 2005. Role of extracytoplasmic leucine rich repeat proteins in plant defence mechanisms. Microbiol Res. 160(1):83–94. [DOI] [PubMed] [Google Scholar]
- Stafford CA, Walker GP, Ullman DE. 2011. Infection with a plant virus modifies vector feeding behavior. Proc Natl Acad Sci U S A. 108(23):9350–9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd JC, Ammar ED, Redinbaugh MG, Hoy C, Hogenhout SA. 2010. Plant host range and leafhopper transmission of Maize fine streak virus. Phytopathology 100:1138–1145. [DOI] [PubMed] [Google Scholar]
- Tor M, Lotze MT, Holton N. 2009. Receptor-mediated signalling in plants: molecular patterns and programmes. J Exp Bot. 60:3645–3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu JC, Ford RE. 1970. Free amino acids in soybeans infected with Soybean mosaic virus, Bean pod mottles virus, or both. Phytopathology 60:660–664. [Google Scholar]
- Valle EM, Boggio SB, Heldt HW. 1998. Free amino acid composition of phloem sap and growing fruit of Lycopersicon esculentum. Plant Cell Physiol. 39:458–461. [Google Scholar]
- Walters HJ. 1969. Beetle transmission of plant viruses. Adv Virus Res. 15:339–363. [DOI] [PubMed] [Google Scholar]
- Wang RY, Gergerich RC, Kim KS. 1992. Noncirculative transmission of plant viruses by leaf feeding beetles. Phytopathology 82:946–950. [Google Scholar]
- Wang RY, Gergerich RC, Kim KS. 1994. The relationship between feeding and virus retention time in beetle transmission of plant viruses. Phytopathology 84(9):995–998. [Google Scholar]
- Weibull J, Ronquist F, Brishammar S. 1990. Free amino acid composition of leaf exudates and phloem sap: a comparative study in oats and barley. Plant Physiol. 92:222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson T, Ashford D, Pritchard J, Douglas A. 1997. Honeydew sugars and osmoregulation in the pea aphid Acyrthosiphon pisum. J Exp Biol. 200(Pt 15):2137–2143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.