Aneuploidy is the leading cause of infertility, abortion, stillbirth and congenital birth defects in humans. A well-known example is trisomy 21 (a.k.a Down syndrome). An unusually high frequency of aneuploidy pregnancies occurs in humans compared with other species, and ∼90% of this aberrancy derives from errors in female meiosis.1 Moreover, in human females, the frequency of aneuploidy rises with increasing maternal age, with a basal level of ∼10% in younger women and 50% or more in women nearing the end of their reproductive lifespan. This so-called “maternal age effect” is often attributed to loss of sister chromatid cohesion in older women.1 However, the basal level in young women is still very high as compared with human males or other species, implying an intrinsic defect in the basic meiotic program in females.
A critical event in meiosis is the formation of DNA crossovers (COs) between the maternal and paternal versions of each chromosome (“homologs”). COs promote genetic diversity. However, they also play an important mechanical role for proper chromosome segregation. When inter-homolog COs are absent or sub-optimally located, they will impair the development of tension on centromere/kinetochore complexes, thus promoting chromosome mis-segregation.2
Early comparisons show that in human female aneuploidy, the mis-segregating chromosomes exhibit unique CO patterns as compared with regularly-segregating chromosomes (below).1 It has remained mysterious whether unique features of the CO formation process in female meiosis contribute to this difference and/or whether chromosomes with certain CO configurations are differentially prone to mis-segregation at meiosis I.
To address this issue, we analyzed patterns of COs, as marked by MLH1 foci along spread meiotic pachytene chromosomes, in both human spermatocytes and primary oocytes. Two female-specific characteristics emerged.3 (1) In females, there is a higher frequency of chromosomes that lack even a single CO, despite the fact that females have longer chromosome axes and thus more COs. (2) CO number scales with chromosome axis length in both sexes; however, the absolute density of COs (number per µm axis length) is significantly lower in females than males. Both effects can be simply explained by assuming that some fraction of COs are missing in female meiosis as compared with the number expected. To explore this hypothesis, and to investigate possible reason(s) for “missing COs," we applied our well-established CO simulation program, which can accurately and quantitatively model CO patterns in many organisms.4-6
By combining simulation analysis with experimental data, we could document several effects.3 (1) The recombination process is very similar in both sexes, most notably including CO interference, which ensures that COs do not occur very close together. (2) Males exhibit a typical and efficient CO recombination process as other analyzed organisms. (3) In contrast, and most importantly, in human females, the number and positions of CO recombinational interactions are established with even spacing along chromosomes as in males, but now ∼25% of those “designated” interactions fail to mature into actual COs (Fig. 1). This conclusion is confirmed by analysis of genetically-defined CO patterns along chromosome 21. This and other analyses exclude the possibility that female-specific CO patterns result from defects at earlier stages of the recombination process (e.g. recombination initiation or susceptibility of recombinational interactions to CO designation) and/or are an artifact of MLH1 focus analysis.
Figure 1.
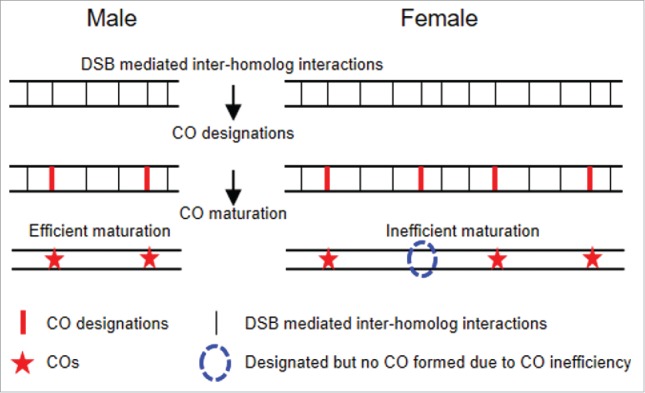
Human females, but not males, have a specific inefficiency of crossover maturation in meiosis. Human males and females have a similar recombination process as other studied organisms. Recombination initiates by programmed DNA double-strand break (DSBs). Ensuing DSB-mediated inter-homolog interactions create undifferentiated “CO precursors." A CO designation process then acts, giving sequential CO-designated sites, each accompanied by spreading of interference to either side. CO designations tend to be evenly spaced along the chromosomes.4-6 Compared to human males, human female meiotic chromosomes have longer axes (and smaller loops) and, in correlation, more DSBs and CO precursors. Ensuing CO designation and crossover interference are the same in both sexes. However, specifically in human females, ∼25% of CO designations fail to mature into COs. This maturation defect leads to lower CO density, higher numbers of chromosomes that lack even one CO, and other observed female-specific effects. (Note: horizontal bars indicate homologous chromosomes).
Exactly how does CO maturation inefficiency compromise meiosis I homolog segregation (and thus promote aneuploidy)?
One important consideration is that inefficient CO maturation comes into play after CO sites are designated.3 Thus, the consequence of a maturation defect is to directly “subtract” events from the array of evenly-spaced COs that would otherwise have occurred. Such subtraction produces 2 types of atypical configurations. (1) A fraction of chromosomes with one or 2 CO designations will now acquire zero COs. (2) Another fraction of chromosomes, especially those with only a small number of CO designations, will now acquire an “irregular” distribution of COs. These 2 situations lead, respectively, to absence, or a sub-optimal level, of tension on homolog centromere/kinetochore complexes and thereby perturb regular homolog segregation.
A second important issue is the relationship of CO maturation inefficiency to effects that come into play in older women. Here the important point is that CO formation is completed (at pachytene) whereas maternal age effects come into play during arrest at the stage immediately after pachytene. Many considerations focus on loss of sister cohesion as an age-dependent effect.1 This effect is possible because a CO can link a pair of homologs together only if sister connections are present centromere-distal to the CO site. CO maturation inefficiency tends to increase the frequency of homolog pairs whose COs occur far from the centromere and thus are more susceptible to age-dependent loss of cohesion. Other possibilities for age-dependent factors that influence mis-segregation have also been suggested. In any case, CO maturation inefficiency sets up the baseline for aneuploidy, as seen in younger women. Then, CO maturation inefficiency and age-dependent factors (e.g., cohesin loss) would work together to promote a higher level of segregation errors in older women.3
A third important consideration is that the level of tension that is imposed upon or sensed or transmitted by homolog centromere/kinetochore complexes can be decreased by several other factors (e.g., short chromosome length; presence of only one chromosome arm; occurrence of chromosome interlockings).3 Thus, effects of CO maturation inefficiency will also interact, potentially synergistically, with these age-independent factors, as well as age-dependent factors, to influence segregation fidelity.
The findings of Wang et al. (ref. 3) raise interesting questions for further study. (1) Why do human females exhibit CO maturation inefficiency, which would seem to be a sub-optimal feature? Perhaps this feature is indeed deleterious and remains present because the period of hominid evolution has simply not been long enough to eliminate it. On the other hand, perhaps CO maturation inefficiency is an actively selected feature with an intrinsic evolutionary benefit(s). Several possible models can be envisioned. Or, conceivably, this is an evolutionarily neutral trait despite its significant consequences. (2) Aneuploidy levels are also elevated in younger men.7 It would be interesting to know whether CO maturation inefficiency contributes to this effect. (3) At what stage of the meiotic recombination process does CO maturation inefficiency occur? And what molecule(s) is/are specifically involved? Our analyses show that the defect must arise at step(s) after a subset of recombination intermediates are specifically designated to be COs but before those intermediates are finally converted into completed CO products. Detailed comparisons of the cytological focus patterns for recombination factors that act during the relevant period, such as RNF212, MSH4/5, Hei10 and CNTD1, in both males and females, would provide valuable information as to which stage(s) might be important.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
Support for N.K. was provided by NIH R01 GM044794.
References
- [1].Nagaoka SI, Hassold TJ, Hunt PA. Human aneuploidy: mechanisms and new insights into an age-old problem. Nat Rev Genet 2012; 13:493-504; PMID:22705668; https://doi.org/ 10.1038/nrg3245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Watanabe Y. Geometry and force behind kinetochore orientation: lessons from meiosis. Nat Rev Mol Cell Biol 2012; 13:370-82; PMID:22588367; https://doi.org/ 10.1038/nrm3349 [DOI] [PubMed] [Google Scholar]
- [3].Wang S, Hassold T, Hunt P, White MA, Zickler D, Kleckner N, Zhang L. Inefficient crossover maturation underlies elevated aneuploidy in human female meiosis. Cell 2017; 168:977-89; PMID:28262352; https://doi.org/ 10.1016/j.cell.2017.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wang S, Zickler D, Kleckner N, Zhang L. Meiotic crossover patterns: Obligatory crossover, interference and homeostasis in a single process. Cell Cycle 2015; 14:305-14; PMID:25590558; https://doi.org/ 10.4161/15384101.2014.991185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].White M, Wang S, Zhang L, Kleckner N. Modeling of meiotic CO interference as inspired by the beam-film model. Methods Mol Biol 2017; 1471:305-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhang L, Liang Z, Hutchinson J, Kleckner N. Crossover patterning by the beam-film model: analysis and implications. PLoS Genet 2014; 10:e1004042; PMID:24497834; https://doi.org/ 10.1371/journal.pgen.1004042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Steiner B, Masood R, Rufibach K, Niedrist D, Kundert O, Riegel M, Schinzel A. An unexpected finding: younger fathers have a higher risk for offspring with chromosomal aneuploidies. Eur J Hum Genet 2015; 23:466-72; PMID:25005732; https://doi.org/ 10.1038/ejhg.2014.122 [DOI] [PMC free article] [PubMed] [Google Scholar]


