Abstract
Background
Carbapenems are widely used for the management of bloodstream infections (BSIs) caused by extended-spectrum β-lactamase-producing Enterobacteriaceae (ESBL-PE). However, the wide use of carbapenems has been associated with carbapenem-resistant Enterobacteriaceae development.
Methods
We searched the PubMed and Scopus databases (last search date was on June 1, 2016) looking for studies that reported mortality in adult patients with ESBL-PE BSIs that were treated with carbapenems or β-lactam/β-lactamase inhibitors (BL/BLIs).
Results
Fourteen studies reported mortality data in adult patients with ESBL-PE BSI that were treated with carbapenems or BL/BLIs. Among them, 13 studies reported extractable data on empiric therapy, with no statistically significant difference in mortality of patients with ESBL-PE BSI that were treated empirically with carbapenems (22.1%; 121 of 547), compared with those that received empiric BL/BLIs (20.5%; 109 of 531; relative risk [RR], 1.05; 95% confidence interval [CI], 0.83–1.37; I2 = 20.7%; P = .241). In addition, 7 studies reported data on definitive therapy. In total, 767 patients (79.3%) received carbapenems and 199 patients (20.6%) received BL/BLIs as definitive therapy, and there was again no statistically significant difference (RR, 0.62; 95% CI, 0.25–1.52; I2 = 84.6%; P < .001). Regarding specific pathogens, the use of empiric BL/BLIs in patients with BSI due to ESBL-Escherichia coli was not associated with a statistically significant difference in mortality (RR, 1.014; 95% CI, 0.491–2.095; I2 = 62.5%; P = .046), compared with the use of empiric carbapenems.
Conclusions
These data do not support the wide use of carbapenems as empiric therapy, and BL/BLIs might be effective agents for initial/empiric therapy for patients with BSI caused by likely ESBL-PE, and especially ESBL-E coli.
Keywords: β-lactam/β-lactamase inhibitor (BL/BLIs), bloodstream infection (BSI), carbapenems, extended-spectrum β-lactamase (ESBL)
The incidence of infections caused by extended-spectrum β-lactamase-producing Enterobacteriaceae (ESBL-PE) is increasing [1–3], and, according to a 2013 report from the US Centers of Disease Control and Prevention, 19% of healthcare-related Enterobacteriaceae infections are caused by ESBL-PE [4]. Bloodstream infections (BSI) caused by ESBL-PE, in particular, are associated with high mortality, prolonged hospital stay, and increased hospital costs [5, 6], and for these infections, carbapenems are currently considered the first-line therapy [5, 7, 8]. However, the use of carbapenems has been linked to the development of carbapenem-resistant Enterobacteriaceae [8]. The efficacy of β-lactam/β-lactamase inhibitor (BL/BLIs) combinations, such as piperacillin/tazobactam, in ESBL-PE BSI treatment is reported, but it is controversial [9, 10]. We conducted a systematic review and meta-analysis to compare the empiric use of BL/BLIs with that of carbapenems for ESBL-PE BSI.
METHODS
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [11].
Data Source and Searches
A systematic search of the PubMed and Scopus databases was conducted (last day of access: June 1, 2016), using the terms “(ESBL OR (Extended spectrum β lactamase) OR (Extended spectrum β lactamase)) AND (bacteremia OR bacteraemia OR blood OR bloodstream OR septicemia OR sepsis).” Two researchers (M. E. F. and M. M.) screened the identified articles by reading through their title and abstract. Relevant studies in English were reviewed in full text. We supplemented our search by searching the references lists of the eligible studies.
Study Selection
Articles were considered eligible for inclusion if they reported data on the mortality of adult patients with ESBL-PE BSI that received monotherapy with carbapenems or BL/BLIs. For studies that reported outcomes at multiple time points, data on the longest follow-up period were used. Case reports and conference abstracts were not considered.
Definitions
We defined as empiric therapy the use of antimicrobial agents administered based on clinical suspicion and before the results of blood cultures and susceptibility tests become available. The antibiotic treatment administered based on susceptibility results was considered as definitive therapy.
Data Collection
Our primary outcome was to compare the mortality of patients with ESBL-PE BSI that received carbapenems to the mortality of patients with ESBL-PE BSI that received BL/BLIs. Two researchers (M. E. F. and M. M.) independently extracted the data from eligible studies into separate Microsoft Excel 2010 spreadsheets (Microsoft Corporation, Redmond, WA), and discrepancies were resolved by consensus. The following data were extracted: study mid-year, country, study design, ESBL-PE species, number of patients with ESBL-BSI that received empiric and/or definitive therapy with carbapenems or BL/BLIs, and, among them, the number of patients who died. Studies were grouped according to World Health Organization regions [12].
Quality Assessment
All included studies were evaluated using the Newcastle-Ottawa Quality Assessment Scale (NOS) for methodological quality [13]. The NOS is a star-based system that evaluates studies at 3 levels: selection of the exposed and nonexposed cohort, comparability of cohorts, and outcomes. A maximum of 7 stars could be assigned to each study, because the field “Comparability of cohorts on the basis of the design or analysis” was not applicable in our analysis. We considered the study appropriate for inclusion if it received at least 4 of 7 stars (of 7 maximum).
Data Synthesis and Analysis
For subgroup and sensitivity analyses, we divided the included studies that provided relevant data into 2 groups. In the first group, the researchers retrospectively reviewed patient charts and included patients with ESBL-PE BSI, and the isolated pathogens were susceptible to the carbapenems or BL/BLIs. In the second group, the researchers did not use the sensitivity test results to select the included patients.
For statistical analysis, we calculated pooled risk ratios (RRs) and 95% confidence intervals (CIs) for comparison. Data were excluded from our analysis if both comparison sides reported zero-event (death) [14]. I2 and X-square were used to evaluate the statistical heterogeneity among studies. The I2 values of <25%, 25%–50%, or >50% indicates low, moderate, or high heterogeneity, respectively. We used the Egger’s test (ET) to test for publication bias due to small study effect. For direct comparisons, we used a fixed-effects model (Mantel-Haenszel) in absence of heterogeneity, otherwise we used random-effects model with the DerSimonian and Laird approach. For sensitivity analysis we used meta-regression analyses. The Stata version 14 software package (Stata Corporation) was used to perform the statistical analysis. A P value less than 0.05 was considered as statistically significant.
RESULTS
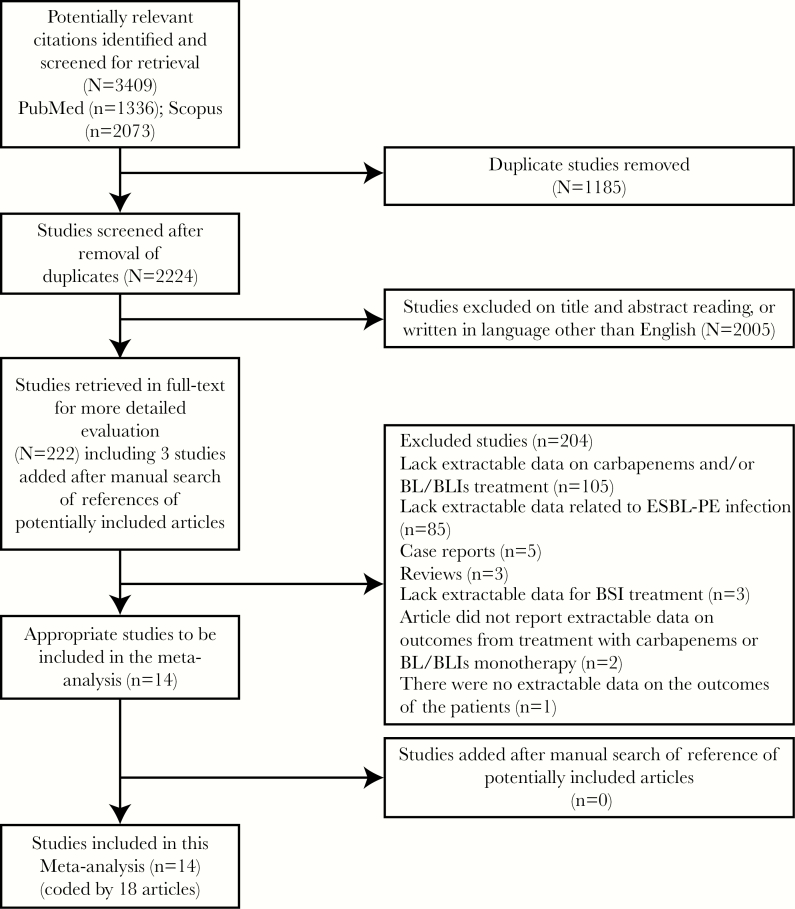
By the last search date (June 1, 2016), we found 3409 articles (1336 articles from PubMed and 2073 articles from Scopus). After removing duplicates and excluding articles by reviewing their abstracts and titles, we accessed 222 articles in full text (including 3 articles that were added later after reviewing the references list of potential included papers). Of these, 204 articles were excluded: 105 articles lacked extractable data on carbapenems and/or BL/BLIs treatment, 85 articles lacked extractable data related to ESBL-PE infection, 5 articles were case reports, 3 articles were reviews, 3 articles lacked extractable data for BSI treatment, 2 articles did not report extractable data on outcomes from treatment with carbapenems or BL/BLIs monotherapy, and in 1 article there were no extractable data on the outcomes of adult patients. As a result, we identified 14 studies, coded by 18 articles, that fulfilled our inclusion criteria (Figure 1). By searching the references list of the included articles, no further studies met our inclusion criteria. All of the included studies were of high quality (registered ≥4 stars of 7 stars on NOS) (Supplementary Data).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram. Abbreviations: BL/BLIs, β-lactam/β-lactamase inhibitor; BSI, bloodstream infection; ESBL-PE, extended-spectrum β-lactamase-producing Enterobacteriaceae.
Of the 14 included studies (that fulfilled our inclusion criteria) [15–28], 11 were retrospective [15, 17–19, 21–25, 27, 28] and 3 were prospective [16, 20, 26] (Table 1). One study was multicenter and performed in 12 countries (Table 1) [15], 6 studies were performed in the Western Pacific region (4 in China [2 of them were performed in Taiwan], 1 in Republic of Korea, and 1 in Singapore) [16, 18, 21, 22, 24, 27], 4 studies were performed in the European region (1 in Italy, 2 in Spain, and 1 in Turkey) [20, 23, 26, 28], and 3 studies were performed in the region of the Americas (1 in Canada and 2 in United States of America) [17, 19, 25]. The isolated pathogens were Escherichia coli in 5 studies [16, 20, 23, 26, 27], Enterobacter cloacae in 2 studies [22, 25], E coli or Klebsiella pneumoniae in 5 studies [17–19, 21, 24], and E coli or K pneumoniae or Proteus mirabilis in 1 study [28]. The isolated pathogens were not specified in 1 study [15]. In addition, 8 studies reported mortality at 30 days [15, 16, 18, 20, 21, 24, 26, 27], 2 studies reported mortality at 28 days [22, 25], 1 study reported mortality at 21 days [28], 1 study reported mortality at 14 days [23], and 2 studies reported outcomes until hospital discharge [17, 19]. A summary of the number and percentage of patients with ESBL-PE BSI categorized according to the source of BSI is provided in the Supplementary Data.
Table 1.
Characteristics of Included Studiesa
| Author (Mid-Year) | Country | Study Design | Type of Bacteria | Mortality (Days) | Patients Received BL/BLIs as Definitive Therapy | Patients Received Carbapenems as Definitive Therapy | Patients Treated Empirically With BL/BLIs | Patients Treated Empirically With Carbapenems |
|---|---|---|---|---|---|---|---|---|
| Deceased/studied | Deceased/studied | Deceased/studied | Deceased/studied | |||||
| Gutiérrez- Gutiérrez et al (2009) [15] | Multicenterb | Retrospective | Enterobacteriaceae | 30 | 9/92 | 71/509 | 30/170 | 39/195 |
| Bin et al (2004) [16] | China | Prospective | Escherichia coli | 30 | 0/2 | 0/8 | 0/2 | 0/3 |
| Chaubey et al (2004) [17] | Canada | Retrospective | E coli or Klebsiella pneumoniae | NA | 6/28 | 4/30 | 6/16 | 0/10 |
| Cheng et al (2006) [18] | China (Taiwan) | Retrospective | E coli or K pneumoniae | 30 | NA | NA | 0/4 | 10/39 |
| Chopra et al (2007) [19] | United States of America | Retrospective | E coli or K pneumoniae | NA | 7/18 | 12/33 | NA | NA |
| Gudiol et al (2007) [20] | Spain | Prospective | E coli | 30 | 2/2 | 3/14 | 3/6 | 2/5 |
| Kang et al (2009) [21] | Republic of Korea | Retrospective | E coli or K pneumoniae | 30 | NA | NA | 8/36 | 21/78 |
| Lee et al (2005) [22] | China (Taiwan) | Retrospective | Enterobacter cloacae | 28 | 3/3 | 5/53 | 1/13 | 4/24 |
| Metan et al (2005) [23] | Turkey | Retrospective | E coli | 14 | NA | NA | 5/7 | 7/22 |
| Ng et al (2012) [24] | Singapore | Retrospective | E coli or K pneumoniae | 30 | NA | NA | 29/94 | 17/57 |
| Qureshi et al (2007) [25] | United States of America | Retrospective | E cloacae | 28 | NA | NA | 1/4 | 0/8 |
| Rodŕıguez- Bano et al (2004) [26] | Spain | Prospective | E coli | 30 | 5/54 | 20/120 | 7/72 | 6/31 |
| To et al (2008) [27] | China | Retrospective | E coli | 30 | NA | NA | 15/74 | 14/47 |
| Tumbarello et al (2002) [28] | Italy | Retrospective | E coli or K pneumoniae or Proteus mirabilis | 21 | NA | NA | 4/33 | 1/28 |
Abbreviations: BL/BLIs, β-lactam/β-lactamase inhibitor; BSI, bloodstream infection; ESBL, extended spectrum β-lactamase; ESBL-PE, ESBL-producing Enterobacteriaceae; NA, not applicable.
aCollected data (first author, mid year, country, study design, ESBL-PE species, number of patients with ESBL-PE BSI that received empiric and/or definitive therapy with carbapenems or BL/BLIs, and among them, the number of patients who died).
bAfrican Region (South Africa), Western Pacific Region (Australia and China [Taiwan]), European region (Germany, Greece, Israel, Italy, Spain, and Turkey), in the region of the Americas (Argentina, Canada, and United States).
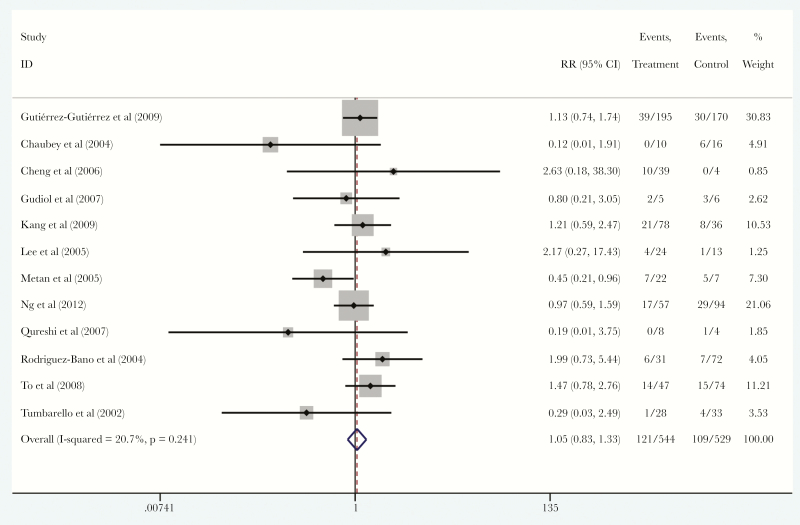
Among the 14 included studies, we found 13 studies [15–18, 20–28] that reported data on empiric therapy for patients with ESBL-PE BSI. These studies included a total of 1078 patients, among them 547 (50.7%) received carbapenems and 531 (49.3%) received BL/BLIs as empiric monotherapy. Among those patients, 121 patients (22.1%) in the carbapenems group and 109 patients (20.5%) in the BL/BLIs group died. There was no statistically significant difference in the mortality of patients that were treated with carbapenems compared with BL/BLIs (RR, 1.05; 95% CI, 0.83–1.37; I2 = 20.7%; P = .241), without a small study effect (ET = 0.053, PET = 0.928) (Figure 2).
Figure 2.
Forest plot of included studies. Relative risk (RR) of mortality among patients with extended-spectrum β-lactamase-producing Enterobacteriaceae bloodstream infections that were treated with empiric carbapenems versus empiric β-lactam/β-lactamase inhibitors. Abbreviation: CI, confidence interval.
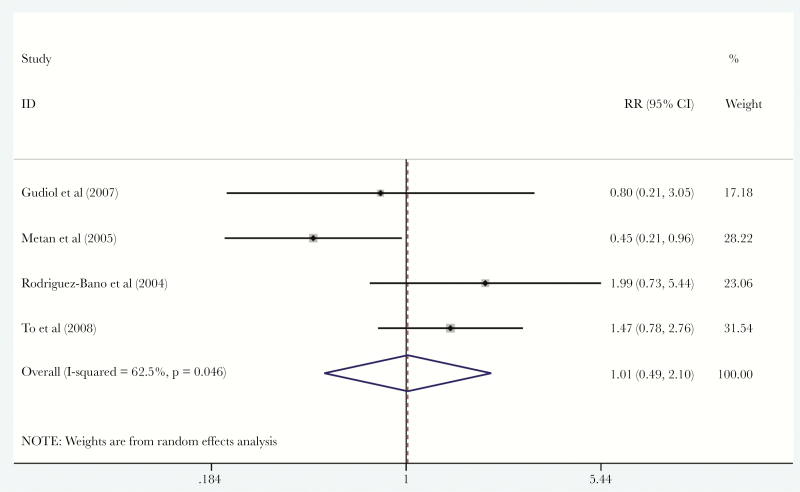
Five studies [16, 20, 23, 26, 27] reported data on empiric therapy for patients with ESBL-E coli BSI. We found no statistically significant difference in mortality of patients with ESBL-E coli BSI that were treated with empiric carbapenems (26.8%; 108 patients and 29 of them died) versus empiric BL/BLIs (18.6%; 161 patients and 30 of them died) (RR, 1.01; 95% CI, 0.49–2.10; I2 = 62.5%; P = .046) (ET = −0.37, PET = 0.929) (Figure 3). Furthermore, 2 studies [22, 25] reported data on empiric therapy for patients with ESBL-E cloacae BSI. Among them, the mortality of patients that were treated with empiric carbapenems was 12.5% (32 patients and 4 of them died) and empiric BL/BLIs was 11.7% (17 patient and 2 of them died). We were not able to estimate the mortality of patients with BSI caused by other ESBL-PE, such as ESBL-K pneumoniae or ESBL-P mirabilis, that were treated with carbapenems versus BL/BLIs, because such data were not reported and not extractable.
Figure 3.
Forest plot of included studies. Relative risk (RR) of mortality of patients with extended-spectrum β-lactamase Escherichia coli bloodstream infections that were treated with empiric carbapenems versus empiric β-lactam/β-lactamase inhibitors. Abbreviation: CI, confidence interval.
Five studies [17–19, 21, 24] included ESBL-E coli BSI and ESBL-K pneumoniae BSI cases without providing distinct data for each pathogen. Among them, 4 studies provided data on empiric therapy. There was no statistically significant difference in mortality of patients that were treated with empiric carbapenems 26% (184 patients and 48 of them died) versus empiric BL/BLIs 28.6% (150 patients and 43 of them died) (RR, 1.02; 95% CI, 0.83–1.25; I2 = 52.2%; P = .099). One study [28] included ESBL-E coli BSI, ESBL-K pneumoniae BSI, and ESBL-P mirabilis BSI cases without providing distinct data for each pathogen. Among them, the mortality of patients that were treated with empiric carbapenems was 3.5% (28 patients and 1 of them died) and empiric BL/BLIs was 5.6% (33 patients and 4 of them died). In addition, 1 study reported combined data regarding empiric therapy for patients with ESBL-PE BSI, without species specification [15]. Among them, the mortality of patients that were treated with empiric carbapenems was 20.0% (195 patients and 39 of them died) and empiric BL/BLIs was 17.6% (170 patients and 30 of them died).
As described under Methods, we divided the 13 studies that provided relevant data into 2 groups. In the first group (4 studies) [18, 26–28], the authors retrospectively reviewed patient charts and included patients with BSI, and the isolated pathogens were susceptible to carbapenems or BL/BLIs. Within the first group, we found that 145 patients were treated empirically with carbapenems (31 of them died; 21.3%) and 183 patients were treated empirically with BL/BLIs (26 of them died; 14.2%). In the second group (9 studies) [15–17, 20–25], the authors did not use the sensitivity test results to select the included patients. Within the second group, 402 patients were treated empirically with carbapenems (90 of them died; 22.3%) and 348 patients were treated empirically with BL/BLIs (83 of them died; 23.8%). In both the first and second group, there was no statistically significant difference in mortality among patients that were treated empirically with carbapenems compared with BL/BLIs: first group (RR, 1.416; 95% CI, 0.859–2.336); and second group (RR, 0.959; 95% CI, 0.734–1.252).
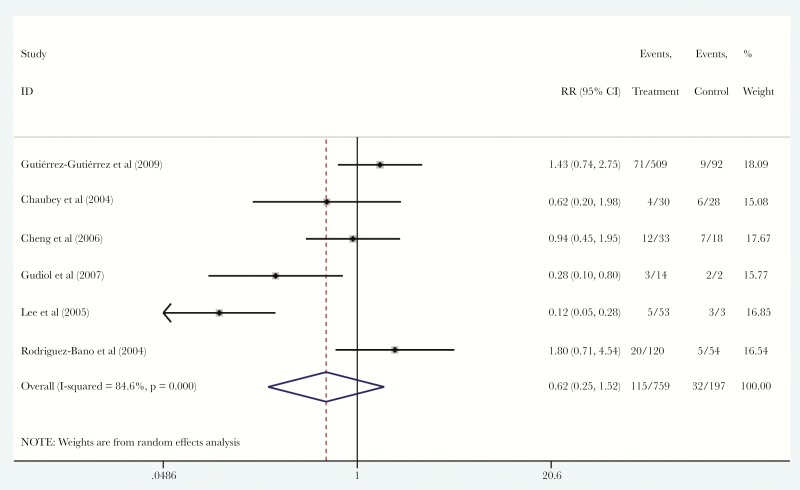
Seven studies [15–17, 19, 20, 22, 26] also reported data on definitive therapy for patients with ESBL-PE BSI. In total, 767 patients (79.3%) received carbapenems and 199 patients (20.6%) received BL/BLIs as definitive therapy. Among them, 115 patients (15.2%) in the carbapenems group and 32 patients (16.2%) in the BL/BLIs group died. There was no statistically significant difference in the mortality of patients that were treated with carbapenems compared with BL/BLIs as definitive therapy (RR, 0.62; 95% CI, 0.25–1.52; I2 = 84.6%; P < .001), without small study effect (ET = −3.13, PET = 0.367) (Figure 4).
Figure 4.
Forest plot of included studies. Relative risk (RR) of mortality among patients with extended-spectrum β-lactamase-producing Enterobacteriaceae bloodstream infections that were treated with definitive therapy with carbapenems versus definitive therapy with β-lactam/β-lactamase inhibitors. Abbreviation: CI, confidence interval.
In addition, in 3 studies [16, 20, 26] that reported data on definitive therapy for patients with ESBL-E coli BSI, the mortality of patients that were treated with carbapenems was 15.1% (142 patients and 23 of them died), whereas among those that received BL/BLIs the mortality was 12.0% (58 patients and 7 of them died). Moreover, 1 study [22] reported data on definitive therapy for patients with ESBL-E cloacae BSI, and the mortality of patients that were treated with carbapenems was 9.4% (53 patients and 5 of them died), whereas among those that received BL/BLIs it was 100% (3 patients and all 3 died). In addition, 2 studies [17, 19] reported combined data on definitive therapy for patients with ESBL-E coli BSI and ESBL-K pneumoniae BSI without providing distinct data for each pathogen, and the mortality of patients that were treated with carbapenems was 25.3% (63 patients and 16 of them died), whereas among those that received BL/BLIs it was 28.2% (46 patients and 13 of them died). Finally, in 1 study that reported combined data on ESBL-PE BSI, without species specification, the mortality of patients that were treated with definitive therapy with carbapenems was 13.9% (509 patients and 71 of them died), whereas among those that received BL/BLIs as definitive therapy it was 9.7% (92 patients and 9 of them died) [15].
DISCUSSION
Infections caused by ESBL-PE are associated with high mortality, and carbapenems are the treatment of choice for these infections [6, 8]. However, use of carbapenems increases the risk for carbapenem-resistant Enterobacteriaceae development and is related to adverse effects [8]. In this systematic review and meta-analysis, we found no statistically significant difference in the mortality of patients with ESBL-PE BSI that were treated empirically with BL/BLIs compared with those treated empirically with carbapenems. Therefore, initial/empiric BL/BLIs may constitute reasonable alternatives to initial/empiric carbapenems for the management of likely ESBL-PE BSI. However, as speculated previously, but has yet to be proven, this conclusion might be more applicable and reasonable probably in not critically ill patients. This is because the included studies are retrospective and nonrandomized, and the treating medical team had the freedom to choose and decide whether to use BL/BLIs or carbapenems for each patient, and it is possible that the patients with more severe illness were treated with carbapenems rather than BL/BLIs. Therefore, the favorable effect of BL/BLIs on outcomes of patients with ESBL-PE BSI might be because the patients that were treated with BL/BLIs were less severely ill.
In our analysis, the mortality of patients with ESBL-E coli BSI that were treated with empiric BL/BLIs (18.6 %) was lower compared with the mortality of patients with ESBL-E coli BSI that were treated with empiric carbapenems (26.8%); however, the difference in mortality did not reach statistical significance. These findings are in alignment with previous reports that propose considering BL/BLIs as treatment for ESBL-E coli BSI [5]. For example, in one multicenter study, piperacillin-tazobactam showed favorable effect on mortality of patients with ESBL-E coli BSI compared with carbapenems [5, 29]. Most notably, in our study, the mortality was also lower among patients with ESBL-E coli BSI that were treated with definitive therapy of BL/BLIs. However, as stated above, in all studies, it is unclear whether the carbapenems were primarily administered to patients with more comorbidities or a more severe clinical picture.
Furthermore, 5 studies included ESBL-E coli BSI and ESBL-K pneumoniae BSI cases without providing distinct data for each pathogen. Among them, 4 studies provided data on empiric therapy. Although statistically not significant, we found that the mortality of patients that were treated with empiric BL/BLIs was higher compared with the mortality of patients that were treated with empiric carbapenems. These results might be attributed to the outcomes of patients with ESBL-K pneumoniae BSI, rather than outcomes of patients with ESBL-E coli BSI. This speculation is based on our aforementioned data that showed a lower mortality in patients with ESBL-E coli BSI that were treated with empiric BL/BLIs. In addition, such a result has been reported previously, and in at least 1 study, the BL/BLIs were found to be inferior compared with cabapenems as treatment for patients with ESBL-K pneumoniae BSI [30]. Therefore, for patients at risk for BSI due to ESBL-K pneumoniae (or ESBL-E cloacae [22]) and for those with a recent urinary tract infection or other infection by these pathogens, clinicians might consider empiric carbapenems treatment.
It is noteworthy to mention that the majority of the included studies did not specify the outcomes among immunocompromised patients, and we were not able to extract such data that can be used to construct a useful conclusion. It also must be noted that a high ESBL-PE inoculum may impact negatively on the antimicrobial efficacy of piperacillin-tazobactam. This phenomenon is known as inoculum effect [9, 31–33]. Among patients with BSIs, it can be speculated that the higher bacterial inoculum volume is encountered in immunocompromised patients [34]. Therefore, empiric BL/BLIs might not be a proper antibiotic for immunocompromised patients with likely ESBL-PE BSI.
Regarding study limitations, our findings are based mainly on retrospective and nonrandomized studies. Moreover, we were unable to perform further stratification of outcomes based on patient comorbidities due to lack of sufficient data. Finally, distinct outcomes of BSI caused by pathogens such as ESBL-K pneumoniae and ESBL-P mirabilis were either very limited or not reported [15, 35–37]. In this context, we anticipate that the MERINO, randomized, controlled trial that compares meropenem versus piperacillin-tazobactam for definitive treatment of BSIs due to ESBL-producing E coli and Klebsiella spp [38] will provide useful information.
CONCLUSIONS
In conclusion, we found no statistically significant difference in mortality of adults with ESBL-PE BSI that were treated empirically with carbapenems compared with those that were treated with BL/BLIs. These data do not support the wide use of empiric carbapenems, and BL/BLIs seem to be effective for the management of patients with likely ESBL-PE BSI, and especially ESBL-E coli BSI, while waiting for susceptibility results. However, the efficacy BL/BLIs may vary based on the specific pathogen, and the future studies should account for severity of illness, immune suppression, and include adequate number of patients with BSI due to ESBL pathogens other than E coli, and especially K pneumoniae.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgement
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Paterson DL, Bonomo RA. Extended-spectrum beta-lactamases: a clinical update. Clin Microbiol Rev 2005; 18:657–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jacoby GA, Munoz-Price LS. The new beta-lactamases. N Engl J Med 2005; 352:380–91. [DOI] [PubMed] [Google Scholar]
- 3. De Rosa FG, Pagani N, Fossati L et al. The effect of inappropriate therapy on bacteremia by ESBL-producing bacteria. Infection 2011; 39:555–61. [DOI] [PubMed] [Google Scholar]
- 4. US Department of Health and Human Services Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States Available at: https://www.cdc.gov/drugresistance/biggest_threats.html 2013. Accessed 31 December 2016.
- 5. Harris PN, Tambyah PA, Paterson DL. β-lactam and β-lactamase inhibitor combinations in the treatment of extended-spectrum β-lactamase producing Enterobacteriaceae: time for a reappraisal in the era of few antibiotic options? Lancet Infect Dis 2015; 15:475–85. [DOI] [PubMed] [Google Scholar]
- 6. Schwaber MJ, Navon-Venezia S, Kaye KS et al. Clinical and economic impact of bacteremia with extended-spectrum-beta-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother 2006; 50:1257–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pitout JD, Laupland KB. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis 2008; 8:159–66. [DOI] [PubMed] [Google Scholar]
- 8. Schwaber MJ, Carmeli Y. Carbapenem-resistant Enterobacteriaceae: a potential threat. JAMA 2008; 300:2911–3. [DOI] [PubMed] [Google Scholar]
- 9. Perez F, Bonomo RA. Editorial commentary: Bloodstream infection caused by extended-spectrum β-lactamase-producing Gram-negative bacteria: how to define the best treatment regimen? Clin Infect Dis 2015; 60:1326–9. [DOI] [PubMed] [Google Scholar]
- 10. Tamma PD, Han JH, Rock C et al. Carbapenem therapy is associated with improved survival compared with piperacillin-tazobactam for patients with extended-spectrum β-lactamase bacteremia. Clin Infect Dis 2015; 60:1319–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moher D, Liberati A, Tetzlaff J et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med 2009; 3:e123–30. [PMC free article] [PubMed] [Google Scholar]
- 12. World Health Organization (WHO). WHO regional offices. Available at: http://www.who.int/about/regions/en/. Accessed 31 December 2016. [Google Scholar]
- 13. Wells GA, Shea B, O’Connell D et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 31 December 2016.
- 14. Bonovas S, Fiorino G, Allocca M et al. Biologic therapies and risk of infection and malignancy in patients with inflammatory bowel disease: a systematic review and network meta-analysis. Clin Gastroenterol Hepatol 2016; 14:1385–97.e10. [DOI] [PubMed] [Google Scholar]
- 15. Gutiérrez-Gutiérrez B, Pérez-Galera S, Salamanca E et al. A multinational, preregistered cohort study of β-lactam/β-lactamase inhibitor combinations for treatment of bloodstream infections due to extended-spectrum-β-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother 2016; 60:4159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bin C, Hui W, Renyuan Z et al. Outcome of cephalosporin treatment of bacteremia due to CTX-M-type extended-spectrum beta-lactamase-producing Escherichia coli. Diagn Microbiol Infect Dis 2006; 56:351–7. [DOI] [PubMed] [Google Scholar]
- 17. Chaubey VP, Pitout JD, Dalton B et al. Clinical outcome of empiric antimicrobial therapy of bacteremia due to extended-spectrum beta-lactamase producing Escherichia coli and Klebsiella pneumoniae. BMC Res Notes 2010; 3:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cheng WL, Hsueh PR, Lee CC et al. Bacteremic pneumonia caused by extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae: appropriateness of empirical treatment matters. J Microbiol Immunol Infect 2016; 49:208–15. [DOI] [PubMed] [Google Scholar]
- 19. Chopra T, Marchaim D, Veltman J et al. Impact of cefepime therapy on mortality among patients with bloodstream infections caused by extended-spectrum-β-lactamase-producing Klebsiella pneumoniae and Escherichia coli. Antimicrob Agents Chemother 2012; 56:3936–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gudiol C, Calatayud L, Garcia-Vidal C et al. Bacteraemia due to extended-spectrum beta-lactamase-producing Escherichia coli (ESBL-EC) in cancer patients: clinical features, risk factors, molecular epidemiology and outcome. J Antimicrob Chemother 2010; 65:333–41. [DOI] [PubMed] [Google Scholar]
- 21. Kang CI, Park SY, Chung DR et al. Piperacillin-tazobactam as an initial empirical therapy of bacteremia caused by extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae. J Infect 2012; 64:533–4. [DOI] [PubMed] [Google Scholar]
- 22. Lee CC, Lee NY, Yan JJ et al. Bacteremia due to extended-spectrum-beta-lactamase-producing Enterobacter cloacae: role of carbapenem therapy. Antimicrob Agents Chemother 2010; 54:3551–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Metan G, Altinbas A, Zarakolu P et al. Predictors of mortality in patients with bacteremia of unknown source due to extended spectrum beta-lactamase producing Escherichia coli. J Chemother 2009; 21:448–51. [DOI] [PubMed] [Google Scholar]
- 24. Ng TM, Khong WX, Harris PN et al. Empiric piperacillin-tazobactam versus carbapenems in the treatment of bacteraemia due to extended-spectrum beta-lactamase-producing Enterobacteriaceae. PLoS One 2016; 11:e0153696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Qureshi ZA, Paterson DL, Pakstis DL et al. Risk factors and outcome of extended-spectrum β-lactamase-producing Enterobacter cloacae bloodstream infections. Int J Antimicrob Agents 2011; 37:26–32. [DOI] [PubMed] [Google Scholar]
- 26. Rodríguez-Baño J, Navarro MD, Retamar P et al. β-lactam/β-lactam inhibitor combinations for the treatment of bacteremia due to extended-spectrum β-lactamase-producing Escherichia coli: a post hoc analysis of prospective cohorts. Clin Infect Dis 2012; 54:167–74. [DOI] [PubMed] [Google Scholar]
- 27. To KK, Lo WU, Chan JF et al. Clinical outcome of extended-spectrum beta-lactamase-producing Escherichia coli bacteremia in an area with high endemicity. Int J Infect Dis 2013; 17:e120–4. [DOI] [PubMed] [Google Scholar]
- 28. Tumbarello M, Sanguinetti M, Montuori E et al. Predictors of mortality in patients with bloodstream infections caused by extended-spectrum-beta-lactamase-producing Enterobacteriaceae: importance of inadequate initial antimicrobial treatment. Antimicrob Agents Chemother 2007; 51:1987–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Peralta G, Lamelo M, Alvarez-García P et al. Impact of empirical treatment in extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella spp. bacteremia. A multicentric cohort study. BMC Infect Dis 2012; 12:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Paterson DL, Ko WC, Von Gottberg A et al. Antibiotic therapy for Klebsiella pneumoniae bacteremia: implications of production of extended-spectrum beta-lactamases. Clin Infect Dis 2004; 39:31–7. [DOI] [PubMed] [Google Scholar]
- 31. López-Cerero L, Picón E, Morillo C et al. Comparative assessment of inoculum effects on the antimicrobial activity of amoxycillin-clavulanate and piperacillin-tazobactam with extended-spectrum beta-lactamase-producing and extended-spectrum beta-lactamase-non-producing Escherichia coli isolates. Clin Microbiol Infect 2010; 16:132–6. [DOI] [PubMed] [Google Scholar]
- 32. Craig WA, Bhavnani SM, Ambrose PG. The inoculum effect: fact or artifact? Diagn Microbiol Infect Dis 2004; 50:229–30. [DOI] [PubMed] [Google Scholar]
- 33. Harada Y, Morinaga Y, Kaku N et al. In vitro and in vivo activities of piperacillin-tazobactam and meropenem at different inoculum sizes of ESBL-producing Klebsiella pneumoniae. Clin Microbiol Infect 2014; 20:O831–9. [DOI] [PubMed] [Google Scholar]
- 34. Koh AY, Köhler JR, Coggshall KT et al. Mucosal damage and neutropenia are required for Candida albicans dissemination. PLoS Pathog 2008; 4:e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nguyen HM, Shier KL, Graber CJ. Determining a clinical framework for use of cefepime and β-lactam/β-lactamase inhibitors in the treatment of infections caused by extended-spectrum-β-lactamase-producing Enterobacteriaceae. J Antimicrob Chemother 2014; 69:871–80. [DOI] [PubMed] [Google Scholar]
- 36. Ambrose PG, Bhavnani SM, Jones RN. Pharmacokinetics-pharmacodynamics of cefepime and piperacillin-tazobactam against Escherichia coli and Klebsiella pneumoniae strains producing extended-spectrum beta-lactamases: report from the ARREST program. Antimicrob Agents Chemother 2003; 47:1643–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zelenitsky SA, Ariano RE, Zhanel GG. Pharmacodynamics of empirical antibiotic monotherapies for an intensive care unit (ICU) population based on Canadian surveillance data. J Antimicrob Chemother 2011; 66:343–9. [DOI] [PubMed] [Google Scholar]
- 38. Harris PN, Peleg AY, Iredell J et al. Meropenem versus piperacillin-tazobactam for definitive treatment of bloodstream infections due to ceftriaxone non-susceptible Escherichia coli and Klebsiella spp (the MERINO trial): study protocol for a randomised controlled trial. Trials 2015; 16:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.