Abstract
Iron overload is a concern for patients who require repeated red-blood-cell transfusions due to conditions such as sickle cell disease, thalassemia, or myelodysplastic syndromes. The recommended treatment for removing excess iron in these patients is iron chelation therapy. Currently available iron chelators include deferoxamine, which is administered by injection, and deferasirox and deferiprone, both of which are administered orally. Adherence to iron chelator therapy is an important consideration and may be affected by side effects. A new formulation of deferasirox, a film-coated tablet (FCT), has the potential to improve adherence by offering greater flexibility in administration compared with the original formulation of deferasirox, a dispersible tablet (DT) for oral suspension. This review provides an overview of the currently available iron chelator formulations, with a focus on a comparison between deferasirox DT for oral suspension and deferasirox FCT. The new formulation may be associated with fewer side effects and has increased bioavailability. In addition, alternative strategies for iron chelation, such as combining two different iron chelators, will be discussed.
Keywords: deferasirox, deferiprone, deferoxamine, iron chelation, myelodysplastic syndromes, sickle cell disease, thalassemia
Introduction
Iron overload is a concern for patients who require repeated red-blood-cell (RBC) transfusions due to inherited or acquired conditions such as sickle cell disease (SCD), thalassemia, or myelodysplastic syndromes (MDS) [1–3]. Iron homeostasis is carefully balanced through the absorption and recirculation of iron [4,5]. Ferroportin is present on the luminal surface of intestinal epithelium and macrophages and allows for the export of iron into the bloodstream [5,6]. This pathway is carefully controlled by hepcidin, and low levels of hepcidin result in an increase in available iron [5,7]. Disorders leading to iron overload occur in two distinct settings. In one form, the disruption of hepcidin regulation of iron absorption from the gastrointestinal (GI) tract leads to an increase in serum iron [7]. This action is often the result of a genetic mutation, such as hereditary hemochromatosis, a condition resulting from mutations in genes that normally induce hepcidin expression, with severity inversely correlated with levels of hepcidin [2,6–8]. Studies in mouse models have shown that an artificial increase in hepcidin expression prevents hemochromatosis in thalassemia and other anemias that are complicated by secondary iron overload while decreased hepcidin production leads to increased iron absorption thereby increasing the iron burden of transfusion therapy [9]. Low hepcidin expression is also seen in non–transfusion-dependent thalassemia (NTDT) due to ineffective erythropoiesis [10]. In the other setting, iron overload occurs with RBC transfusion therapy in which hemoglobin iron from transfused RBCs accumulates predominately in the reticuloendothelial system [4,6].
The human body lacks an efficient mechanism for removing excess iron since iron is regulated at the level of intestinal absorption, and trafficking is through the reticuloendothelial system [1,4]. While the adult body contains approximately 4 g of iron, transfusion of 4–5 units of packed erythrocytes can add up to 1 g of additional iron [1,4]. Transfused iron bypasses the normal regulation of iron absorption via hepcidin in the GI tract [1,6]. In addition, the increased iron load in the blood from chronic RBC transfusions exceeds the binding capacity of transferrin and other binding proteins, leading to non-transferrin-bound iron (NTBI) [2]. The accumulation of iron in the liver, heart, central nervous system, and endocrine organs can lead to organ damage [1–4]. Specifically, NTBI promotes the formation of reactive oxygen species and has been shown to mediate organ damage at the cellular level [11]. Elevated levels of NTBI have been associated with clinical heart disease in patients with thalassemia [12], and elevated levels of NTBI and labile plasma iron (the subset of NTBI involved in redox cycling) have been associated with elevated aspartate aminotransferase (AST) levels in patients with hemochromatosis [13]. Another study showed that iron levels were correlated to AST and alanine aminotransferase (ALT) levels in patients with acquired anemias (mostly MDS) [14].
Iron levels are typically assessed by serum ferritin or by measuring liver iron concentration (LIC) via liver biopsy. Notably, magnetic resonance imaging (MRI) is being performed with increasing frequency to quantitatively measure LIC and provides a noninvasive approach to determining iron levels [1–3]. MRI offers several advantages over traditional approaches to iron measurement [15]. Because it is noninvasive, MRI is preferred by patients for frequent assessments, and it allows for direct evaluation of target organs, with results that are equivalent to or better than those obtained via biopsy [16,17]. Additionally, MRI may provide information on the fibrotic stage of the liver, potentially identifying patients who still require biopsy for histologic analysis [17]. Removal of iron requires either phlebotomy or iron chelation, but phlebotomy is not usually an option for patients with transfusion-dependent anemias or NTDT [2,4]. Therapy with iron chelators such as deferoxamine, deferasirox, or deferiprone (Table 1) is the primary way to remove excess iron in transfusion-dependent anemias and NTDT [1–3,18–22]. While SCD and thalassemia are inherited conditions and, as such, affect both pediatric and adult patients, MDS primarily affects older adults [1–3]. Because of these differences in disease demographics, factors related to administration of drugs associated with iron overload, and adherence to those drugs, may vary among these conditions.
Table 1.
| Deferoxamine | Deferasirox | Deferiprone | |
|---|---|---|---|
| Structure |
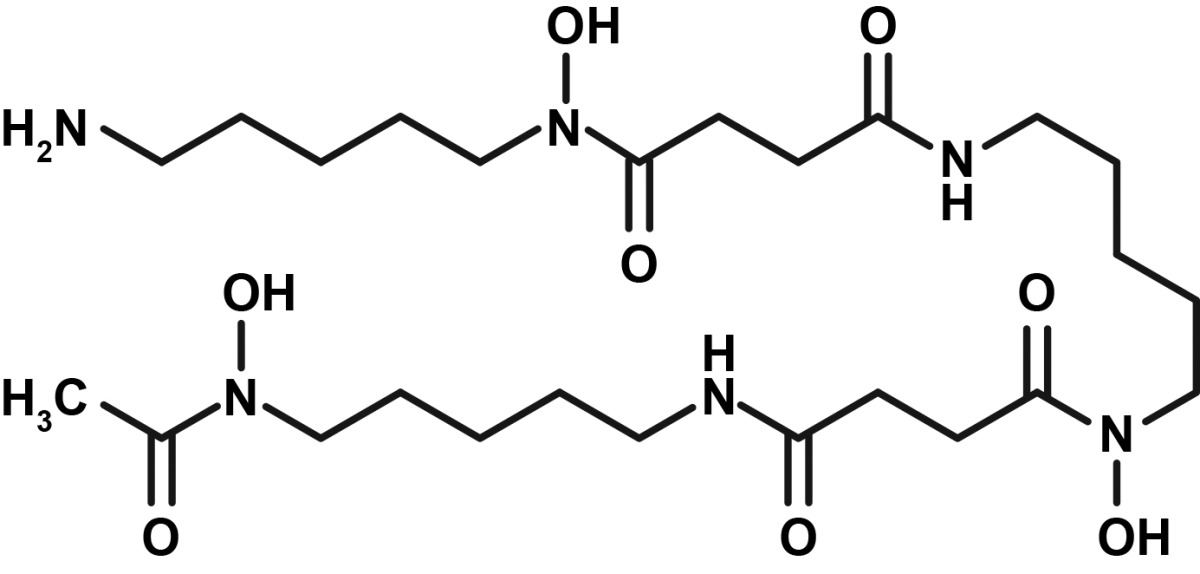
|
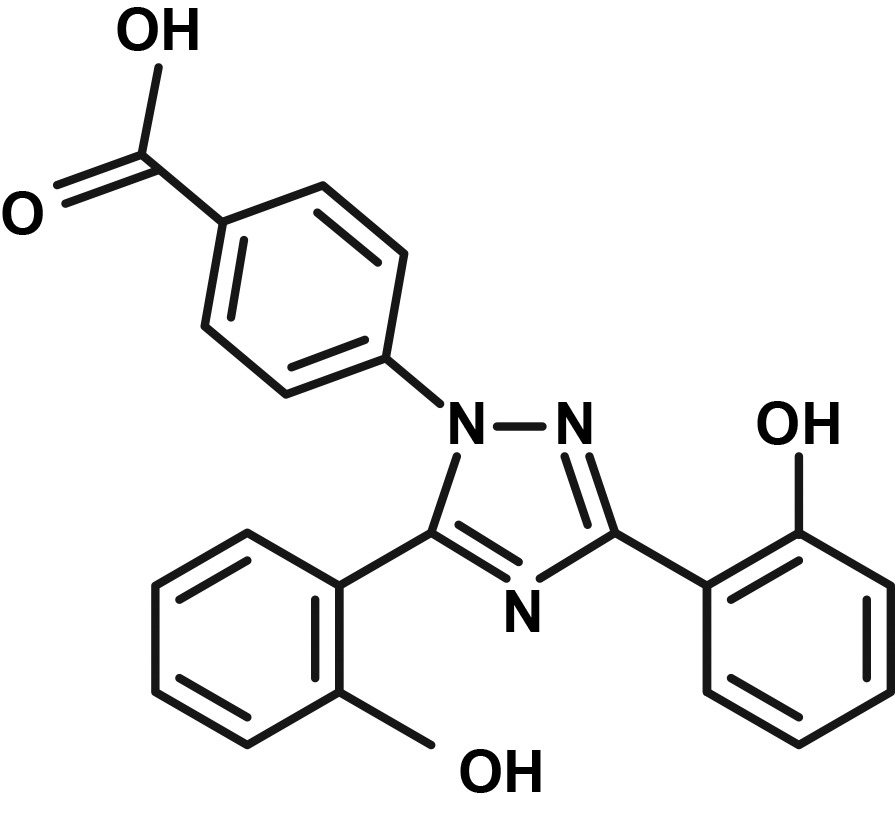
|
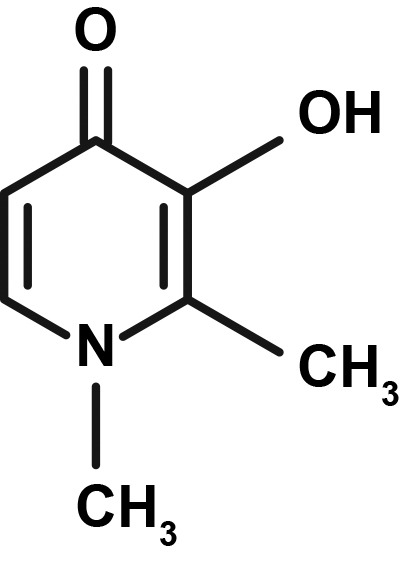
|
| Administration route | Subcutaneousa | Oral | Oral |
| Recommended dose for transfusional iron overload | 1000–2000 mg (20–40 mg/kg/day) | Deferasirox DT Starting dose: 20 mg/kg/day Maximum dose: 40 mg/kg/day Deferasirox FCT Starting dose: 14 mg/kg/day Maximum dose: 28 mg/kg/day |
25–33 mg/kg TID (75–99 mg/kg/day) |
| Half-life | 18–20 minutes | 8–16 hoursb | 1.9 hours |
| Approximate daily iron cleared | 0.05–0.19 mg Fe/kg body weight/day | 0.1–0.5 mg Fe/kg body weight/dayb | 0.03–0.4 mg/kg body weight/day |
| Elimination | Primarily urine | Primarily fecalb | Primarily urine |
DT, dispersible tablet; FCT, film-coated tablet; TID, 3 times a day.
Recommended; may also be administered intravenously and intramuscularly.
Studies were performed using deferasirox DT for oral suspension.
This review will provide an overview of the currently available formulations of iron chelation therapy (ICT) and strategies for their effective use, with a focus on transitioning patients from the deferasirox dispersible tablet (DT) for oral suspension to the new deferasirox film-coated tablet (FCT).
Iron chelation therapy
Deferoxamine
Deferoxamine, an injectable iron chelator, was approved in the late 1960s and is indicated for chronic iron overload in patients aged 3 years and older [18]. Daily subcutaneous (SC) administration over 8–12 hours is recommended for chronic iron overload, but it can also be administered intravenously (IV) or intramuscularly (IM) [18]. The most common adverse events (AEs) associated with deferoxamine are injection-site reactions [18]. Deferoxamine has also been associated with a possible increase in eye and hearing disorders, particularly in older patients, but most cases were reversible [18].
Deferasirox
Deferasirox, initially approved in the United States in 2005, is indicated for the treatment of chronic iron overload due to blood transfusions in patients aged 2 years and older and for the treatment of chronic iron overload due to NTDT in patients aged 10 years and older [19,20]. Deferasirox is available in two different once-daily oral formulations: deferasirox DT for oral suspension (the original formulation) [19] and deferasirox FCT (a newer formulation approved in 2015) [20]. Deferasirox FCT was approved based on efficacy and safety data for deferasirox DT [20]. One year of treatment with deferasirox DT demonstrated dose-dependent reductions in LIC and serum ferritin in pediatric and adult patients with transfusion-dependent β-thalassemia (TDT) and SCD and in adult patients with lower-risk MDS [23–25]. In Phase II and Phase III clinical trials of deferasirox DT, the most common AEs were transient GI effects (15.2%–45.6% of patients), including abdominal pain, nausea, vomiting, diarrhea, and constipation; skin rash (8.7%–13.6% of patients); and mild increases in serum creatinine (36.4%–39.7% of patients) [23–25].
Deferiprone
Deferiprone, a tablet administered orally three times daily, was approved by the US Food and Drug Administration (FDA) in 2011 and is indicated for the treatment of transfusional iron overload due to thalassemia syndromes when current chelation therapy is inadequate [21]. In clinical trials, the most common AEs with deferiprone were GI events, including nausea, vomiting, and abdominal pain; chromaturia; arthralgia; increased ALT; and neutropenia [21]. Patients taking deferiprone must have weekly monitoring of neutrophil counts due to the risk of agranulocytosis, which can be fatal [21].
Adherence to ICT
Adherence to treatment is important to achieve maximum benefit from any medication, and this is no less true for iron overload. Adherence is defined as the extent to which a patient acts in accordance with the prescribed interval and dose of a dosing regimen [26]. Randomized controlled studies have identified a clear dose response for both efficacy and safety parameters for iron chelating agents in patients treated for iron overload [23–25]. As patients allocated to the highest dose of chelating agent had the best outcomes in these trials, it seems reasonable to assume that enhanced compliance with ICT would be of benefit to patients with iron overload. Despite this need, adherence remains suboptimal, and the cause is likely to be multifactorial. Of note, ICT generally does not lead to any perceptible short-term benefit; treatment is often long term, and, until recently, the agents have been difficult to administer.
The importance of adherence is supported by studies showing increased morbidity and mortality in patients with poorer adherence to ICT [27]. Two long-term observational studies following consecutive patients with TDT showed that survival was significantly improved for patients with better compliance to deferoxamine [28,29]. In the first study (N=257; duration 30 years), Gabutti and Piga found that patients with >70% compliance (58% of patients) had a survival rate of 98% compared with a survival rate of 46% for poorly chelated patients (p<0.000001) [28]. In the second study (N=45; duration 15 years), Efthimiadis and colleagues found that patients with excellent compliance (20% of patients; ICT 6–7 days/week) had a 15-year cumulative survival of 100% compared with 52% survival for patients with poor compliance (47% of patients; ICT ≤3 days/week; p=0.018) [29]. In an analysis using a Markov model based on multiple data sources, including the observational study from Gabutti and Piga mentioned above, adequate compliance to deferoxamine was associated with a decreased risk of complications, including cardiac disease (60% vs 96%), diabetes (9% vs 54%), hypogonadism (47% vs 81%), and hypothyroidism (14% vs 28%) compared with typical compliance (i.e., compliance observed in clinical practice) [30]. In addition, estimated life expectancy was longer (49.3 vs 28.0 years), and the lifetime cost for complications, such as cardiac disease and endocrinopathies, was lower ($22,199 vs $55,620) in patients with adequate compliance [30]. It should be noted that in most cases, the deaths and complications in noncompliant patients were not directly attributed to untreated iron overload. Instead, the increased mortality and complications may simply be a surrogate marker for patients who are sicker or noncompliant in general.
Improved compliance to ICT has also been associated with improvement in markers of iron overload, namely, decreased levels of serum ferritin, in a study of patients with thalassemia or SCD taking deferoxamine (N=45), and decreased cardiac iron overload as measured by increases in T2* MRI in β-thalassemia patients taking deferasirox (N=36) or deferoxamine (N=15) [30,31]. In the latter study, although compliance was not directly measured, the authors attributed the improvements to better patient compliance related to having cardiac iron overload measured and/or receiving care at a specialized thalassemia treatment center [32].
Although studies of adherence to ICT outside of randomized clinical trials have shown varying results, adherence to deferasirox is generally higher than adherence to deferoxamine. In a sample of adolescent and adult patients from the Thalassemia Longitudinal Cohort study, adherence was significantly higher for patients receiving deferasirox (N=494) than for patients receiving deferoxamine (N=171) (96% vs 92%; p<0.001) [33]. In a cohort of Medicaid patients with SCD taking ICT (N=404), the rate of treatment discontinuation was higher for deferoxamine than for deferasirox (84% vs 63% at 12 months; 95% vs 80% at 18 months) [34]. In a cohort of Medicaid patients with SCD (N=728) or thalassemia (N=218), more patients with SCD were adherent to deferasirox than to deferoxamine (66% vs 43%; p<0.001), but patients with thalassemia had similar rates of adherence to both therapies [35]. Although the percentage of patients considered adherent in this study was the same for both SCD and thalassemia patients (63% for each) [35], this result may have been due in part to the comparable socioeconomic profile for the patients (all were receiving Medicaid). In the author’s clinical experience, adherence appears to be notably worse for SCD, which primarily affects individuals of African descent. In addition to socioeconomic factors, another potential reason for poorer adherence in this population was increased GI toxicity due to lactose being a component of the original deferasirox formulation [19].
While the reasons for poor adherence may not be fully defined, AEs may be a significant component [36,37]. In two studies of deferasirox that evaluated patient-reported outcomes, injection-site reactions were cited as being one of the reasons for preferring deferasirox to deferoxamine [36,37]. Tolerability issues, such as the injection-site reactions associated with deferoxamine and the GI side effects associated with deferasirox, are a concern for older patients with MDS that impact adherence [38].
Rationale for an alternative deferasirox formulation
Due to concerns about the palatability, tolerability, and convenience of the original deferasirox DT for oral suspension formulation and the potential implications for patient adherence, a short-term study was conducted to evaluate alternative modes of oral administration [39]. The study included 65 adult and pediatric patients, the majority of whom had been receiving prior treatment with deferasirox DT [39]. Following a 4-week run-in phase with deferasirox DT administered per label (30 minutes before a meal; dispersed in water, orange juice, or apple juice), patients were able to choose alternative oral administration options for a 12-week assessment phase [39]. Patient palatability ratings were generally improved with alternative modes of administration compared with the 4-week run-in phase, and fewer patients experienced GI side effects during the first 4 weeks of the assessment phase than during the 4-week run-in phase (19% vs 37%, p=0.05) [39].
Despite the potential benefits associated with tablet formulations, it should be noted that children younger than age 6 years may have difficulty swallowing tablets due in part to their developmental stage [40]. Moreover, some older adults have mechanical issues that prevent them from being able to swallow tablets [40]. Crushing a tablet and mixing it with soft food may be a useful alternative for patients with swallowing difficulties [40]. For older patients with dysphagia, soft foods are preferable to liquids because there is a lower risk of aspiration [40].
Deferasirox DT vs deferasirox FCT
The newer deferasirox formulation, deferasirox FCT, offers greater convenience and flexibility in administration compared with deferasirox DT (Table 2). Deferasirox FCT can be taken in a single step: swallowed whole with water or other beverages, either on an empty stomach or with a light meal (<7% fat and ~250 calories) [20]. Deferasirox DT, in contrast, requires administration on an empty stomach at least 30 minutes prior to a meal [19]. When taken with a low-fat meal, there is a modest effect on the bioavailability and maximum plasma concentration of deferasirox FCT (reductions of 11% and 16%, respectively) [20]. However, taking deferasirox DT with food or deferasirox FCT with a high-fat meal results in increased bioavailability of the drug and is not recommended [19,20]. Compared with deferasirox FCT, deferasirox DT requires more preparation: measuring a specified amount of water, apple juice, or orange juice (3.5 oz for <1 g deferasirox; 7 oz for ≥1 g deferasirox); stirring the tablet(s) in suspension until completely dispersed; consuming the suspension; and resuspending any residue in a small amount of liquid and consuming immediately [19]. Unlike deferasirox DT, deferasirox FCT does not contain lactose or sodium lauryl sulfate [19,20]; this aspect may result in fewer GI side effects with deferasirox FCT compared with deferasirox DT [41]. Like deferasirox DT, it is preferable to administer deferasirox FCT at the same time each day [19,20]. When transitioning patients from deferasirox DT to deferasirox FCT, it is important to note that the dose for deferasirox FCT is approximately 30% lower, rounded to the nearest whole tablet, because the bioavailability of deferasirox FCT is higher than that of deferasirox DT [20]. Deferasirox FCT is available in 90, 180, and 360 mg tablets [20]. Table 1 shows dosing conversions for the two different deferasirox formulations. For patients who have difficulty swallowing tablets, deferasirox FCT may be crushed and mixed with soft foods, such as yogurt or apple sauce [20].
Table 2.
Deferasirox formulations: Key differences.
| Dispersible tablet for oral suspension [19] (deferasirox [Exjadea]) | Film-coated tablet [20] (deferasirox [Jadenua]) | |
|---|---|---|
| Dosage forms and strengths |
|
|
| Administration |
|
|
| Starting doseb |
|
|
| Titration incrementsb |
|
|
| Maximum doseb |
|
|
| Inactive ingredients |
|
|
NF, National Formulary grade; NTDT, non–transfusion-dependent thalassemia.
Exjade and Jadenu are registered trademarks of Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
The dose of deferasirox film-coated tablet should be ~30% lower than the dose of deferasirox dispersible tablet, rounded to the nearest whole tablet.
Preferably at the same time each day.
Do not use commercial serrated crushers for a single 90-mg tablet; the complete dose should be consumed immediately.
A recently completed Phase II study (clinicaltrials.gov registry number NCT02125877) compared deferasirox DT with deferasirox FCT in patients with TDT or MDS (very low, low, or intermediate risk) who had iron overload and required deferasirox DT (≥30 mg/kg/day for those with TDT or ≥20 mg/kg/day for those with MDS). Safety, GI side effects, palatability, satisfaction, and compliance were also assessed. The results of this study showed that the FCT formulation of deferasirox was well tolerated. In patients with prior deferasirox DT exposure, fewer GI AEs were seen with deferasirox FCT than with deferasirox DT. Compliance of patients receiving deferasirox FCT was enhanced, and these patients continued on treatment for longer times. Additionally, patients receiving deferasirox FCT had greater reductions in serum ferritin levels than patients receiving deferasirox DT. More patients were satisfied with the new deferasirox FCT formulation than with the deferasirox DT formulation at all visits. Patient-reported outcomes were also improved with deferasirox FCT compared with deferasirox DT [42].
Monitoring recommendations for patients taking deferasirox FCT
Monitoring to determine the titration of doses for deferasirox FCT should follow the same schedule as for deferasirox DT, but the dose adjustment increments are lower for deferasirox FCT (5–10 mg/kg for deferasirox DT; 3.5–7 mg/kg for deferasirox FCT) [19,20]. Patients with transfusional iron overload, including those with TDT, should have serum ferritin monitored monthly, and dose adjustments should be made every 3–6 months if needed based on serum ferritin trends [19,20]. Although serum ferritin measurements are widely used due to their ease of implementation and low cost, they may not provide an accurate representation of a patient’s iron load in susceptible organs, as serum ferritin is an indirect measure of iron load that can be influenced by factors such as inflammation, oxidative stress, and hepatic dysfunction [15]. The LIC more accurately predicts total body iron stores [43]. If at all feasible, LIC monitoring should be performed on a yearly basis in patients who undergo regular transfusion therapy [44]. Additionally, patients with NTDT should have serum ferritin monitored monthly and LIC monitored every 6 months (or if serum ferritin <300 μg/L), and dose adjustments should be made every 6 months if needed based on LIC trends [19,20].
As with deferasirox DT, serum creatinine should be measured and creatinine clearance determined in duplicate prior to initiating treatment with deferasirox FCT in new patients [19,20]. Serum creatinine should also be monitored weekly during the first month of treatment and then monthly [19,20]. AST, ALT, and bilirubin should be monitored prior to treatment initiation, every 2 weeks during the first month of treatment and then monthly [19,20].
Patients taking either deferasirox formulation may require dose adjustments in response to renal or hepatic toxicity during treatment. In patients with transfusional iron overload, for increases in serum creatinine (≥33% elevation after repeat testing within 1 week for adults and adolescents), dose reductions are recommended (10 mg/kg for deferasirox DT and 7 mg/kg for deferasirox FCT) [19,20]. Treatment should be discontinued if serum creatinine is greater than two times the age-appropriate upper limit of normal or creatinine clearance is less than 40 mL/min [19,20]. For patients with NTDT, treatment with deferasirox should be interrupted when LIC is less than 3 mg Fe/g dw, and LIC should be monitored. Treatment should be restarted when LIC rises again to more than 5 mg Fe/g dw [19,20]. For both categories, dose reductions or interruptions should also be considered for severe or persistent elevations in AST, ALT, or bilirubin [19,20].
The decision to initiate ICT is based on the number of transfusions a patient has been given as well as serum ferritin levels and/or LIC [1–3,45]. Iron overload may occur after as few as 10 transfusions. Treatment that offers greater flexibility and the potential for greater adherence are the orally administered formulations of ICT: deferasirox DT, deferasirox FCT, and deferiprone FCT. All have shown proven efficacy [19–21].
The decision to have a patient transition from deferasirox DT to the new formulation, deferasirox FCT, should be based on a number of factors, including the patient’s response to deferasirox DT and serum ferritin levels. In the ECLIPSE trial, validated methods showed that patient compliance was higher with deferasirox FCT than with DT. Patients reported better palatability, enhanced compliance, and improved satisfaction with deferasirox FCT than with DT. Long-term studies will be important to confirm that these parameters translate into improved clinical outcomes, including improved survival [42].
In addition to the on-label use of iron chelators, several alternative strategies have been employed to optimize ICT. Although deferasirox DT is indicated for once-daily dosing, using divided daily doses may help to decrease GI toxicity [2]. For patients who require more aggressive treatment, combination regimens have been assessed in multiple studies and may be an option. In the prospective Phase II HYPERION trial, the combination of deferasirox and deferoxamine was investigated in 60 patients (59 β-thalassemia major; 1 Diamond-Blackfan anemia) with severe transfusional myocardial siderosis and was found to be safe and effective in removing cardiac and liver iron [46]. Per the study design, which assumed better long-term adherence with monotherapy than with combination therapy, 15 patients switched to deferasirox monotherapy after achieving a reduction in cardiac iron as measured by T2* MRI [46]. A pilot Phase II study performed in 22 patients with thalassemia also showed reductions in cardiac and liver iron as well as NTBI with deferasirox and deferoxamine [47]. Deferiprone has also been used both sequentially and concomitantly with deferoxamine and concomitantly with deferasirox in randomized, published clinical trials [48–50]. In a sequential study, deferiprone plus deferoxamine treatment significantly decreased serum ferritin compared with deferiprone alone during 5 years of treatment; however, there were no significant differences in survival, AEs, or costs [48]. In comparison to the standard monotherapy of deferoxamine, combination treatment with deferoxamine plus deferiprone reduced myocardial iron and improved the ejection fraction and endothelial function of patients with thalassemia major with mild to moderate cardiac iron loading [49]. Deferiprone in combination with either deferoxamine or deferasirox was also compared with the standard chelation monotherapy of deferoxamine. Combination treatment with deferoxamine plus deferiprone reduced myocardial iron and improved the ejection fraction and endothelial function in thalassemia major patients with mild to moderate cardiac iron loading. The two combination regimens were equally effective in reducing iron overload and improving quality of life. Deferiprone in combination with deferasirox was superior in improving cardiac T2*, treatment compliance, and patient satisfaction with no greater incidence of AEs [50].
Conclusions
Multiple studies have demonstrated that patients with higher adherence to ICT have improved morbidity and mortality. Among the iron chelators available for use, the oral options are associated with improved adherence. The new FCT formulation of deferasirox has the potential to increase adherence to a greater degree than the original oral suspension formulation since it can be administered in a single step without preparation and with less stringent food restrictions. Moreover, deferasirox FCT is more palatable, with fewer of the GI side effects that may be experienced with the original oral suspension formulation; these therapeutic attributes can lead to greater compliance and thus decrease the complications associated with iron overload.
Other means of promoting adherence include the use of combination and sequential chelating agents that may also improve efficacy and decrease the incidence of AEs. In the future, these combinations may be refined allowing for intensification of therapy in individuals with a high iron burden or a dose-limiting toxicity of a single agent and possibly providing them with a degree of chelation every day [51].
Additional future possibilities include decreasing transfusional iron burden by decreasing the need or frequency of blood transfusions. Agents that may decrease transfusional requirements include those that target ineffective hematopoiesis, such as JAK2 inhibitors, and those that decrease the overproduction of immature erythroid cells in patients with thalassemia [52], eventually leading to an increase in hemoglobin production in this class of patients. Hopefully, the next few years will bring us the knowledge and means to enhance the lives of patients with iron overload, either through improved chelation therapy or alternative means of decreasing transfusional requirements, in at least some portion of these populations.
Acknowledgments
The author was responsible for all content and editorial decisions and received no honoraria related to the development of this publication. The author performed the research, writing, and review of all drafts of this manuscript and approved the final draft.
Abbreviations
- AE
adverse event
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- DT
dispersible tablet
- FCT
film-coated tablet
- FDA
US Food and Drug Administration
- GI
gastrointestinal
- ICT
iron chelation therapy
- IM
intramuscular
- IV
intravenous
- LIC
liver iron concentration
- MDS
myelodysplastic syndromes
- MRI
magnetic resonance imaging
- NF
National Formulary
- NTBI
non-transferrin-bound iron
- NTDT
non–transfusion-dependent thalassemia
- RBC
red blood cell
- SC
subcutaneous
- SCD
sickle cell disease
- TDT
transfusion-dependent thalassemia
Footnotes
Disclosure and potential conflicts of interest: Nirmish R. Shah, MD, is a member of a Novartis Pharmaceuticals Corporation speakers’ bureau. The international Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interest form for the author is available for download at: http://www.drugsincontext.com/wp-content/uploads/2017/06/dic.212502-COI.pdf.
Funding Declaration: Editorial assistance was provided by Jennifer Lee, PhD, of Phase Five Communications, Inc., supported by Novartis Pharmaceuticals Corporation, which had no other involvement in the development of this publication.
Correct attribution: Copyright © 2017 Shah NR. https://doi.org/10.7573/dic.212502. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 3.0.
Article URL: http://www.drugsincontext.com/advances-iron-chelation-therapy-transitioning-new-oral-formulation
Provenance: submitted; externally peer reviewed
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: 14 Weller Street, London, SE1 1QU, UK.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252772009.
For all manuscript and submissions enquiries, contact the Editorial office: dic.editorial@bioexcelpublishing.com
For all permissions, rights and reprints, contact David Hughes: david.hughes@bioexcelpublishing.com
Peer review comments to author: 27 February 2017
References
- 1.National Heart, Lung, and Blood Institute. Evidence-based management of sickle cell disease: expert panel report. 2014. [Last accessed: 20 December 2016]. Available at: https://www.nhlbi.nih.gov/sites/www.nhlbi.nih.gov/files/sickle-cell-disease-report.pdf.
- 2.Children’s Hospital and Research Center Oakland. Standards of care guidelines for thalassemia. 2012. [Last accessed: 20 December 2016]. Available at http://thalassemia.com/documents/SOCGuidelines2012.pdf.
- 3.National Comprehensive Cancer Network (NCCN) NCCN clinical practice guidelines in oncology (NCCN Guidelines®) Myelodysplastic Syndromes. Version 2. 2017. [Last accessed: 3 January 2017]. Available at: http://www.nccn.org/professionals/physician_gls/pdf/mds.pdf. [DOI] [PubMed]
- 4.Andrews NC. Disorders of iron metabolism. N Engl J Med. 1999;341(26):1986–95. doi: 10.1056/NEJM199912233412607. Erratum in: N Engl J Med. 2000;342(5):364. http://dx.doi.org/10.1056/NEJM199912233412607. [DOI] [PubMed] [Google Scholar]
- 5.Kohgo Y, Ikuta K, Ohtake T, Torimoto Y, Kato J. Body iron metabolism and pathophysiology of iron overload. Int J Hematol. 2008;88(1):7–15. doi: 10.1007/s12185-008-0120-5. http://dx.doi.org/10.1007/s12185-008-0120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganz T, Nemeth E. Iron metabolism: interactions with normal and disordered erythropoiesis. Cold Spring Harb Perspect Med. 2012;2(5):a011668. doi: 10.1101/cshperspect.a011668. http://dx.doi.org/10.1101/cshperspect.a011668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrews NC. Closing the iron gate. N Engl J Med. 2012;366(4):376–7. doi: 10.1056/NEJMcibr1112780. http://dx.doi.org/10.1056/NEJMcibr1112780. [DOI] [PubMed] [Google Scholar]
- 8.Roy CN, Andrews NC. Anemia of inflammation: the hepcidin link. Curr Opin Hematol. 2005;12(2):107–11. doi: 10.1097/00062752-200503000-00001. http://dx.doi.org/10.1097/00062752-200503000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Nicolas G, Viatte L, Lou DQ, Lou DQ, Bennoun M, Beaumont C, Kahn A, Andrews NC, Vaulont S. Constitutive hepcidin expression prevents iron overload in a mouse model of hemochromatosis. Nat Genet. 2003;34(1):97–101. doi: 10.1038/ng1150. http://dx.doi.org/10.1038/ng1150. [DOI] [PubMed] [Google Scholar]
- 10.Gardenghi S, Marongiu MF, Ramos P, Guy E, Breda L, Chadburn A, Liu Y, Amariglio N, Rechavi G, Rachmilewitz EA, Breuer W, Cabantchik ZI, Wrighting DM, Andrews NC, de Sousa M, Giardina PJ, Grady RW, Rivella S. Ineffective erythropoiesis in beta-thalassemia is characterized by increased iron absorption mediated by down-regulation of hepcidin and up-regulation of ferroportin. Blood. 2007;109(11):5027–35. doi: 10.1182/blood-2006-09-048868. http://dx.doi.org/10.1182/blood-2006-09-048868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brissot P, Ropert M, Le Lan C, Loréal O. Non-transferrin bound iron: a key role in iron overload and iron toxicity. Biochim Biophys Acta. 2012;1820(3):403–10. doi: 10.1016/j.bbagen.2011.07.014. http://dx.doi.org/10.1016/j.bbagen.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Piga A, Longo F, Duca L, Roggero S, Vinciguerra T, Calabrese R, Hershko C, Cappellini MD. High nontransferrin bound iron levels and heart disease in thalassemia major. Am J Hematol. 2009;84(1):29–33. doi: 10.1002/ajh.21317. http://dx.doi.org/10.1002/ajh.21317. [DOI] [PubMed] [Google Scholar]
- 13.Le Lan C, Loréal O, Cohen T, Ropert M, Glickstein H, Lainé F, Pouchard M, Deugnier Y, Le Treut A, Breuer W, Cabantchik ZI, Brissot P. Redox active plasma iron in C282Y/C282Y hemochromatosis. Blood. 2005;105(11):4527–31. doi: 10.1182/blood-2004-09-3468. http://dx.doi.org/10.1182/blood-2004-09-3468. [DOI] [PubMed] [Google Scholar]
- 14.Jensen PD, Jensen FT, Christensen T, Nielsen JL, Ellegaard J. Relationship between hepatocellular injury and transfusional iron overload prior to and during iron chelation with desferrioxamine: a study in adult patients with acquired anemias. Blood. 2003;101(1):91–6. doi: 10.1182/blood-2002-06-1704. http://dx.doi.org/10.1182/blood-2002-06-1704. [DOI] [PubMed] [Google Scholar]
- 15.Quinn CT, St Pierre TG. MRI measurements of iron load in transfusion-dependent patients: implementation, challenges, and pitfalls. Pediatr Blood Cancer. 2016;63(5):773–80. doi: 10.1002/pbc.25882. http://dx.doi.org/10.1002/pbc.25882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gandon Y, Olivié D, Guyader D, Aubé C, Oberti F, Sebille V, Deugnier Y. Non-invasive assessment of hepatic iron stores by MRI. Lancet. 2004;363(9406):357–62. doi: 10.1016/S0140-6736(04)15436-6. http://dx.doi.org/10.1016/S0140-6736(04)15436-6. [DOI] [PubMed] [Google Scholar]
- 17.Clark PR, Chua-Anusorn W, St Pierre TG. Proton transverse relaxation rate (R2) images of liver tissue; mapping local tissue iron concentrations with MRI [corrected] Magn Reson Med. 2003;49(3):572–5. doi: 10.1002/mrm.10378. http://dx.doi.org/10.1002/mrm.10378. [DOI] [PubMed] [Google Scholar]
- 18.Desferal (deferoxamine mesylate) prescribing information. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2011. [Google Scholar]
- 19.Exjade (deferasirox) prescribing information. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2016. [Google Scholar]
- 20.Jadenu (deferasirox) prescribing information. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2016. [Google Scholar]
- 21.Ferriprox (deferiprone) prescribing information. Rockville, MD: ApoPharma USA, Inc; 2015. [Google Scholar]
- 22.Cappellini MD, Cohen A, Eleftheriou A, Piga A, Porter J, Taher A. Guidelines for the clinical management of thalassaemia. 2nd revised edition [Internet] Nicosia (CY): Thalassaemia International Federation; 2008. [Last accessed: 20 December 2016]. Iron overload. Available at: http://www.ncbi.nlm.nih.gov/books/NBK173958/ [PubMed] [Google Scholar]
- 23.Cappellini MD, Cohen A, Piga A, Bejaoui M, Perrotta S, Agaoglu L, Aydinok Y, Kattamis A, Kilinc Y, Porter J, Capra M, Galanello R, Fattoum S, Drelichman G, Magnano C, Verissimo M, Athanassiou-Metaxa M, Giardina P, Kourakli-Symeonidis A, Janka-Schaub G, Coates T, Vermylen C, Olivieri N, Thuret I, Opitz H, Ressayre-Djaffer C, Marks P, Alberti D. A phase 3 study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with beta-thalassemia. Blood. 2006;107(9):3455–62. doi: 10.1182/blood-2005-08-3430. http://dx.doi.org/10.1182/blood-2005-08-3430. [DOI] [PubMed] [Google Scholar]
- 24.Vichinsky E, Onyekwere O, Porter J, Swerdlow P, Eckman J, Lane P, Files B, Hassell K, Kelly P, Wilson F, Bernaudin F, Forni GL, Okpala I, Ressayre-Djaffer C, Alberti D, Holland J, Marks P, Fung E, Fischer R, Mueller BU, Coates T Deferasirox in Sickle Cell Investigators. A randomised comparison of deferasirox versus deferoxamine for the treatment of transfusional iron overload in sickle cell disease. Br J Haematol. 2007;136(3):501–8. doi: 10.1111/j.1365-2141.2006.06455.x. http://dx.doi.org/10.1111/j.1365-2141.2006.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porter J, Galanello R, Saglio G, Neufeld EJ, Vichinsky E, Cappellini MD, Olivieri N, Piga A, Cunningham MJ, Soulières D, Gattermann N, Tchernia G, Maertens J, Giardina P, Kwiatkowski J, Quarta G, Jeng M, Forni GL, Stadler M, Cario H, Debusscher L, Della Porta M, Cazzola M, Greenberg P, Alimena G, Rabault B, Gathmann I, Ford JM, Alberti D, Rose C. Relative response of patients with myelodysplastic syndromes and other transfusion-dependent anaemias to deferasirox (ICL670): a 1-yr prospective study. Eur J Haematol. 2008;80(2):168–76. doi: 10.1111/j.1600-0609.2007.00985.x. http://dx.doi.org/10.1111/j.1600-0609.2007.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, Wong PK. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44–7. doi: 10.1111/j.1524-4733.2007.00213.x. http://dx.doi.org/10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 27.Huang V, Luini C, El-Ali A, Kessabi S. Iron chelation therapy: a review of the literature on the issues and importance of adherence to treatment in iron overload. Blood. 2015;126(23):4748. [Google Scholar]
- 28.Gabutti V, Piga A. Results of long-term iron-chelating therapy. Acta Haematol. 1996;95(1):26–36. doi: 10.1159/000203853. http://dx.doi.org/10.1159/000203853. [DOI] [PubMed] [Google Scholar]
- 29.Efthimiadis GK, Hassapopoulou HP, Tsikaderis DD, Karvounis HI, Giannakoulas GA, Parharidis GE, Louridas GE. Survival in thalassaemia major patients. Circ J. 2006;70(8):1037–42. doi: 10.1253/circj.70.1037. http://dx.doi.org/10.1253/circj.70.1037. [DOI] [PubMed] [Google Scholar]
- 30.Delea TE, Edelsberg J, Sofrygin O, Thomas SK, Baladi JF, Phatak PD, Coates TD. Consequences and costs of noncompliance with iron chelation therapy in patients with transfusion-dependent thalassemia: a literature review. Transfusion. 2007;47(10):1919–29. doi: 10.1111/j.1537-2995.2007.01416.x. http://dx.doi.org/10.1111/j.1537-2995.2007.01416.x. [DOI] [PubMed] [Google Scholar]
- 31.Payne KA, Desrosiers MP, Caro JJ, Baladi JF, Lordan N, Proskorovsky I, Ishak K, Rofail D. Clinical and economic burden of infused iron chelation therapy in the United States. Transfusion. 2007;47(10):1820–9. doi: 10.1111/j.1537-2995.2007.01398.x. http://dx.doi.org/10.1111/j.1537-2995.2007.01398.x. [DOI] [PubMed] [Google Scholar]
- 32.Cassinerio E, Roghi A, Pedrotti P, Brevi F, Zanaboni L, Graziadei G, Pattoneri P, Milazzo A, Cappellini MD. Cardiac iron removal and functional cardiac improvement by different iron chelation regimens in thalassemia major patients. Ann Hematol. 2012;91(9):1443–9. doi: 10.1007/s00277-012-1480-8. http:/dx.doi.org/10.1007/s00277-012-1480-8. [DOI] [PubMed] [Google Scholar]
- 33.Trachtenberg FL, Gerstenberger E, Xu Y, Mednick L, Sobota A, Ware H, Thompson AA, Neufeld EJ, Yamashita R Thalassemia Clinical Research Network. Relationship among chelator adherence, change in chelators, and quality of life in thalassemia. Qual Life Res. 2014;23(8):2277–88. doi: 10.1007/s11136-014-0671-2. http://dx.doi.org/10.1007/s11136-014-0671-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Armstrong EP, Skrepnek GH, Sasane M, Snodgrass SM, Ballas SK. Long-term persistency and costs associated with the use of iron chelation therapies in the treatment of sickle cell disease within Medicaid programs. J Med Econ. 2013;16(1):10–18. doi: 10.3111/13696998.2012.723081. http://dx.doi.org/10.3111/13696998.2012.723081. [DOI] [PubMed] [Google Scholar]
- 35.Vekeman F, Sasane M, Cheng WY, Ramanakumar AV, Fortier J, Qiu Y, Duh MS, Paley C, Adams-Graves P. Adherence to iron chelation therapy and associated healthcare resource utilization and costs in Medicaid patients with sickle cell disease and thalassemia. J Med Econ. 2016;19(3):292–303. doi: 10.3111/13696998.2015.1117979. http://dx.doi.org/10.3111/13696998.2015.1117979. [DOI] [PubMed] [Google Scholar]
- 36.Cappellini MD, Bejaoui M, Agaoglu L, Porter J, Coates T, Jeng M, Lai ME, Mangiagli A, Strauss G, Girot R, Watman N, Ferster A, Loggetto S, Abish S, Cario H, Zoumbos N, Vichinsky E, Opitz H, Ressayre-Djaffer C, Abetz L, Rofail D, Baladi JF. Prospective evaluation of patient-reported outcomes during treatment with deferasirox or deferoxamine for iron overload in patients with beta-thalassemia. Clin Ther. 2007;29(5):909–17. doi: 10.1016/j.clinthera.2007.05.007. http://dx.doi.org/10.1016/j.clinthera.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 37.Vichinsky E, Pakbaz Z, Onyekwere O, Porter J, Swerdlow P, Coates T, Lane P, Files B, Mueller BU, Coïc L, Forni GL, Fischer R, Marks P, Rofail D, Abetz L, Baladi JF. Patient-reported outcomes of deferasirox (Exjade, ICL670) versus deferoxamine in sickle cell disease patients with transfusional hemosiderosis. Substudy of a randomized open-label phase II trial. Acta Haematol. 2008;119(3):133–41. doi: 10.1159/000125550. http://dx.doi.org/10.1159/000125550. [DOI] [PubMed] [Google Scholar]
- 38.Porter JB, Evangeli M, El-Beshlawy A. Challenges of adherence and persistence with iron chelation therapy. Int J Hematol. 2011;94(5):453–60. doi: 10.1007/s12185-011-0927-3. http://dx.doi.org/10.1007/s12185-011-0927-3. [DOI] [PubMed] [Google Scholar]
- 39.Goldberg SL, Giardina PJ, Chirnomas D, Esposito J, Paley C, Vichinsky E. The palatability and tolerability of deferasirox taken with different beverages or foods. Pediatr Blood Cancer. 2013;60(9):1507–12. doi: 10.1002/pbc.24561. http://dx.doi.org/10.1002/pbc.24561. [DOI] [PubMed] [Google Scholar]
- 40.Liu F, Ranmal S, Batchelor HK, Orlu-Gul M, Ernest TB, Thomas IW, Flanagan T, Tuleu C. Patient-centred pharmaceutical design to improve acceptability of medicines: similarities and differences in paediatric and geriatric populations. Drugs. 2014;74(16):1871–89. doi: 10.1007/s40265-014-0297-2. http://dx.doi.org/10.1007/s40265-014-0297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nolte F, Angelucci E, Breccia M, Gattermann N, Santini V, Vey N, Hofmann WK. Updated recommendations on the management of gastrointestinal disturbances during iron chelation therapy with Deferasirox in transfusion dependent patients with myelodysplastic syndrome – emphasis on optimized dosing schedules and new formulations. Leuk Res. 2015;39(10):1028–33. doi: 10.1016/j.leukres.2015.06.008. http://dx.doi.org/10.1016/j.leukres.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 42.Taher AT, Origa R, Perrotta S, Kourakli A, Ruffo GB, Kattamis A, Goh AS, Cortoos A, Huang V, Weill M, Merino Herranz R, Porter JB. New film-coated tablet formulation of deferasirox is well tolerated in patients with thalassemia or lower risk MDS: results of the randomized, phase II ECLIPSE study. Am J Hematol. 2017 Jan 31; doi: 10.1002/ajh.24668. http://dx.doi.org/10.1002/ajh.24668. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Angelucci E, Brittenham GM, McLaren CE, Ripalti M, Baronciani D, Giardini C, Galimberti M, Polchi P, Lucarelli G. Hepatic iron concentration and total body iron stores in thalassemia major. N Engl J Med. 2000;343(5):327–31. doi: 10.1056/NEJM200008033430503. http://dx.doi.org/10/1056/NEJM200008033430503. [DOI] [PubMed] [Google Scholar]
- 44.Hoffbrand AV, Taher A, Cappellini MD. How I treat transfusional iron overload. Blood. 2012;120(18):3657–69. doi: 10.1182/blood-2012-05-370098. http://dx.doi.org/10.1182/blood-2012-05-370098. [DOI] [PubMed] [Google Scholar]
- 45.Ault P, Jones K. Understanding iron overload: screening, monitoring, and caring for patients with transfusion-dependent anemias. Clin J Oncol Nurs. 2009;13(5):511–17. doi: 10.1188/09.CJON.511-517. http://dx.doi.org/10.1188/09.CJON.511-517. [DOI] [PubMed] [Google Scholar]
- 46.Aydinok Y, Kattamis A, Cappellini MD, El-Beshlawy A, Origa R, Elalfy M, Kilinç Y, Perrotta S, Karakas Z, Viprakasit V, Habr D, Constantinovici N, Shen J, Porter JB HYPERION Investigators. Effects of deferasirox-deferoxamine on myocardial and liver iron in patients with severe transfusional iron overload. Blood. 2015;125(25):3868–77. doi: 10.1182/blood-2014-07-586677. http://dx.doi.org/10.1182/blood-2014-07-586677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lal A, Porter J, Sweeters N, Ng V, Evans P, Neumayr L, Kurio G, Harmatz P, Vichinsky E. Combined chelation therapy with deferasirox and deferoxamine in thalassemia. Blood Cells Mol Dis. 2013;50(2):99–104. doi: 10.1016/j.bcmd.2012.10.006. http://dx.doi.org/1016/j.bcmd.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maggio A, Vitrano A, Capra M, Cuccia L, Gagliardotto F, Filosa A, Romeo MA, Magnano C, Caruso V, Argento C, Gerardi C, Campisi S, Violi P, Malizia R, Cianciulli P, Rizzo M, D’Ascola DG, Quota A, Prossomariti L, Fidone C, Rigano P, Pepe A, D’Amico G, Morabito A, Gluud C. Long-term sequential deferiprone-deferoxamine versus deferiprone alone for thalassaemia major patients: a randomized clinical trial. Br J Haematol. 2009;145(2):245–54. doi: 10.1111/j.1365-2141.2009.07609.x. http://dx.doi.org/10.1111/j.1365-2141.2009.07609.x. [DOI] [PubMed] [Google Scholar]
- 49.Tanner MA, Galanello R, Dessi C, Smith GC, Westwood MA, Agus A, Roughton M, Assomull R, Nair SV, Walker JM, Pennell DJ. A randomized, placebo-controlled, double-blind trial of the effect of combined therapy with deferoxamine and deferiprone on myocardial iron in thalassemia major using cardiovascular magnetic resonance. Circulation. 2007;115(14):1876–84. doi: 10.1161/CIRCULATIONAHA.106.648790. http://dx.doi.org/10.1161/CIRCULATIONAHA.106.648790. [DOI] [PubMed] [Google Scholar]
- 50.Elalfy MS, Adly AM, Wali Y, Tony S, Samir A, Elhenawy YI. Efficacy and safety of a novel combination of two oral chelators deferasirox/deferiprone over deferoxamine/deferiprone in severely iron overloaded young beta thalassemia major patients. Eur J Haematol. 2015;95(5):411–20. doi: 10.1111/ejh.12507. http://dx.doi.org/10.1111/ejh.12507. [DOI] [PubMed] [Google Scholar]
- 51.Sheth S. Iron chelation: an update. Curr Opin Hematol. 2014;21(3):179–85. doi: 10.1097/MOH.0000000000000031. http://dx.doi.org/10.1097/MOH.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 52.Saliba AN, Harb AR, Taher AT. Iron chelation therapy in transfusion-dependent thalassemia patients: current strategies and future directions. J Blood Med. 2015;6:197–209. doi: 10.2147/JBM.S72463. http://dx.doi.org/10.2147/JBM.S72463. [DOI] [PMC free article] [PubMed] [Google Scholar]

