ABSTRACT
Hypoxia is an inherent impediment to cancer therapy. Palbociclib, a highly selective inhibitor for CDK4/6, has been tested in numerous clinical trials and has been approved by the FDA. We previously reported that CDK inhibitors can destabilize HIF1α regardless of the presence of hypoxia and can sensitize tumor cells to TRAIL through dual blockade of CDK1 and GSK-3β. To translate this knowledge into a cancer therapeutic strategy, we investigated the therapeutic effects and molecular mechanisms of CDK inhibition against colon cancer cells under normoxia and hypoxia. We found that palbociclib sensitizes colon cancer cells to hypoxia-induced apoptotic resistance via deregulation of HIF-1α accumulation. In addition to inhibition of cell proliferation, we observed that palbociclib promotes colon cancer cell death regardless of the presence of hypoxia at a comparatively high concentration via regulating ERK/GSK-3β signaling and GSK-3β expression. Furthermore, palbociclib synergized with irinotecan in a variety of colon cancer cell lines with various molecular subtypes via deregulating irinotecan-induced Rb phosphorylation and reducing HIF-1α accumulation under normoxia or hypoxia. Collectively, our findings provide a novel combination therapy strategy against hypoxic colon cancer cells that may be further translated in the clinic.
KEYWORDS: apoptosis, colon cancer, Hypoxia, Prinotecan, Palbociclib
Introduction
Colorectal cancer (CRC) is one of the most common cancers and one of the leading causes of cancer mortality worldwide. Although the therapeutic regimens of cytotoxic drugs, FOLFOX and FOLFIRI have improved overall and progression-free survival of advanced CRC, the therapeutic responses vary individually. There is a need to develop additional molecularly-targeted therapies for advanced CRC.
Various biologic roles of CDK4/6 have been identified. Besides regulating cell cycle progression through the pRb/E2F pathway and the G1-S checkpoint,1 non-cell-cycle effects of CDK4/6 include direct activation of vascular endothelial growth factor (VEGF) transcription, promotion of angiogenesis and nuclear factor (NF)-κB activation via the p65 transcription factor.2-4 PD0332991 (palbociclib) is a highly selective small molecular inhibitor of CDK4 and 6. Palbociclib was approved by the FDA in 2015 in combination with letrozole as initial hormone- plus CDK-targeted therapy for post-menopausal women with ER(+)/Her2(−) advanced breast cancer.5 The therapeutic effects of palbociclib have been demonstrated in numerous clinical trials for breast cancer, NSCLC, GBM, lymphoma, and leukemia, in combination with 5-FU and oxaliplatin in solid malignancies (NCT01522989) or with cetuximab in head and neck cancer.6,7 A combined pharmacological inhibition of MEK and CDK4/6 led to substantial synergy in KRAS-mutated NSCLC in vivo.8 Synergy of palbociclib with a MEK inhibitor has also been demonstrated in KRAS mutant colon cancer in vivo.9,10 Vemurafenib-resistant tumors remain sensitive to palbociclib suggesting that initial treatment with vemurafenib followed by palbociclib with or without mTOR inhibitors might provide an approach to overcome recurrence of vemurafenib-resistant, metastatic tumors.11 In brief, these studies indicate combinatorial administration of a CDK4/6 inhibitor with other chemo- or targeted-therapies could provide a promising treatment against various malignancies.
Hypoxia is an inherent impediment to tumor treatment in most solid tumors, and contributes to a poor prognosis as well as resistance to chemo/radiotherapy. HIF-1α is a transcription factor that mediates the adaptive response to oxygen deprivation, is involved in crucial aspects of cancer biology, including angiogenesis, cell survival, glucose metabolism and invasion by hypoxic tumor cells.12 Therefore, targeting HIF-1α provides a rational target for anti-cancer therapy and may combat drug resistance. However, the regulatory mechanisms and effects of HIF-1α are complex and involve multiple pathways in hypoxic tumor cells. For example, it was recently reported that CDKs regulate lysosomal degradation of HIF-1α to promote cell cycle progression.13 PI3K/AKT-mTOR and GSK-3β signals regulate HIF-1α in an oxygen-independent manner.14 ERK1/2 proteins are involved in the regulation of HIF-1α synthesis and transcriptional activation via phosphorylation of the co-activator CBP/p300.15
We recently reported that a family of nucleoside analogs (sangivamycin-like molecules, SLMs), act as inhibitors of CDKs, can sensitize tumor cells to TRAIL mediated cell death in vivo through dual blockade of CDK1 and GSK-3β and destabilize HIF-1α regardless of VHL or p53 mutation status or the presence of hypoxia.16,17 To translate this knowledge into a novel cancer therapeutic strategy, we investigated the anti-tumor effects and mechanisms of CDK inhibition in CRC with or without chemotherapy under hypoxia. We investigated the therapeutic effect of the CDK4/6 inhibitor palbociclib in combination with chemotherapies used to treat CRC to explore a potentially novel and effective treatment against advanced disease. Our data suggest that palbociclib, a highly selective inhibitor of CDK4/6, is a potent cytotoxic agent via deregulation of the accumulation of HIF-1α under either normoxia or hypoxia while simultaneously reducing the constitutive expression of GSK-3β under hypoxia. We found that the CDK4/6 inhibitor palbociclib can effectively synergize with CPT11 (irinotecan) against CRC under hypoxia. Furthermore, addition of palbociclib to CPT11 deregulates CPT11-induced CDK6 and Rb phosphorylation that could account for palbociclib synergy with CPT11against CRC under hypoxia. Collectively, these results indicate that palbociclib synergizes with conventional therapy that could be further tested in the clinic against advanced CRC.
Materials and methods
Chemicals and reagents
Palbociclib was purchased from MedKoo Biosciences (Research Triangle Park, NC), and was solubilized in PBS at a storage concentration of 10 mM. 5-fluorouracil, oxaliplatin and irinotecan solutions were obtained from Hospira (Lake Forest, IL). Penicillin/Streptomycin, DMEM, McCoy's 5A, PBS and trypsin were purchased from Cellgro (Manassas, VA). Fetal bovine serum was obtained from Genimi Bio-products (Broderick, CA).
The following antibodies were used: GSK-3β, phospho-GSK-3β (Ser9), caspase-3, cleaved-caspase-3, PARP, cleaved-PARP, AKT, phospho-AKT (Ser473), Rb, phospho-Rb (Ser780), ERK1/2 and phospho-ERK1/2 (Thr202/Tyr204) (Cell Signaling Technology, Beverly, MA); phospho-CDK6 and CDK6 (Santa Cruz Biotechnology, Santa Cruz, CA); HIF-1α and CDK1 (BD PharMingen, San Diego, CA).
Cell lines and culture conditions
The human colorectal cancer cell lines HT-29, RKO, DLD-1 and SW-480 were purchased from the American Type Culture Collection (ATCC, Manassas, VA). HCT-116 was generously provided by Dr. Bert Vogelstein (Johns Hopkins University, Baltimore, MD). All cell lines were cultured in their ATCC-recommended media supplemented with 10% (v/v) fetal bovine serum with or without chemotherapy agents at 37°C within a 95% humidified atmosphere containing 5% carbon dioxide in an incubator. Hypoxia experiments were performed at 0.5–1% O2 using the INVIVO2 hypoxia workstation (TOUCAN Technologies).
Western blotting analysis
Cells were collected and lysed using protein lysis buffer. Total protein was collected and quantified using the Bio-Rad Bradford reagent (Bio-Rad Laboratories, Hercules, CA). Equal amounts of protein were separated in SDS-PAGE gels (NuPage Bis-Tris Gels, Invitrogen, Carlsbad, CA) using the XCell system (Invitrogen). The separated proteins were transferred to PVDF membranes (Millipore) using a transfer apparatus (Bio-Rad). After blocking with 10% (w/v) nonfat-milk in phosphate-buffered saline (PBS), the blots were incubated with primary antibodies at 4°C overnight, and subsequently incubated with the corresponding fluorescent secondary antibodies, and the bands were visualized using Odyssey Infrared Imaging System (LI-COR Bioscience, Lincoln, NE).
Cell viability assay and cell cycle profile
Cells were seeded in 96-well dark plates at a density of 2000–6000 cells per well and incubated overnight to allow proper attachment. Subsequently the media were replaced using media with or without chemotherapeutic agents and treatment was continued for 48 hours. CellTiterGlo bioluminescence agent (Promega Corporation, Madison, WI) was used to quantify cell viability according to the manufacturer's instruction.
Cells were collected at the indicated time points after the corresponding treatment and fixed with ice-cold 70% ethanol at 4°C overnight. After washing twice with PBS, the fixed cells were re-suspended using 1% (w/v) bovine serum albumin in PBS. Then the prepared cells were stained with propidium iodide (Sigma) at a final concentration of 0.1 mg/ml for 15 minutes and DNA content was measured using an Epics Elite flow cytometer (Beckman Coulter, Fullerton, CA).
Dose-response assessment and synergy assessment
Half-maximal inhibitory concentration (IC50) value is a critical index of the dose-response curve. In this study, it was used to assess single drug inhibition in cell viability experiments after treatment of 48 hours in vitro. Prism statistical software (GraphPad Software, La Jolla, CA) was used to calculate the IC50 values and to plot dose-response curves.
The combinatorial inhibitory effects of multiple drugs against CRC in vitro were assessed using a quantitative analysis of dose-response relationships as described previously18: multiples of the IC50 of each drug were used in non-constant ratio combinations and compared against the predicted effects for each drug alone. Combining effects of multiple doses was assessed by combination index (CI)—quantitative measurements based on the mass-action law of the degree of drug interaction that allows determination of synergism and antagonism for a given end point of the effect measurement based on Chou-Talalay analysis.19 If the sum of the 2 fractional terms is equal to 1, an additive effect is indicated. If the CI value is smaller than 1, synergism is indicated, and if the CI value is greater than 1, antagonism is indicated. CI < 0.5 indicates very strong synergy. Compusyn software was used to calculate combination index values.
Statistical analysis
All data are shown as the average ± standard deviation (Mean ± SD) and each experiment was repeated at least 3 times independently. Measurements were analyzed by the Student's t-test (2-tailed, paired or unpaired) using SPSS software. A P-value of less than 0.05 was considered to be statistically significant.
Results
Palbociclib sensitizes colorectal cancer (CRC) cells to hypoxia-induced drug resistance
Five-fluorouracil (5-FU) and irinotecan (CPT11) are conventional chemotherapies used to treat patients with CRC. We assessed the sensitivity of CRC to palbociclib, irinotecan (CPT11) and 5-FU in CRC cell lines with various molecular genetic subtypes under normoxic as well as hypoxic conditions (Fig. 1). The data show that hypoxia induces resistance in 5 different CRC cell lines to 5-FU (Fig 1B, 165.4 ± 161.1 μM for the IC50 value under normoxia for the entire cell line panel versus 428.3 ± 336.3 μM for the IC50 value under hypoxia, again for the entire panel). By contrast to 5-FU, hypoxia has minimal impact on the sensitivity of 5 different CRC cell lines to palbociclib (Fig 1A and B, 7.88 ± 1.96 μM for the IC50 values under normoxia for the entire panel vs. 9.09 ± 2.68 μM for the IC50 values under hypoxia, again for the entire panel) and irinotecan (Fig 1C, 53.5 ± 31.7 μM for the IC50 value under normoxia vs. 55.6 ± 28.9 μM for the IC50 values under hypoxia). We previously reported that hypoxia induces apoptotic resistance to 5-FU in CRC cells lacking p53.16 Irinotecan belongs to the family of camptothecins (CPTs) analogs, which have a topoisomerase I (Top I) inhibitory activity, and it has been reported that hypoxia-induced accumulation of carboxylesterase increases tumor sensitivity to irinotecan (CPT-11).20 Additionally irinotecan reduces tumor angiogenesis and tumor growth via inhibiting HIF-1α protein accumulation or translation in hypoxic glioma cells.21 However, the effect and mechanism of palbociclib is not clear against CRC under hypoxia.
Figure 1.
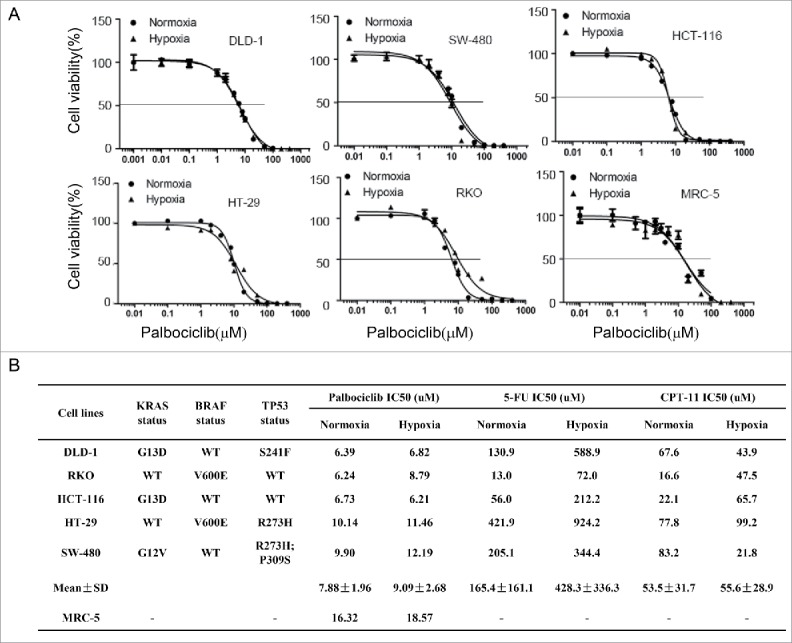
Hypoxia has minimal impact on colorectal cancer (CRC) cell viability after palbociclib or irinotecan treatment, as compared with 5-FU. (A) Dose-response curves of palbociclib against a panel of 5 human colon cancer cell lines under either normoxia or hypoxia, after treatment of 48 hours. (B) Tables of IC50 values with a panel of 5 human colon cancer cell lines with various molecular genetic subtypes under normoxia and hypoxia after treatment of 48 hours. Cell viability was detected with CellTiter-Glo assay. Prism statistical software was used to calculate IC50 values and plot dose-response curve. Each experiment was repeated 4 times.
Palbociclib promotes cell death of CRC regardless of the presence of hypoxia
It was previously demonstrated that palbociclib could arrest the transition of the G1-S phase of the cell cycle at a concentration range from 0.08 μM to 10 μM in Colo-205 human colon cancer cells under normoxia.22 Besides its anti-proliferative effect in a variety of malignancies, palbociclib inhibits the epithelial-mesenchymal transition (EMT) and metastasis in breast cancer cells via downregulation of the c-Jun/COX-2 pathway at approximately 10 μM.23 High concentrations of palbociclib were recently used to allow for tissue perfusion, and a significant increase in the percentage of cleaved-caspase 3-positive cells was observed in human HCC samples. Moreover, palbociclib-treated mice showed a remarkable reduction in tumor volume, indicating that palbociclib treatment can be effective against liver tumors in vivo.24 These findings suggest that the anti-tumor effects and mechanisms of palbociclib could be more complicated than the primary CDK inhibitory activities. To further investigate how palbociclib may affect cell viability in CRC, cell death was evaluated by DNA fragment analysis (sub-G1 analysis). Our data show that palbociclib promotes cell death in CRC cells under either normoxia (Fig 2A and C) or hypoxia (Fig 2B and D) at 10 μM. Immunoblotting detected PARP/cleaved-PARP and Caspase-3/cleaved-caspase-3 and confirmed that palbociclib promoted CRC cell apoptosis under either normoxic or hypoxic conditions (Fig 2E). In addition, hypoxia might delay the G1-phase arrest observed with palbociclib under hypoxic vs. normoxic conditions (Fig 2B and A). Interestingly, palbocicilib increased the percentage of CRC cells in the G2-phase at 10 μM under either normoxia (Fig 2A) or hypoxia (Fig 2B) after an early period of treatment.
Figure 2.
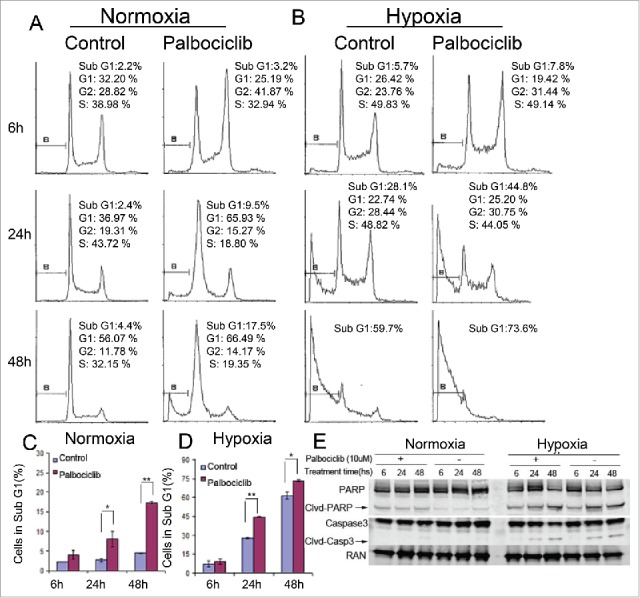
Effects of Palbociclib on cell death and cell cycle in CRC cell lines. RKO colon cancer cells were treated at the indicated time points with palbociclib (10 μM) under normoxic (A and C) or hypoxic (B and D) conditions. Representative curves are shown in the upper panels (A and B). Bar diagrams (C and D) represent Sub-G1 distribution within the cell cycle profiles. Data are shown as Mean ± SD (n = 3, Student's t-test, 2-tailed, “*” represents P < 0.05, “**” represents P < 0.01 (E) Immunoblotting shows cleavage of apoptosis-related proteins with or without palbociclib under either normoxia (Left panels) or hypoxia (Right panels). Cell death and cell cycle alterations are shown using flow cytometry. Cell death after treatment of 48 hours under hypoxia was significant. Thus there were not enough cells to support cell cycle analysis (B).
Palbocicilib destabilizes HIF-1α and impacts GSK-3β, ERK1/2 and Akt signaling in CRC differentially under hypoxia vs. normoxia
Treatment with a CDK4/6 inhibitor reduces the formation of the CDK4/6-CyclinD1 complex, and, as a result the phosphorylation of Rb is downregulated at the Ser780 and 795 sites so that Rb-positive cells arrest in the G1-phase of the cell cycle.22 We confirmed the activity of palbociclib as a highly selective inhibitor of CDK4/6. Palbocicilib efficiently deregulated the phosphorylation of CDK6 and Rb under either normoxic or hypoxic conditions (Fig 3). However, Hypoxia induced Rb phosphorylation and attenuated palbociclib-induced Rb phosphorylation at a time-dependent manner. We further investigated the anti-tumor mechanisms of palbociclib in CRC by western blotting. Our previous studies showed that CDK inhibitors can destabilize HIF-1α regardless of the presence of hypoxia and the combinatorial inhibition of GSK-3β and CDK1 kinases could reverse the apoptotic resistance of hypoxic CRC cells.16,17 We found that palbociclib destabilizes HIF-1α under either normoxic or hypoxic conditions (Fig 3). Palbociclib reduced GSK-3β expression under hypoxia and activated Akt signaling under normoxia (Fig 3), although these roles of palbociclib were not obvious under normoxia for GSK-3β or hypoxia for Akt (Fig 3). Furthermore, palbociclib inactivated inhibitory GSK-3β signaling under normoxia and simultaneously activated ERK1/2 signals in an identical time-dependent manner under hypoxic conditions. Thus, the trends for palbociclib effects on ERK1/2 and GSK-3β were different under hypoxia vs. normoxia (Fig 3).
Figure 3.
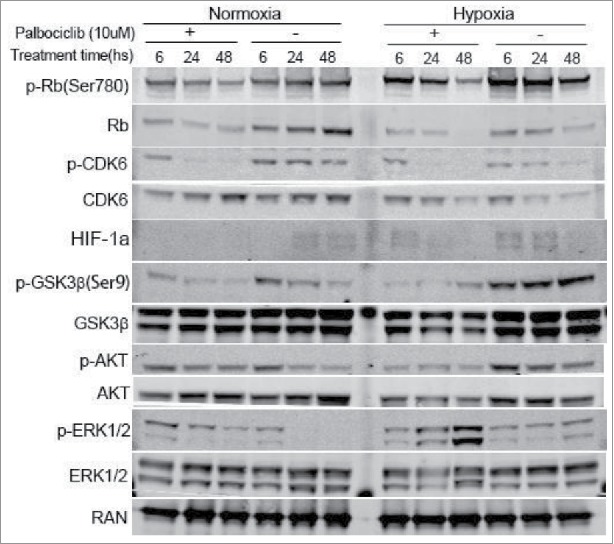
Palbociclib therapeutic effects against CRC cells via regulation of various cellular signaling pathways under normoxia and hypoxia. RKO colon cancer cells were treated at the indicated time points with palbociclib (10 μM) under normoxia (Left panels) and hypoxia (Right panels). Whole cell lysates were separated in 4–12% SDS-PAGE gels.
Palbociclib synergizes with CPT11 and reduces the viability of CRC cells under hypoxia
There are previous reports combining palbociclib with 5-FU and oxaliplatin in solid malignancies (NCT01522989) or with cetuximab in head and neck cancer under normoxia.6,7 Our assessment of IC50 values indicated that the CDK4/6 inhibitor palbociclib reduces the viability of CRC cells regardless of the presence of hypoxia (Fig 1). 5-FU, oxaliplatin and irinotecan are the backbone standard chemotherapy agents used to treat patients with advanced CRC. We assessed the therapeutic effect of palbociclib alone or in combination with these chemotherapy agents under hypoxia in vitro. To quantitatively analyze the combinatorial effects of palbociclib with these conventional CRC chemotherapies, we constructed isobolograms using palbociclib, 5-FU, oxaliplatin and irinotecan. The data shows that palbociclib can synergize with a variety of chemotherapy agents in vitro under hypoxia (Fig. 4A, B, C and D). Among these chemotherapy agents, CPT11 (irinotecan) showed a highly significant synergy with palbociclib in CRC cell lines at various doses under hypoxia (Fig 4A and B). However, the underlying mechanism of palbociclib synergy with CPT11 is not clear.
Figure 4.
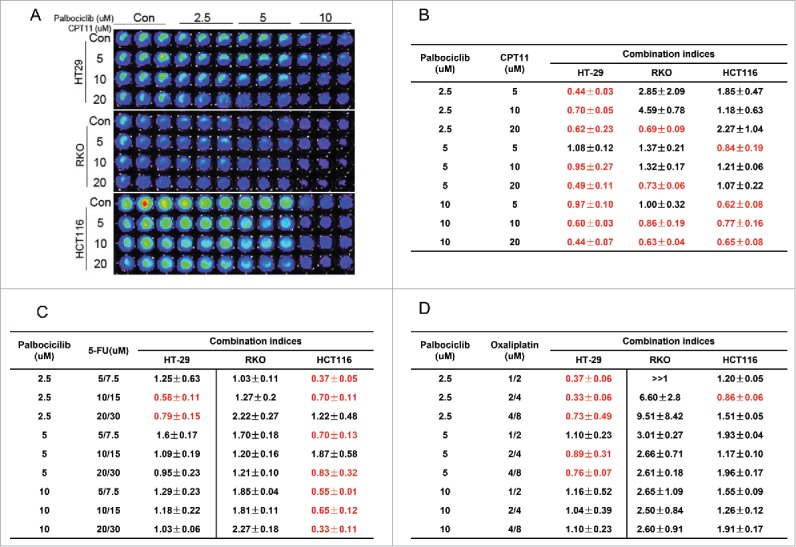
Synergy assessment of palbociclib in combination with a variety of chemotherapeutic agents in CRC cell lines under hypoxia. HT-29, RKO and HCT-116 CRC cell lines were treated with the indicated concentrations of palbociclib in combination with CPT11 (A and B), 5-FU (C) or Oxaliplatin (D) for 48 hours in various non-constant ratios/doses. (A) Representative fluorescent images of palbociclib in combination with CPT11 in a panel of 3 CRC cell lines are shown. Combination index (CI) <1, = 1 and >1 respectively indicate synergism, additivity or antagonism. CI < 0.5 indicates very strong synergy. Red colored numbers highlight synergy at the indicated concentrations.
Palbociclib synergy with CPT11 and cell death occurs though a variety of mechanisms under hypoxia
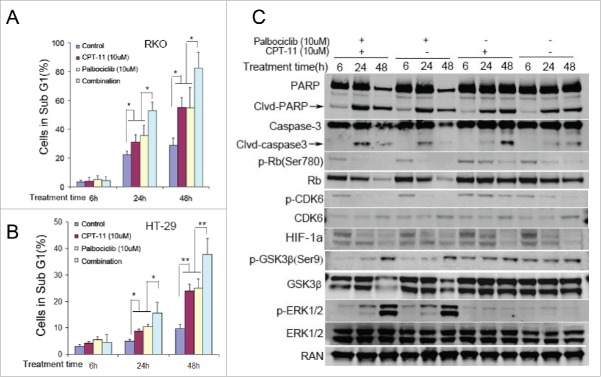
CPT11 (irinotecan), as a cytotoxic chemotherapeutic agent against CRC, is a potent and selective inhibitor of DNA topoisomerase I (Top1). CPT11 can induce DNA double-strand breakage and trigger a DNA-damage response leading to cell death. CPT11 in combination with other chemotherapies can improve therapeutic effects and reduce hematological and digestive toxicity by reducing the treatment dose of single drug in gastric cancer.25 Our data shows that palbociclib promotes cell death at 10 μM in CRC despite the presence of hypoxia (Fig 2). We assessed the effect of palbociclib in combination with CPT11 on cell death in vitro by DNA fragment analysis. The results show that palbociclib in combination with CPT11 significantly increases cell death under hypoxia after treatment of 24 hours, as compared with single drug treatment and control (Fig 5A and B). Combined administration of palbociclib with CPT11 significantly increases the ratio of caspase-cleaved protein/total protein after treatment of 24 hours, as compared with respectively single drug administration and control (Fig 5C). Immunoblotting showed that a single administration of CPT11 upregulates the phosphorylation of CDK6 and Rb, whereas addition of palbociclib to CPT11 treatment decreases the phosphorylation level of CDK6 and Rb under hypoxia regardless of whether CPT11 is administered (Fig. 5C). Single agent palbociclib or CPT11-combined with palbociclib significantly reduced HIF-1α by 24 hours, as compared with single agent CPT11 or control (Fig. 5C). Palbociclib deregulated the phosphorylation of GSK-3β and upregulated the phosphorylation of ERK1/2 in a time-dependent manner regardless of the administration of CPT11 (Fig. 5C).
Figure 5.
Palbociclib synergizes with CPT11 against CRC cell lines via deregulating CPT-11-induced Rb activation and further reducing HIF-1α accumulation under hypoxia. RKO (A and C) and HT-29 (B) CRC cell lines were treated with or without palbociclib (10 μM) in combination with CPT11 (10 μM) at the indicated time points under hypoxia. Bar diagrams (A and B) depict the sub-G1 percentage in the cell cycle profiles. Data are shown as Mean ± SD (n = 3, Student's t-test, 2-tailed, “*”represents P < 0.05, “**” represents P < 0.01). (C) Immunoblotting shows protein levels (as indicated) with or without chemotherapy under either normoxia (Left panels) or hypoxia (Right panels).
Discussion
Our previous study indicated that CDK1 stabilizes HIF-1α to promote tumor growth,17 whereas inhibition of CDK1 increases the apoptotic sensitivity of hypoxic cells via deregulating HIF-1α.16 Our present data indicates that hypoxia has little impact on the sensitivity of CRC cells to palbociclib (Fig. 1). We found that palbociclib destabilizes HIF-1α in colon cancer cells under either normoxic or hypoxic conditions (Fig. 3), suggesting that palbociclib may sensitize CRC cells to hypoxia-induced apoptotic resistance (Fig. 1). 5-FU is the backbone of chemotherapy against CRC. It has been reported that hypoxia induces 5-FU resistance in gastrointestinal cancer, whereas this role of hypoxia may be reversed via inhibition of a HIF-1α signal regulating glycolysis.26 Our previous study reported that hypoxia induces apoptotic resistance to 5-FU in CRC cells lacking p53.16 We confirmed that hypoxia rapidly induces 5-FU resistance in diverse CRC cell lines (Fig 1). Irinotecan is also part of the backbone chemotherapy against advanced CRC, and hypoxia-induced accumulation of carboxylesterase increases tumor sensitivity to irinotecan (CPT-11).20 CPT11 can reduce angiogenesis and tumor growth via inhibiting HIF-1α protein accumulation or translation in hypoxic glioma cells.21 We found that CPT11 exerts its therapeutic effect in CRC cells regardless of the presence of hypoxia (Fig. 1). Collectively, hypoxia-induced drug resistance represents a formidable issue in CRC therapy, and our findings shed light on a potential therapeutic strategy against hypoxic tumor cells.
High concentrations of palbociclib have been used to allow for tissue perfusion, where a significant increase in cleaved-caspase 3-positive cells was observed in human HCC samples. Single agent palbociclib-treated mice showed a remarkable reduction in tumor volume, indicating that palbociclib treatment can be effective in liver tumors in vivo.24 Our data indicates that palbociclib promotes apoptosis at 10 μM regardless of the presence of the presence of hypoxia (Fig. 1 and 2). These findings suggest that the therapeutic effect and mechanism of palbociclib is more complex than what is presently understood. Glycogen synthase kinase-3β is an essential serine/threonine kinase in cell survival and NF-kB activation.27 Inhibition of GSK-3β induces glioma cell death.28 ERK1/2 upregulating pGSK-3β (Tyr216) mediates basic fibroblast growth factor (FGF)-induced apoptosis in neuroblastoma cells, whereas blockade of GSK-3β and ERK1/2 protects cells from FGF2-induced apoptosis.29 Atractylenolide I (AT-I), owning the activities of p21activator and CDK2 inhibitor isolated from atractylodis macrocephalae rhizome, induces apoptosis and G1-S arrest in melanoma via deregulating pGSK-3β (Ser9), whereas blockade of GSK-3β reverses AT-1-induced apoptosis and G1-S arrest.30 These findings are consistent with our finding that palbociclib promotes apoptosis in CRC cells, and simultaneously activates ERK1/2 signals and deregulates inhibitory pGSK-3β (Ser9). Moreover, palbociclib reduces GSK-3β expression under hypoxia (Fig. 3), which may explain why more CRC cell death is observed under hypoxia (Fig. 2). In addition, we found that hypoxia induced Rb activation and reduced the inhibition of palbociclib on Rb phosphorylation (Fig 3). Consequently, hypoxia may delay palbociclib-induced G1-phase arrest (Fig. 2). We also found that palbociclib can arrest cells in the G2-phase even prior its noted effect of G1-phase arrest (Fig. 2). This is not consistent with a prior study,22 and the reason could be one 24-hour time point was evaluated and different cell lines were previously used. P276–00, a cyclin-dependent kinase inhibitor, inhibits HIF-1α and induces G2/M arrest under hypoxia in prostate cancer cells.31 Some studies have reported that ERK- or JNK/MAPK activation induces G2/M phase arrest and apoptosis in solid tumors.32-36 We found that palbociclib destabilizes HIF-1α and activates ERK1/2 under either normoxic or hypoxic conditions (Fig. 3). These mechanisms could explain the effects of palbociclib on G2/M phase arrest and apoptosis in CRC cells (Fig. 1 and 2).
Numerous studies and clinical trials have indicated that palbociclib synergizes with conventional chemotherapy or targeted agents and improves the efficacy of tumor treatment.5-11 We analyzed the combinatorial effects of palbociclib with the commonly used chemotherapy agents against CRC, as a prelude to possible translation. Chou-Talalay analysis revealed that palbociclib can synergize with a variety of chemotherapies under hypoxia (Fig. 4). Among these chemotherapy agents, irinotecan showed comprehensive and often potent synergy with palbociclib in CRC cells with diverse molecular genetic sub-types under hypoxia. We found that the combinatorial administration of irinotecan with palbociclib further reduced HIF-1α in hypoxic CRC cells (Fig. 5). Our finding is consistent with studies indicating that irinotecan synergizes with a variety of chemotherapies via regulating HIF-1α signaling in hypoxic tumor cells37,38 Additionally, we found that single agent irinotecan administration increases Rb phosphorylation, whereas addition of palbociclib reduces irinotecan-induced Rb phosphorylation. Thus, palbociclib addition can reverse irinotecan-induced cell-cycle progression in hypoxic CRC cells (Fig. 5).
Our studies have translational significance and suggest possible testing of a CDK4/6 inhibitor in combination with CPT11 (irinotecan) in CRC therapy. The observed effects and mechanistic insights involving HIF-1α, pRb, GSK-3β, Akt, and ERK1/2 further support the proposed clinical trial rationale. In particular the strategy combining a CDK4/6 inhibitor with irinotecan is expected to target both normoxic as well hypoxic tumor cell populations that may contribute to conventional therapy resistance or tumor relapse. The combination of a CDK4/6 inhibitor plus irinotecan (CPT11) merits further investigation in the clinic for patients with advanced colorectal cancer and potentially other tumor types.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was presented in part at the 2016 annual AACR meeting in New Orleans. W.S.E-D. is an American Cancer Society Research Professor.
References
- [1].Manning AL, Dyson NJ. RB: mitotic implications of a tumour suppressor. Nat Rev Cancer 2012; 12(3):220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Abedin ZR, Ma Z, and Reddy EP. Increased angiogenesis in Cdk4- (R24C/R24C): Apc(+/Min) intestinal tumors. Cell Cycle 2010; 9(12):2456-63; PMID:20603602; https://doi.org/ 10.4161/cc.9.12.12055 [DOI] [PubMed] [Google Scholar]
- [3].Kollmann K, Heller G, Schneckenleithner C, Warsch W, Scheicher R, Ott RG, Schäfer M, Fajmann S, Schlederer M, Schiefer AI, et al.. A kinase-independent function of CDK6 links the cell cycle to tumor angiogenesis. Cancer Cell 2013. 24(2):167-81; PMID:23948297; https://doi.org/ 10.1016/j.ccr.2013.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Handschick K, Beuerlein K, Jurida L, Bartkuhn M, Müller H, Soelch J, Weber A, Dittrich-Breiholz O, Schneider H, Scharfe M, et al.. Cyclin-dependent kinase 6 is a chromatin-bound cofactor for NF-κB-dependent gene expression. Mol Cell 2014. ;53(2):193-208; https://doi.org/ 10.1016/j.molcel.2013.12.002 [DOI] [PubMed] [Google Scholar]
- [5].Beaver JA, Amiri-Kordestani L, Charlab R, Chen W, Palmby T, Tilley A, Zirkelbach JF, Yu J, Liu Q, Zhao L, et al.. FDA Approval: Palbociclib for the Treatment of Postmenopausal Patients with Estrogen Receptor-Positive, HER2-Negative Metastatic Breast Cancer. Clin Cancer Res 2015; 21(21): 4760-6; PMID:26324739; https://doi.org/ 10.1158/1078-0432.CCR-15-1185 [DOI] [PubMed] [Google Scholar]
- [6].Franco J, Witkiewicz AK, Knudsen ES. CDK4/6 inhibitors have potent activity in combination with pathway selective therapeutic agents in models of pancreatic cancer. Oncotarget 2014; 5 (15):6512-25; https://doi.org/ 10.18632/oncotarget.2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Michel L, Ley J, Wildes TM, Schaffer A, Robinson A, Chun SE, Lee W, Jr Lewis J, Trinkaus K, Adkins D. Phase I trial of palbociclib, a selective cyclin dependent kinase 4/6 inhibitor, in combination with cetuximabin patients with recurrent/metastatic head and neck squamous cell carcinoma.Oral Oncol. 2016; 58:41-8; https://doi.org/ 10.1016/j.oraloncology.2016.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tao Z, Le Blanc JM, Wang C, Zhan T, Zhuang H, Wang P, Yuan Z, Lu B. Coadministration of Trametinib and Palbociclib Radiosensitizes KRAS-Mutant Non-Small Cell Lung Cancers In Vitro and In Vivo.Clin Cancer Res 2016; 22(1):122-33; https://doi.org/ 10.1158/1078-0432.CCR-15-0589 [DOI] [PubMed] [Google Scholar]
- [9].Ziemke EK, Dosch JS, Maust JD, Shettigar A, Sen A, Welling TH, Hardiman KM, Sebolt-Leopold JS. Sensitivity of KRAS Mutant Colorectal Cancers to Combination Therapy That Cotargets MEK and CDK4/6.Clin Cancer Res 2016; 22(2):405-14; https://doi.org/ 10.1158/1078-0432.CCR-15-0829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lee MS, Helms TL, Feng N, Gay J, Chang QE, Tian F, Wu JY, Toniatti C, Heffernan TP, Powis G, Kwong LN, Kopetz S. Efficacy of the combination of MEK and CDK4/6 inhibitors in vitro and in vivo in KRAS mutant colorectal cancer models. Oncotarget 2016; 7(26):39595-39608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yoshida A., Lee E.K., and Diehl J.A., Induction of Therapeutic Senescence in Vemurafenib-Resistant Melanoma by Extended Inhibition of CDK4/6. Cancer Res 2016; 76(10):2990-3002; PMID:26988987; https://doi.org/ 10.1158/0008-5472.CAN-15-2931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rebucci M. and Michiels C., Molecular aspects of cancer cell resistance to chemotherapy. Biochem Pharmacol 2013; 85(9):1219-26; PMID:23435357; https://doi.org/ 10.1016/j.bcp.2013.02.017 [DOI] [PubMed] [Google Scholar]
- [13].Hubbi ME, Gilkes DM, Hu H, Kshitiz Ahmed I, Semenza GL Cyclin-dependent kinases regulate lysosomal degradation of hypoxia-inducible factor- 1α to promote cell-cycle progression. Proc Natl Acad Sci U S A 2014; 111(32):E3325-34; https://doi.org/ 10.1073/pnas.1412840111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Flügel D, Görlach A, Michiels C, Kietzmann T. Glycogen synthase kinase 3 phosphorylates hypoxia-inducible factor-1alpha and mediates its destabilization in a VHL-independent manner. Mol Cell Biol 2007; 27(9):3253-65; https://doi.org/ 10.1128/MCB.00015-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sang N, Stiehl DP, Bohensky J, Leshchinsky I, Srinivas V, Caro J., MAPK signaling up-regulates the activity of hypoxia-inducible factors by its effects on p300. J Biol Chem, 2003. 278(16): p. 14013-9; PMID:12588875; https://doi.org/ 10.1074/jbc.M209702200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mayes PA, Dolloff NG, Daniel CJ, Liu JJ, Hart LS, Kuribayashi K, Allen JE, Jee DI, Dorsey JF, Liu YY, et al.. Overcoming hypoxia-induced apoptotic resistance through combinatorial inhibition of GSK-3β and CDK1. Cancer Res 2011; 71(15):5265-75; https://doi.org/ 10.1158/0008-5472.CAN-11-1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Warfel NA, Dolloff NG, Dicker DT, Malysz J, El-Deiry WS. CDK1 stabilizes HIF-1α via direct phosphorylation of Ser668 to promote tumor growth. Cell Cycle 2013; 12(23):3689-701; https://doi.org/ 10.4161/cc.26930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gallant JN, Allen JE, Smith CD, Dicker DT, Wang W, Dolloff NG, Navaraj A, El-Deiry WS, Quinacrine synergizes with 5-fluorouracil and other therapies in colorectal cancer. Cancer Biol Ther 2011; 12(3):239-51; PMID:21725213; https://doi.org/ 10.4161/cbt.12.3.17034 [DOI] [PubMed] [Google Scholar]
- [19].Chou T.C., Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev 2006; 58(3):621-81; PMID:16968952; https://doi.org/ 10.1124/pr.58.3.10 [DOI] [PubMed] [Google Scholar]
- [20].Matzow T, Cowen RL, Williams KJ, Telfer BA, Flint PJ, Southgate TD, Saunders MP. Hypoxia-targeted over-expression of carboxylesterase as a means of increasing tumour sensitivity toirinotecan (CPT-11). J Gene Med 2007; 9(4):244-52; https://doi.org/ 10.1002/jgm.1016 [DOI] [PubMed] [Google Scholar]
- [21].Rapisarda A, Uranchimeg B, Sordet O, Pommier Y, Shoemaker RH, Melillo G. Topoisomerase I-mediated inhibition of hypoxia-inducible factor 1: mechanism and therapeutic implications. Cancer Res 2004; 64(4):1475-82; PMID:14983893; https://doi.org/ 10.1158/0008-5472.CAN-03-3139 [DOI] [PubMed] [Google Scholar]
- [22].Fry DW, Harvey PJ, Keller PR, Elliott WL, Meade M, Trachet E, Albassam M, Zheng X, Leopold WR, Pryer NK, Toogood PL, Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther 2004; 3(11):1427-38; PMID:15542782 [PubMed] [Google Scholar]
- [23].Qin G, Xu F, Qin T, Zheng Q, Shi D, Xia W, Tian Y, Tang Y, Wang J, Xiao X, Deng W, Wang S, Palbociclib inhibits epithelial-mesenchymal transition and metastasis in breast cancer via c-Jun/COX-2 signaling pathway. Oncotarget 2015; 6(39):41794-808; PMID:26540629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bollard J, Miguela V, Ruiz de Galarreta M, Venkatesh A, Bian CB, Roberto MP, Tovar V, Sia D, Molina-Sánchez P, Nguyen CB, Nakagawa S, Llovet JM, Hoshida Y, Lujambio A. Palbociclib (PD-0332991), a selective CDK4/6 inhibitor, restricts tumour growth in preclinical models of hepatocellular carcinoma. Gut 2016; pii:gutjnl-2016-312268; https://doi.org/ 10.1136/gutjnl-2016-312268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Farhat FS. A general review of the role of irinotecan (CPT11) in the treatment of gastric cancer. Med Oncol 2007; 24 (2):137-46. Review; PMID:17848736; https://doi.org/ 10.1007/BF02698032 [DOI] [PubMed] [Google Scholar]
- [26].Xuan Y, Hur H, Ham IH, Yun J, Lee JY, Shim W, Kim YB, Lee G, Han SU, Cho YK. Dichloroacetate attenuates hypoxia-induced resistance to 5-fluorouracil in gastric cancer through theregulation of glucose metabolism. Exp Cell Res 2014; 321(2):219-30; https://doi.org/ 10.1016/j.yexcr.2013.12.009 [DOI] [PubMed] [Google Scholar]
- [27].KP1 Hoeflich, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. 2000; 406(6791):86-90. [DOI] [PubMed] [Google Scholar]
- [28].Kotliarova S, Pastorino S, Kovell LC, Kotliarov Y, Song H, Zhang W, Bailey R, Maric D, Zenklusen JC, Lee J, et al.. Glycogen synthase kinase-3 inhibition induces glioma cell death through c-MYC, nuclear factor-kappaB, and glucose regulation. Cancer Res 2008; 68(16):6643-51; https://doi.org/ 10.1158/0008-5472.CAN-08-0850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ma C, Bower KA, Chen G, Shi X, Ke ZJ, Luo J. Interaction between ERK and GSK3beta mediates basic fibroblast growth factor-induced apoptosis in SK-N-MC neuroblastoma cells. J Biol Chem 2008; 283(14):9248-56; https://doi.org/ 10.1074/jbc.M707316200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ye Y, Chao XJ, Wu JF, Cheng BC, Su T, Fu XQ, Li T, Guo H, Tse AK, Kwan HY, Du J, Chou GX, Yu ZL. ERK/GSK3β signaling is involved in atractylenolide I-induced apoptosis and cell cycle arrest in melanomacells. Oncol Rep 2015; 34(3):1543-8. [DOI] [PubMed] [Google Scholar]
- [31].Manohar SM, Padgaonkar AA, Jalota-Badhwar A, Rao SV, Joshi KS. P276 -00, a cyclin-dependent kinase inhibitor, inhibits HIF-1a and induces G2/M arrest under hypoxia in prostate cancer cells. Prostate Cancer Prostatic Dis 2012; 15(1):15-27; PMID:22083267; https://doi.org/ 10.1038/pcan.2011.51 [DOI] [PubMed] [Google Scholar]
- [32].Sun WJ, Huang H, He B, Hu DH, Li PH, Yu YJ, Zhou XH, Lv Z, Zhou L, Hu TY, et al.. Romidepsin induces G2/M phase arrest via Erk/cdc25C/cdc2/cyclinB pathway and apoptosis induction through JNK/c-Jun/caspase3 pathway in hepatocellular carcinoma cells. Biochem Pharmacol 2016; 127:90-100. pii: S0006-2952(16)30488-9 [DOI] [PubMed] [Google Scholar]
- [33].Wei W, Yu Z, Xie M, Wang W, Luo X. Oxygen-Glucose Deprivation Induces G2/M Cell Cycle Arrest in Brain Pericytes Associated with ERK Inactivation. J Mol Neurosci 2017; 61(1):105-114; https://doi.org/ 10.1007/s12031-016-0844-2 [DOI] [PubMed] [Google Scholar]
- [34].Liu W, Ning R, Chen RN, Huang XF, Dai QS, Hu JH, Wang YW, Wu LL, Xiong J, Hu G, et al.. Aspafilioside B induces G2/M cell cycle arrest and apoptosis by up-regulating H-Ras and N-Ras via ERK and p38 MAPK signaling pathways in human hepatoma HepG2 cells. Mol Carcinog 2016; 55(5):440-57; https://doi.org/ 10.1002/mc.22293 [DOI] [PubMed] [Google Scholar]
- [35].Lv C, Hong Y, Miao L, Li C, Xu G, Wei S, Wang B, Huang C, Jiao B. Wentilactone A as a novel potentia antitumor agent induces apoptosis and G2/M arrest of human lungcarcinoma cells, and is mediated by HRas-GTP accumulation to excessively activate the Ras/Raf/ERK/p53-p21 pathway. Cell Death Dis 2013; 4:e952; https://doi.org/ 10.1038/cddis.2013.484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yin X, Zhang R, Feng C, Zhang J, Liu D, Xu K, Wang X, Zhang S, Li Z, Liu X, Ma H Diallyl disulfide induces G2/M arrest and promotes apoptosis through the p53/p21 and MEK-ERK pathwaysin human esophageal squamous cell carcinoma. Oncol Rep 2014; 32(4):1748-56. [DOI] [PubMed] [Google Scholar]
- [37].Pencreach E, Guérin E, Nicolet C, Lelong-Rebel I, Voegeli AC, Oudet P, Larsen AK, Gaub MP, Guenot D. Marked activity of irinotecan and rapamycin combination toward colon cancer cells in vivo and in vitro is mediated through cooperative modulation of the mammalian target of rapamycin/hypoxia-inducible factor-1alpha axis. Clin Cancer Res 2009; 15(4):1297-307; https://doi.org/ 10.1158/1078-0432.CCR-08-0889 [DOI] [PubMed] [Google Scholar]
- [38].Chintala S, Tóth K, Cao S, Durrani FA, Vaughan MM, Jensen RL, Rustum YM. Se-methylselenocysteine sensitizes hypoxic tumor cells to irinotecan by targeting hypoxia-inducible factor1alpha. Cancer Chemother Pharmacol. 2010; 66(5):899-911; https://doi.org/ 10.1007/s00280-009-1238-8 [DOI] [PMC free article] [PubMed] [Google Scholar]