ABSTRACT
Previously, a patient-derived orthotopic xenograft (PDOX) model was established with a lung metastasis from an osteosarcoma patient which developed after adjuvant cisplatinum (CDDP) treatment. In this model, we previously demonstrated the efficacy of trabectedin (TRAB) and temozolomide (TEM) compared with CDDP. In the present report, osteosarcoma tissue was implanted orthotopically in the distal femur of mice which were randomized into the following groups when tumor volume reached approximately 100 mm3; On day 14 after initiation of treatment, all but CDDP significantly inhibited tumor volume growth compared with untreated controls. Control (G1): 793.7 ± 215.0 mm3; CDDP (G2): 588.1 ± 176.9 mm3; Salmonella typhimurium A1-R (S. typhimurium A1-R) intravenous (i.v.) (G3): 269.7 ± 72.7 mm3; S. typhimurium A1-R intra-arterial (i.a.) (G4): 70.2 ± 18.9 mm3 (CDDP: p = 0.056; S. typhimurium A1-R i.v.: p = 0.0001; S. typhimurium A1-R i.a.: p = 0.00003, all vs. untreated controls). i.a. administration of S. typhimurium A1-R was significantly more effective than either CDDP (p = 0.00007), or i.v. administration of S. typhimurium A1-R (p = 0.00007) and significantly regressed the tumor volume compared with day 0 (p = 0.001). The new model of i.a. administration of S. typhimurium A1-R has great promise for the treatment of recalcitrant osteosarcoma.
KEYWORDS: Osteosarcoma, PDOX, Salmonella typhimurium A1-R, intra-arterial, cisplatinum-resistant, recurrent
Introduction
Toward the goal of precision oncology, our laboratory pioneered the patient-derived orthotopic xenograft (PDOX) nude mouse model with the technique of surgical orthotopic implantation (SOI), including pancreatic,1-4 breast,5 ovarian,6 lung,7 cervical,8 colon,9-11 and stomach cancer,12 sarcoma,13-17 and melanoma.18-20
In a previous study, we evaluated the efficacy of trabectedin (TRAB) and temozolomide (TEM) compared with cisplatinum (CDDP) on a lung-metastatic osteosarcoma PDOX mouse model.21 TEM and TRAB, but not CDDP, significantly inhibited tumor volume compared with the untreated controls. The results of the previous study showed that a PDOX model of an osteosarcoma lung-metastasis that recurred after adjuvant CDDP-treatment, had identified potentially, highly-effective drugs for this recalcitrant disease, while precisely maintaining the CDDP resistance of the tumor in the patient.21
However, more effective therapeutics are needed for metastatic osteosarcoma. Toward this goal, our laboratory developed tumor-targeting Salmonella typhimurium A1-R (S. typhimurium A1-R). S. typhimurium A1-R was effective against primary and metastatic tumors as monotherapy in nude mouse models of major cancers, including prostate,22,23 breast,24-26 lung,27,28 pancreatic,1,29-32 ovarian,33, 34 stomach,35 and cervical cancer.36 In addition, S. typhimurium A1-R was effective against patient-derived orthotopic models (PDOX) of pancreatic cancer,1,32 sarcoma,13,15,37 and melanoma.18-20
Metastatic osteosarcoma is a recalcitrant disease. We previously reported that a patient-derived subcutaneous-transplant nude-mouse model of the ostoeosarcoma lung metastasis that occurred after adjuvant CDDP treatment, was regressed by tumor-targeting S. typhimurium A1-R. The osteosarcoma was only partially sensitive to the molecular-targeting drug sorafenib, which did not arrest its growth. S. typhimurium A1-R was significantly more effective than sorafenib.21, 37
We previously reported the efficacy and safety of intra-portal-vein (iPV) targeting of S. typhimurium A1-R on colon cancer liver metastasis in a nude-mouse orthotopic model.38
In the present report, we demonstrate the high efficacy of intra-arterial (i.a.) administration of S. typhimurium A1-R on the CDDP-resistant, recurrent osteosarcoma in the PDOX model.
Results and discussion
Distribution of S. typhimurium A1-R in the CDDP-resistant relapsed osteosarcoma PDOX mouse model
Distribution of S. typhimurium A1-R was imaged by confocal microscopy with the Olympus FV1000 on day 3 after injection of S. typhimurium A1-R (Fig. 1). The mean fluorescence intensity in the PDOX tumor on day 3 after intra-arterial (i.a.) injection of S. typhimurium A1-R was 1.76 × 106 compared with 1.49 × 105 after intravenous (i.v.) injection (p = 0.000013; Fig. 2A). The fluorescence area in the PDOX tumor on day 3 after i.a. injection of S. typhimurium A1-R was 485.4 ± 103.7 µm2 compared with 51.3 ± 45.7 µm2 after i.v. injection (p < 0.00001; Fig. 2B). In the tibialis anterior muscle of the tumor-bearing limb, fluorescence intensity after i.a. injection S. typhimurium A1-R was 5.18 compared with 2.74 after i.v. injection (p = 0.50). The fluorescence area after i.a. injection S. typhimurium A1-R was 0.01 µm2 compared with 0.02 µm2 after i.v. injection in the tibialis anterior muscle of affected limb (p = 0.42).
Figure 1.
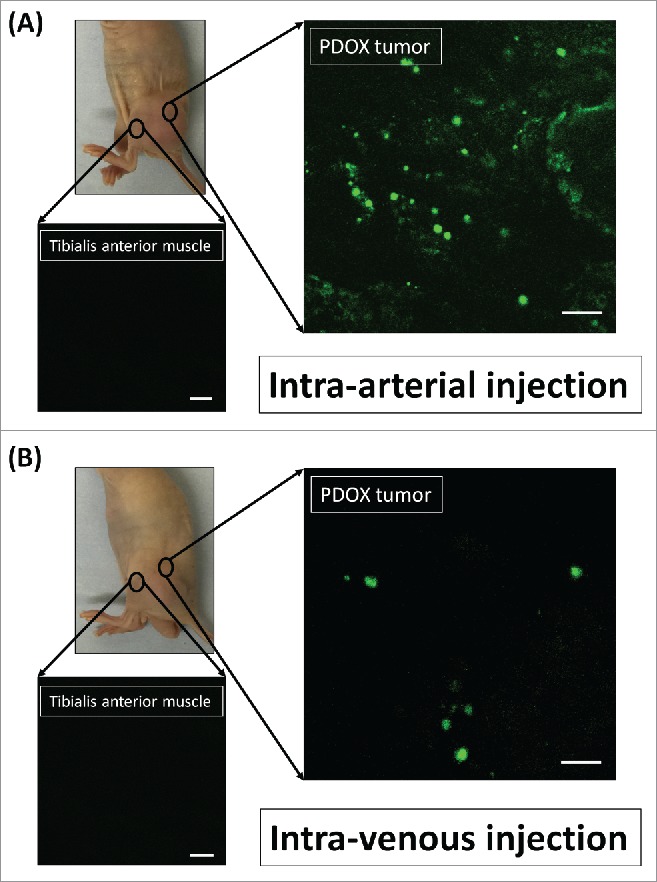
Distribution of fluorescence imaging. (A) S. typhimurium-A1-R-GFP targeting the osteosarcoma PDOX after intra-arterial (i.a.) injection. (B) S. typhimurium A1-R-GFP targeting the osteosarcoma after intravenous (i.v.) injection. Confocal microscopy imaging with the Olympus FV1000 demonstrated S. typhimurium A1-R-GFP targeting the osteosarcoma PDOX. Scale bars: 12.5 µm.
Figure 2.
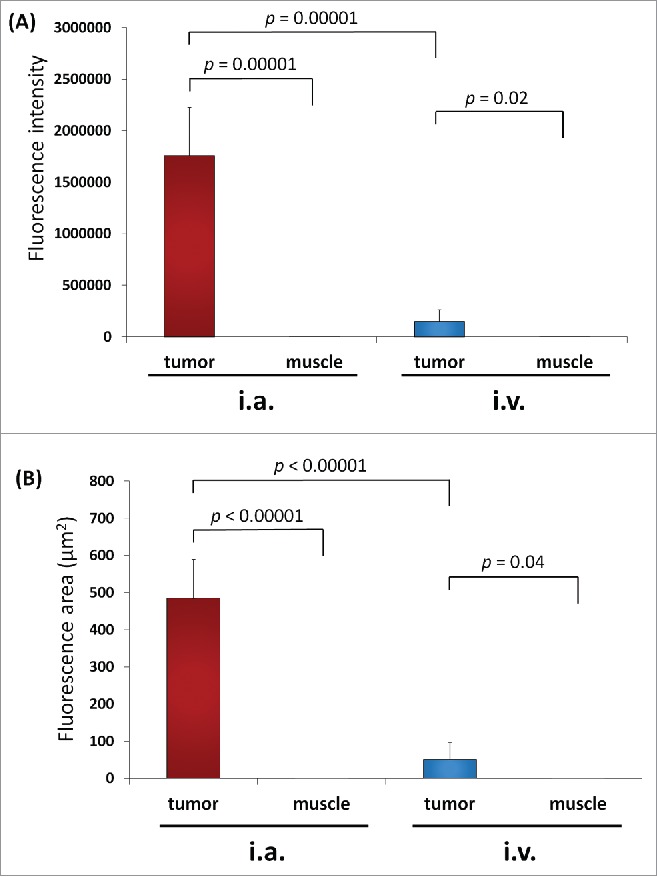
(A) Fluorescence intensity of S. typhimurium A1-R-GFP targeting the osteosarcoma PDOX after i.a. or i.v. administration. (B) Fluorescent area of S. typhimurium A1-R-GFP targeting the osteosarcoma PDOX after i.a. or i.v. administration.
Efficacy of CDDP, S. typhimurium A1-R i.v. and S. typhimurium A1-R i.a. on the CDDP-resistant osteosarcoma PDOX mouse model
On day 14 after initiation of treatment, all but CDDP significantly inhibited tumor volume growth compared with untreated control (G1): 793.7 ± 215.0 mm3; CDDP (G2): 588.1 ± 176.9 mm3; S. typhimurium A1-R i.v. (G3): 269.7 ± 72.7 mm3; S. typhimurium A1-R i.a. (G4): 70.2 ± 18.9 mm3 (CDDP: p = 0.056; S. typhimurium A1-R i.v.: p = 0.0001; S. typhimurium A1-R i.a.: p = 0.00003, all vs. untreated controls). i.a. administration of S. typhimurium A1-R was significantly more effective than either CDDP (p = 0.00007); i.v. administration of S. typhimurium A1-R (p = 0.00007) and significantly regressed the tumor volume compared with day 0 (p = 0.001) (Fig. 3).
Figure 3.
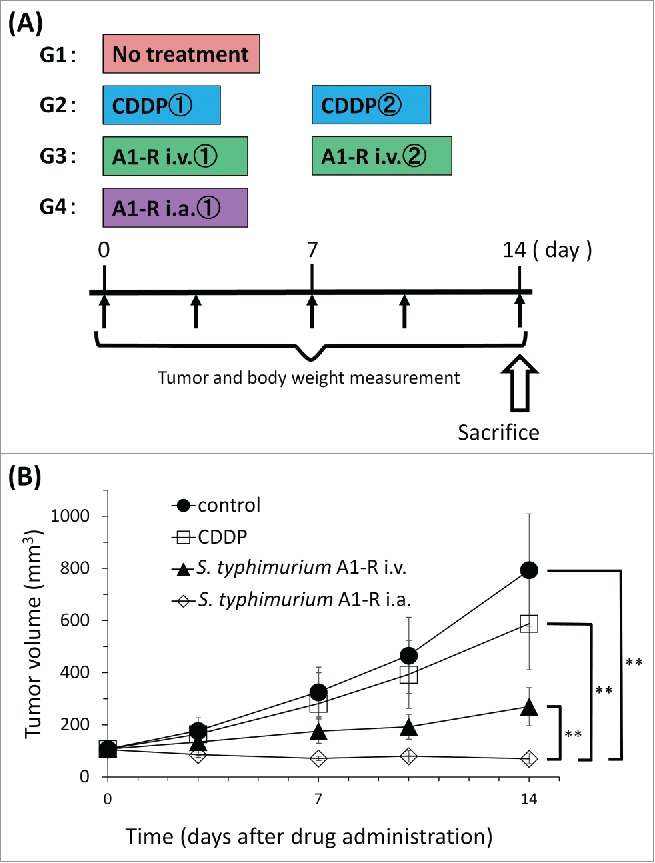
(A) Treatment schema. Mice were treated with CDDP, S. typhimurium A1-R i.v. or S. typhimurium A1-R i.a. CDDP (6 mg/kg/week i.p. for 2 weeks); S. typhimurium A1-R i.v. (5 × 107 CFU/100 μl, i.v., weekly, for 2 weeks); S. typhimurium A1-R i.a. (5 × 105 CFU/100 μl, i.a., once). Tumor volume was measured at the indicated time points after the onset of treatment. n = 8mice/group. (B) Treatment efficacy. *p < 0.05, **p < 0.001.
There were no animal deaths, limb necrosis or paraplegia in any group. The body weight of treated mice were not significantly different in each group (Fig. 4).
Figure 4.
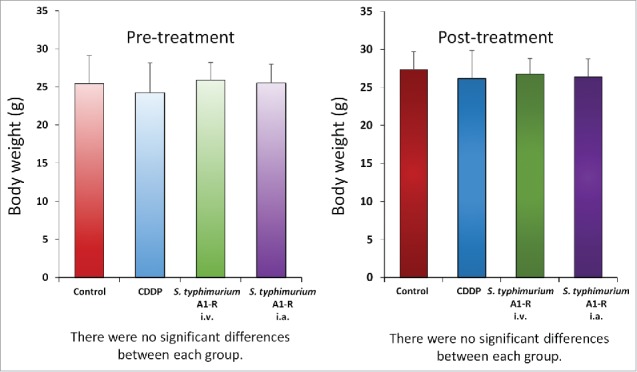
Body weight after various treatments. Bar graphs show body weight in each group at pre-treatment and 2 weeks after drug administration.
Histology of original tumor and PDOX tumors
High power photomicrography of the original patient tumor demonstrated a chondroid matrix occupied by anaplastic cells. The tumor had hypercellular areas populated by anaplastic cells displaying nuclear pleomorphism, coarse and hyperchromatic chromatin and abundant mitotic figures (Fig. 5A). High power photomicrography of the untreated PDOX tumor showed the tumor had a solid and chondroblastic appearance similar to the original patient tumor and had hypercellular areas filled with cancer cells displaying nuclear pleomorphism and mitotic figures (Fig. 5B). The PDOX tumor treated with CDDP comprised viable cells without apparent necrosis or inflammatory changes and had almost the same features as the untreated control (Fig. 5C). The PDOX Tumor treated with i.v. injection of S. typhimurium A1-R showed changes in cancer-cell shape with a necrotic area (Fig. 5D). The i.a. injection of S. typhimurium A1-R-treated tumor showed more extensive tumor necrosis (Fig. 5E).
Figure 5.
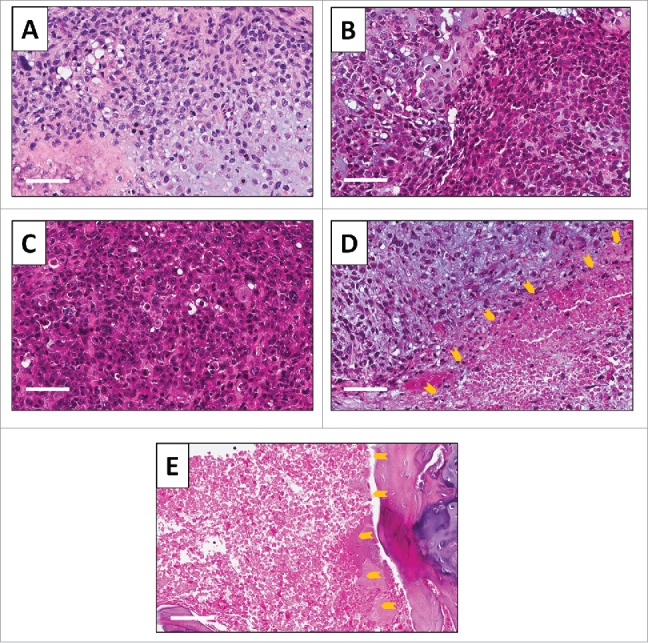
Tumor histology. Hematoxylin and eosin (H&E)-stained section of the (A) original patient tumor; (B) untreated PDOX tumor; (C) PDOX tumor treated with CDDP; (D) PDOX tumor treated with S. typhimurium A1-R i.v.; and (E) PDOX tumor treated with S. typhimurium A1-R i.a.. Necrotic areas are indicated by yellow arrows. White scale bars: 80 µm.
Previously-developed concepts and strategies of highly-selective tumor targeting can take advantage of molecular targeting of tumors, including tissue-selective therapy which focuses on unique differences between normal and tumor tissues.39-44
Materials and methods
Mice
Athymic nu/nu nude mice (AntiCancer Inc., San Diego, CA), 4–6 weeks old, were used in this study. Animals were housed in a barrier facility on a high efficiency particulate arrestance (HEPA)-filtered rack under standard conditions of 12-hour light/dark cycles. The animals were fed an autoclaved laboratory rodent diet. All animal studies were conducted with an AntiCancer Institutional Animal Care and Use Committee (IACUC)-protocol specifically approved for this study and in accordance with the principals and procedures outlined in the National Institute of Health Guide for the Care and Use of Animals under Assurance Number A3873–1. To minimize any suffering of the animals, anesthesia and analgesics were used for all surgical experiments. Animals were anesthetized by subcutaneous injection of a 0.02 ml solution of 20 mg/kg ketamine, 15.2 mg/kg xylazine, and 0.48 mg/kg acepromazine maleate. The response of animals during surgery was monitored to ensure adequate depth of anesthesia. The animals were observed on a daily basis and humanely killed by CO2 inhalation when they met the following humane end point criteria: severe tumor burden (more than 20 mm in diameter), prostration, significant body weight loss, difficulty breathing, rotational motion and body temperature drop.
Patient-derived tumor
The study was previously reviewed and approved by the UCLA Institutional Review Board (IRB #10–001857).37 Written informed consent was obtained from the patient as part of the above-mentioned UCLA Institutional Review Board-approved protocol. A 16-year old patient with localized left distal femoral high grade osteosarcoma underwent CDDP-based neo-adjuvant chemotherapy and limb salvage with distal femoral replacement. One year later, the osteosarcoma recurred with 3 bilateral metachronous pulmonary metastases. The patient was treated with curative surgery at the Division of Surgical Oncology, University of California, Los Angeles (UCLA). The patient did not receive chemotherapy or radiotherapy before lung surgery.37
SOI for establishment of the PDOX osteosarcoma model
The osteosarcoma from the patient was previously established subcutaneously in mice.37 Subcutaneously grown tumors were harvested and cut into small fragments (3–4 mm). After nude mice were anesthetized, a 10 mm skin incision was made on the right thigh, the vastus lateralis muscle was opened and the biceps femoris muscle was split to reach the distal femur. An incision was made in the lateral patello-femoral ligament, sparing the knee joint and then the lateral condyle of the femur was resected. A single 3 to 4 mm tumor fragment was implanted orthotopically into this space to establish a PDOX model. The muscle and wound was closed with 6–0 nylon suture (Ethilon, Ethicon, Inc., NJ, USA) (Fig. 1).
Treatment design
Primary osteosarcomas are fed by an artery. Therefore, in the present study, we compared i.a. administration of S. typhimurium A1-R with i.v. administration in the osteosarcoma PDOX.
The PDOX models were randomized into the following groups when tumor volume reached 100 mm3: G1, control without treatment, n = 8; G2, CDDP (6 mg/kg, intraperitoneal (i.p.) injection, weekly, for 2 weeks, n = 8); G3, i.v. injection of S. typhimurium A1-R (5 × 107 CFU/100 μl, i.v., weekly, for 2 weeks, n = 8); G4, i.a. injection of S. typhimurium A1-R (5 × 105 CFU/100 μl, i.a., once, n = 8). Tumor length, width and mouse body weight were measured twice in a week. Tumor volume was calculated by following formula: Tumor volume (mm3) = length (mm) × width (mm) × width (mm) × 1/2. Data are presented as mean ± SD.
Preparation and administration of S. typhimurium A1-R
GFP-expressing S. typhimurium A1-R bacteria (AntiCancer Inc.,) were grown overnight in LB medium (Fisher Sci., Hanover Park, IL, USA) and then diluted 1:10 in LB medium. Bacteria were harvested at late-log phase, washed with PBS, and then diluted in PBS.22-24
i.a. injection of S. typhimurium A1-R
Nude mice were anesthetized with the ketamine mixture as described above and placed in a right lateral decubitus position. A 20 mm skin incision was made on the left lateral abdomen followed by exposure of the abdominal aorta. S. typhimurium A1-R (5 × 105 CFU in 100 μl PBS) was injected in the abdominal aorta using a 31G needle. After removal of the needle, bleeding was stopped by gently pressing the puncture site with a cotton swab. After injection, the abdomen was closed with non-absorbable sutures.
Confocal microscopy
The FV1000 confocal microscope (Olympus, Tokyo, Japan) was used for high-resolution imaging. Fluorescence images were obtained using the 20 × /0.50 UPlan FLN and 40 × /1.3 oil Olympus UPLAN FLN objectives.45
Distribution of S. typhimurium A1-R
Twelve PDOX mouse models were treated with i.v. administration of S. typhimurium A1-R (5 × 107 CFU/100 μl, i.v., once) or i.a. administration of S. typhimurium A1-R (5 × 105 CFU/100 μl, i.a., once) when the tumor volume reached 500 mm3, (n = 6 mice each). PDOX tumors or the tibialis anterior muscle of the affected limb were resected on day 3. The distribution of S. typhimurium A1-R was determined by confocal imaging with the FV1000.46 Three random fields were accessed in each specimen.
Histological analysis
Fresh tumor samples were fixed in 10% formalin and embedded in paraffin before sectioning and staining. Tissue sections (3 μm) were deparaffinized in xylene and rehydrated in an ethanol series. Hematoxylin and eosin (H&E) staining was performed according to standard protocol. Histological examination was performed with a BHS system microscope. Images were acquired with INFINITY ANALYZE software (Lumenera Corporation, Ottawa, Canada).
Conclusions
The new model of i.a. administration of S. typhimurium A1-R has great promise for the treatment of recalcitrant osteosarcoma.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Hiroshima Y, Zhang Y, Murakami T, Maawy AA, Miwa S, Yamamoto M, Yano S, Sato S, Momiyama M, Mori R, et al.. Efficacy of tumor-targeting Salmonella typhimurium A1-R in combination with anti-angiogenesis therapy on a pancreatic cancer patient-derived orthotopic xenograph (PDOX) and cell line mouse models. Oncotarget 2014; 5:12346-57; PMID:25402324; https://doi.org/ 10.18632/oncotarget.2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fu X, Guadagni F, Hoffman RM. A metastatic nude-mouse model of human pancreatic cancer constructed orthotopically with histologically intact patient specimens. Proc Natl Acad Sci USA 1992; 89:5645-9; PMID:1608975; https://doi.org/ 10.1073/pnas.89.12.5645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hiroshima Y, Maawy A, Zhang Y, Murakami T, Momiyama M, Mori R, Matsuyama R, Katz MH, Fleming JB, Chishima T, et al.. Metastatic recurrence in a pancreatic cancer patient derived orthotopic xenograft (PDOX) nude mouse model is inhibited by neoadjuvant chemotherapy in combination with fluorescence-guided surgery with an anti-CA 19–9-conjugated fluorophore. Plos One 2014; 9:e114310; PMID:25463150; https://doi.org/ 10.1371/journal.pone.0114310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hiroshima Y, Maawy AA, Katz MH, Fleming JB, Bouvet M, Endo I, Hoffman RM. Selective efficacy of zoledronic acid on metastasis in a patient-derived orthotopic xenograph (PDOX) nude-mouse model of human pancreatic cancer. J Surg Oncol 2015; 111:311-15; PMID:25394368; https://doi.org/ 10.1002/jso.23816 [DOI] [PubMed] [Google Scholar]
- [5].Fu X, Le P, Hoffman RM. A metastatic-orthotopic transplant nude-mouse model of human patient breast cancer. Anticancer Res 1993; 13:901-4; PMID:8352558 [PubMed] [Google Scholar]
- [6].Fu X, Hoffman RM. Human ovarian carcinoma metastatic models constructed in nude mice by orthotopic transplantation of histologically-intact patient specimens. Anticancer Res 1993; 13:283-6; PMID:8517640 [PubMed] [Google Scholar]
- [7].Wang X, Fu X, Hoffman RM. A new patient-like metastatic model of human lung cancer constructed orthotopically with intact tissue via thoracotomy in immunodeficient mice. Int J Cancer 1992; 51:992-5; PMID:1639545; https://doi.org/ 10.1002/ijc.2910510621 [DOI] [PubMed] [Google Scholar]
- [8].Hiroshima Y, Zhang Y, Zhang M, Maawy A, Mii S, Yamamoto M, Uehara F, Miwa S, Yano S, Murakami T, et al.. Establishment of a patient-derived orthotopic xenograph (PDOX) model of HER-2-positive cervical cancer expressing the clinical metastatic pattern. PLOS ONE 2015; 10:e0117417; PMID:25689852; https://doi.org/ 10.1371/journal.pone.0117417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fu X, Besterman JM, Monosov A, Hoffman RM. Models of human metastatic colon cancer in nude mice orthotopically constructed by using histologically intact patient specimens. Proc Natl Acad Sci USA 1991; 88:9345-9; PMID:1924398; https://doi.org/ 10.1073/pnas.88.20.9345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Metildi CA, Kaushal S, Luiken GA, Talamini MA, Hoffman RM, Bouvet M. Fluorescently-labeled chimeric anti-CEA antibody improves detection and resection of human colon cancer in a patient-derived orthotopic xenograft (PDOX) nude mouse model. J Surg Oncol 2014; 109:451-8; PMID:24249594; https://doi.org/ 10.1002/jso.23507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hiroshima Y, Maawy A, Metildi CA, Zhang Y, Uehara F, Miwa S, Yano S, Sato S, Murakami T, Momiyama M, et al.. Successful fluorescence-guided surgery on human colon cancer patient-derived orthotopic xenograft mouse models using a fluorophore-conjugated anti-CEA antibody and a portable imaging system. J Laparoendosc Adv Surg Tech A 2014; 24:241-7; PMID:24494971; https://doi.org/ 10.1089/lap.2013.0418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Furukawa T, Kubota T, Watanabe M, Kitajima M, Hoffman RM. Orthotopic transplantation of histologically intact clinical specimens of stomach cancer to nude mice: correlation of metastatic sites in mouse and individual patient donors. Int J Cancer 1993; 53:608-12; PMID:8436434; https://doi.org/ 10.1002/ijc.2910530414 [DOI] [PubMed] [Google Scholar]
- [13].Murakami T, DeLong J, Eilber FC, Zhao M, Zhang Y, Zhang N, Singh A, Russell T, Deng S, Reynoso J, et al.. Tumor-targeting Salmonella typhimurium A1-R in combination with doxorubicin eradicate soft tissue sarcoma in a patient-derived orthotopic xenograft PDOX model. Oncotarget 2016; 7:12783-90; PMID:26859573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hiroshima Y, Zhao M, Zhang Y, Zhang N, Maawy A, Murakami T, Mii S, Uehara F, Yamamoto M, Miwa S, Yano S, et al.. Tumor-targeting Salmonella typhimurium A1-R arrests a chemo-resistant patient soft-tissue sarcoma in nude mice. PLoS One 2015; 10:e0134324; PMID:26237416; https://doi.org/ 10.1371/journal.pone.0134324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kiyuna T, Murakami T, Tome Y, Kawaguchi K, Igarashi K, Zhang Y, Zhao M, Li Y, Bouvet M, Kanaya F, et al.. High efficacy of tumor-targeting Salmonella typhimurium A1-R on a doxorubicin- and dactolisib-resistant follicular dendritic-cell sarcoma in a patient-derived orthotopic xenograft PDOX nude mouse model. Oncotarget 2016; 7:33046-54; PMID:27105519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Murakami T, Singh AS, Kiyuna T, Dry SM, Li Y, James AW, Igarashi K, Kawaguchi K, DeLong JC, Zhang Y, et al.. Effective molecular targeting of CDK4/6 and IGF-1R in a rare FUS-ERG fusion CDKN2A-deletion doxorubicin-resistant Ewing's sarcoma in a patient-derived orthotopic xenograft (PDOX) nude-mouse model. Oncotarget 2016; 7:47556-64; PMID:27286459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hiroshima Y, Zhang Y, Zhang N, Uehara F, Maawy A, Murakami T, Mii S, Yamamoto M, Miwa S, Yano S, et al.. Patient-derived orthotopic xenograft (PDOX) nude mouse model of soft-tissue sarcoma more closely mimics the patient behavior in contrast to the subcutaneous ectopic model. Anticancer Res 2015; 35:697-701; PMID:25667448 [PubMed] [Google Scholar]
- [18].Yamamoto M, Zhao M, Hiroshima Y, Zhang Y, Shurell E, Eilber FC, Bouvet M, Noda M, Hoffman RM. Efficacy of tumor-targeting Salmonella typhimurium A1-R on a melanoma patient-derived orthotopic xenograft (PDOX) nude-mouse model. PLoS One 2016; 11:e0160882; PMID:27500926; https://doi.org/ 10.1371/journal.pone.0160882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kawaguchi K, Murakami T, Chmielowski B, Igarashi K, Kiyuna T, Unno M, Nelson SD, Russell TA, Dry SM, Li Y, et al.. Vemurafenib-resistant BRAF-V600E mutated melanoma is regressed by MEK targeting drug trametinib, but not cobimetinib in a patient-derived orthotopic xenograft (PDOX) mouse model. Oncotarget 2016; 7:71737-43; PMID:27690220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kawaguchi K, Igarashi K, Murakami T, Chmielowski B, Kiyuna T, Zhao M, Zhang Y, Singh A, Unno M, Nelson SD, et al.. Tumor-targeting Salmonella typhimurium A1-R combined with Temozolomide regresses malignant melanoma with a BRAF-V600 mutation in a patient-derived orthotopic xenograft (PDOX) model. Oncotarget 2016; 7:85929-36; PMID:27835903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Igarashi K, Murakami T, Kawaguchi K, Kiyuna T, Miyake K, Zhang Y, Nelson SD, Dry SM, Li Y, Yanagawa J, et al.. A patient-derived orthotopic xenograft (PDOX) mouse model of an osteosarcoma lung metastasis that recurred after cisplatinum treatment was resistant to cisplatinum but highly-sensitive to temozolomide and trabectedin. JCO Precision Oncol, submitted [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhao M, Yang M, Li X-M, Jiang P, Baranov E, Li S, Xu M, Penman S, Hoffman RM. Tumor-targeting bacterial therapy with amino acid auxotrophs of GFP-expressing Salmonella typhimurium. Proc Natl Acad Sci USA 2005; 102:755-60; PMID:15644448; https://doi.org/ 10.1073/pnas.0408422102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhao M, Geller J, Ma H, Yang M, Penman S, Hoffman RM. Monotherapy with a tumor-targeting mutant of Salmonella typhimurium cures orthotopic metastatic mouse models of human prostate cancer. Proc Natl Acad Sci USA 2007; 104:10170-4; PMID:17548809; https://doi.org/ 10.1073/pnas.0703867104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhao M, Yang M, Ma H, Li X, Tan X, Li S, Yang Z, Hoffman RM. Targeted therapy with a Salmonella typhimurium leucine-arginine auxotroph cures orthotopic human breast tumors in nude mice. Cancer Res 2006; 66:7647-52; PMID:16885365; https://doi.org/ 10.1158/0008-5472.CAN-06-0716 [DOI] [PubMed] [Google Scholar]
- [25].Zhang Y, Tome Y, Suetsugu A, Zhang L, Zhang N, Hoffman RM, Zhao M. Determination of the optimal route of administration of Salmonella typhimurium A1-R to target breast cancer in nude mice. Anticancer Res 2012; 32:2501-8; PMID:22753706 [PubMed] [Google Scholar]
- [26].Zhang Y, Miwa S, Zhang N, Hoffman RM, Zhao M. Tumor-targeting Salmonella typhimurium A1-R arrests growth of breast-cancer brain metastasis. Oncotarget 2015; 6:2615-22; PMID:25575815; https://doi.org/ 10.18632/oncotarget.2811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Uchugonova A, Zhao M, Zhang Y, Weinigel M, König K, Hoffman RM. Cancer-cell killing by engineered Salmonella imaged by multiphoton tomography in live mice. Anticancer Res 2012; 32:4331-9; PMID:23060555 [PubMed] [Google Scholar]
- [28].Liu F, Zhang L, Hoffman RM, Zhao M. Vessel destruction by tumor-targeting Salmonella typhimurium A1-R is enhanced by high tumor vascularity. Cell Cycle 2010; 9:4518-24; PMID:21135579; https://doi.org/ 10.4161/cc.9.22.13744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Nagakura C, Hayashi K, Zhao M, Yamauchi K, Yamamoto N, Tsuchiya H, Tomita K, Bouvet M, Hoffman RM. Efficacy of a genetically-modified Salmonella typhimurium in an orthotopic human pancreatic cancer in nude mice. Anticancer Res 2009; 29:1873-8; PMID:19528442 [PubMed] [Google Scholar]
- [30].Yam C, Zhao M, Hayashi K, Ma H, Kishimoto H, McElroy M, Bouvet M, Hoffman RM. Monotherapy with a tumor-targeting mutant of S. typhimurium inhibits liver metastasis in a mouse model of pancreatic cancer. J Surg Res 2010; 164:248-55; PMID:19766244; https://doi.org/ 10.1016/j.jss.2009.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hiroshima Y, Zhao M, Zhang Y, Maawy A, Hassanein MK, Uehara F, Miwa S, Yano S, Momiyama M, Suetsugu A, et al.. Comparison of efficacy of Salmonella typhimurium A1-R and chemotherapy on stem-like and non-stem human pancreatic cancer cells. Cell Cycle 2013; 12:2774-80; PMID:23966167; https://doi.org/ 10.4161/cc.25872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hiroshima Y, Zhao M, Maawy A, Zhang Y, Katz MH, Fleming JB, Uehara F, Miwa S, Yano S, Momiyama M, et al.. Efficacy of Salmonella typhimurium A1-R versus chemotherapy on a pancreatic cancer patient-derived orthotopic xenograft (PDOX). J Cell Biochem 2014; 115:1254-61; PMID:24435915; https://doi.org/ 10.1002/jcb.24769 [DOI] [PubMed] [Google Scholar]
- [33].Matsumoto Y, Miwa S, Zhang Y, Hiroshima Y, Yano S, Uehara F, Yamamoto M, Toneri M, Bouvet M, Matsubara H, et al.. Efficacy of tumor-targeting Salmonella typhimurium A1-R on nude mouse models of metastatic and disseminated human ovarian cancer. J Cell Biochem. 2014; 115:1996-2003; PMID:24924355 [DOI] [PubMed] [Google Scholar]
- [34].Matsumoto Y, Miwa S, Zhang Y, Zhao M, Yano S, Uehara F, Yamamoto M, Hiroshima Y, Toneri M, Bouvet M, et al.. Intraperitoneal administration of tumor-targeting Salmonella typhimurium A1-R inhibits disseminated human ovarian cancer and extends survival in nude mice. Oncotarget 2015; 6:11369-77; PMID:25957417; https://doi.org/ 10.18632/oncotarget.3607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yano S, Zhang Y, Zhao M, Hiroshima Y, Miwa S, Uehara F, Kishimoto H, Tazawa H, Bouvet M, Fujiwara T, et al.. Tumor-targeting Salmonella typhimurium A1-R decoys quiescent cancer cells to cycle as visualized by FUCCI imaging and become sensitive to chemotherapy. Cell Cycle 2014; 13:3958-63; PMID:25483077; https://doi.org/ 10.4161/15384101.2014.964115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hiroshima Y, Zhang Y, Zhao M, Zhang N, Murakami T, Maawy A, Mii S, Uehara F, Yamamoto M, Miwa S, et al.. Tumor-targeting Salmonella typhimurium A1-R in combination with Trastuzumab eradicates HER-2-positive cervical cancer cells in patient-derived mouse models. PLoS One 2015; 10:e0120358; PMID:26047477; https://doi.org/ 10.1371/journal.pone.0120358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Murakami T, Igarashi K, Kawaguchi K, Kiyuna T, Zhang Y, Zhao M, Hiroshima Y, Nelson SD, Dry SM, Li Y, et al.. Tumor-targeting Salmonella typhimurium A1-R regresses an osteosarcoma in a patient-derived xenograph model resistant to a molecular-targeting drug. Oncotarget 2017; 8:8035-42; PMID:28030831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kawaguchi K, Murakami T, Suetsugu A, Kiyuna T, Igarashi K, Hiroshima Y, Zhao M, Zhang Y, Bouvet M, Clary B, et al.. High-efficacy targeting of colon-cancer liver metastasis with Salmonella typhimurium A1-R via intra-portal-vein injection in orthotopic nude-mouse models. Oncotarget 2017; 8:19065-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Blagosklonny MV. Matching targets for selective cancer therapy. Drug Discov Today 2003; 8:1104-7; PMID:14678733; https://doi.org/ 10.1016/S1359-6446(03)02806-X [DOI] [PubMed] [Google Scholar]
- [40].Blagosklonny MV. Teratogens as anti-cancer drugs. Cell Cycle 2005; 4:1518-21; PMID:16258270; https://doi.org/ 10.4161/cc.4.11.2208 [DOI] [PubMed] [Google Scholar]
- [41].Blagosklonny MV. Treatment with inhibitors of caspases, that are substrates of drug transporters, selectively permits chemotherapy-induced apoptosis in multidrug-resistant cells but protects normal cells. Leukemia 2001; 15:936-41; PMID:11417480; https://doi.org/ 10.1038/sj.leu.2402127 [DOI] [PubMed] [Google Scholar]
- [42].Blagosklonny MV. Target for cancer therapy: proliferating cells or stem cells. Leukemia 2006; 20:385-91; PMID:16357832; https://doi.org/ 10.1038/sj.leu.2404075 [DOI] [PubMed] [Google Scholar]
- [43].Apontes P, Leontieva OV, Demidenko ZN, Li F, Blagosklonny MV. Exploring long-term protection of normal human fibroblasts and epithelial cells from chemotherapy in cell culture. Oncotarget 2011; 2:222-33; PMID:21447859; https://doi.org/ 10.18632/oncotarget.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Blagosklonny MV. Tissue-selective therapy of cancer. Br J Cancer 2003; 89:1147-51; PMID:14520435; https://doi.org/ 10.1038/sj.bjc.6601256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Uchugonova A, Duong J, Zhang N, König K, Hoffman RM. The bulge area is the origin of nestin-expressing pluripotent stem cells of the hair follicle. J Cell Biochem 2011; 112:2046-50; PMID:21465525; https://doi.org/ 10.1002/jcb.23122 [DOI] [PubMed] [Google Scholar]
- [46].Kawaguchi K, Igarashi K, Murakami T, Chmielowski B, Kiyuna T, Zhao M, Zhang Y, Singh A, Unno M, Nelson SD, et al.. Tumor-targeting Salmonella typhimurium A1-R combined with temozolomide regresses malignant melanoma with a BRAF-V600E mutation in a patient-derived orthotopic xenograft (PDOX) model. Oncotarget 2016; 7(52):85929-36 [DOI] [PMC free article] [PubMed] [Google Scholar]


