ABSTRACT
Cohesin is a ring-shaped protein complex which comprises the Smc1, Smc3 and Scc1 subunits. It topologically embraces chromosomal DNA to connect sister chromatids and stabilize chromatin loops. It is required for proper chromosomal segregation, DNA repair and transcriptional regulation. We have recently reported that cohesin rings can adopt a “collapsed” rod-like conformation which is driven by the interaction between the Smc1 and Smc3 coiled coil arms and is regulated by post-translational modifications. The “collapsed” conformation plays a role in cohesin ring assembly and its loading on the DNA. Here we speculate about the mechanism of cohesin's conformational transitions in relation to its loading on the DNA and draw parallels with other Smc-like complexes.
KEYWORDS: acetyltransferase, ATPase, chromosomes, cohesin, sister chromatid cohesion
Introduction
In eukaryotic cells, chromosomal DNA is threaded through the proteinaceous cohesin rings. A fraction of cohesin rings embrace 2 sister DNA molecules and function in sister chromatid cohesion. Cohesion between sister chromatids is essential for their proper segregation to daughter cells during cell division1 and for double-strand break repair by homologous recombination.2 Other cohesins stabilize loops within the same chromatid to define the boundaries of chromatin domains and regulate transcription.3 A topological mode of association between cohesin rings and the DNA.4,5 is thought to provide a very stable connection between 2 DNA molecules or 2 parts of the same DNA molecule while the very large size of the rings, which are estimated to be about 35 nm in diameter, ensures minimal interference with DNA metabolism. However, the molecular mechanism of how the cohesin ring entraps sister chromatids remains to be unraveled.
The cohesin ring is composed of the Smc1, Smc3 and Scc1 subunits and associates with the additional Scc3, Pds5 and Wpl1 proteins.6 The Smc1 and Smc3 fold into long anti-parallel intramolecular coiled coils, or arms, which form the circumference of the ring. The coiled coils are flanked by the globular hinge and head domains. The head is composed of the N- and C-terminal regions of the Smc protein which fold together into an ABC-type ATPase domain. The Smc1 and Smc3 heads can sandwich and hydrolyze 2 molcules of ATP and are connected by the Scc1 subunit. The Smc1 and Smc3 hinges associate with each other thus completing the ring. While the interaction between the hinge domains is very stable,7 the heads have a very low affinity for each other even in the presence of non-hydrolyzable ATP.8 This raises the question of how the rings get assembled in the first place.
The “collapsed” form of cohesin ring
We have recently uncovered evidence that the arms of budding yeast Smc1 and Smc3 associate with each other along their lengths, which brings the heads together in the correct orientation and allows them to be connected by Scc1.9 Amino acid substitutions in the coiled coils, which affect association between the Smc arms, disrupt Scc1 recruitment and result in lethality. The Smc coiled coils appear to be highly flexible. The flexibility is probably enhanced by the disordered gaps which intersperse with the coiled coil stretches and can be identified by amino acid sequence analysis. However, when the Smc1 and Smc3 arms are bound together, they form a stable rod-like structure, which we were able to observe by scanning force microscopy (SFM) in solution. The arms in the rod appeared to be tightly intertwined and the overall structure was very compact. Our results are supported by the report from Peters' laboratory that the cohesin coiled coil arms could be cross-linked using a chemical cross-linker indicating their physical proximity to each other.10 Very recently, the rod-like form of human cohesin was also observed by electron microscopy.11 Our ability to follow the individual cohesin complexes over time in solution using SFM further established that the association between the Smc1 and Smc3 arms is not caused by the complexes drying out during the preparation of the sample for electron or scanning force microscopy. The “collapsed” rod-like form of the cohesin ring is likely to facilitate its assembly, intracellular transport and import inside the nucleus and to play a role during cohesin loading on the DNA. Conversely, the Smc arms would need to separate to let the DNA inside the cohesin ring.
The enigmatic role of ATP hydrolysis
The cohesin ring is assembled before its loading on the DNA and therefore an interface between either the heads or the hinges needs to open for the DNA to be imported inside it. Evidence that blocking the opening of the interface between the Smc1 and Smc3 hinge domains prevents the loading of cohesin onto DNA was interpreted to indicate that this interface serves as an entry gate.12 Since loading of cohesin on the DNA in vivo requires ATP hydrolysis by the heads,8,13,14 it was hypothesized that the role of ATP hydrolysis is to facilitate the dissociation of the Smc1 and Smc3 hinge domains. Until now, it was difficult to envision the mechanistic connection between the heads and the hinges since they are separated by a very long coiled coil and do not bind to each other when expressed as individual domains. Our observation of the collapsed form of cohesin ring suggests that the cycle of ATP binding/ATP hydrolysis, which controls engagement/disengagement of the heads,15 is likely to affect the degree to which the arms are coiled around each other. ATP-dependent head engagement promotes dissolution of the Bacillus subtilis Smc rod and is antagonized by the hinge dimerization.16,17 Changes in the degree of ring supercoiling might be the means to transmit the conformational change from the heads to the hinge and force the hinge to open. The cohesin loader protein Scc2, which makes contacts with the Smc arms and with other regions of cohesin,18-21 might also be involved in this process (Fig. 1A). Alternatively, the hinge needs to open and close in the process of unraveling the Smc arms and the requirement for ATP hydrolysis stems at least partially from the necessity to separate the arms to let the DNA inside the ring. The recent development of an in vitro DNA loading assay with purified cohesin and cohesin loader should make it possible to address these hypotheses.19 Interestingly, small quantities of fission yeast19 and human22 cohesin can both be loaded onto DNA in the absence of ATP and the cohesin loader in vitro. The low level of DNA binding of fission yeast cohesin observed in the absence of the cohesin loader is sensitive to DNA linearization, suggesting that cohesin can load topologically without requirement for the cohesin loader in vitro.19 However, loading of fission yeast but not human cohesin was greatly stimulated by ATP and the cohesin loader.19,22,23 Attempts to load budding yeast cohesin have been unsuccessful. It is conceivable that cohesin preparations from different species, which are used for the experiments in vitro, differ in relative amounts of different cohesin conformations, e.g., O-shaped vs rod-like. Purified human cohesin is predominantly O-shaped when visualized by rotary-shadowing electron microscopy10,11 although a variety of conformations, including rods, were observed by negative-stain electron microscopy.11 Budding yeast cohesin is mostly rod-like.9 While we are not aware of any electron microscopy studies of fission yeast cohesin, it is tempting to speculate that this preparation might be comprised predominantly of the partially collapsed rings. It remains to be investigated whether stability of the rod-like conformation of cohesin varies among species and whether this could contribute to the species-specific differences in the efficiency of cohesin loading in vitro.
Figure 1.
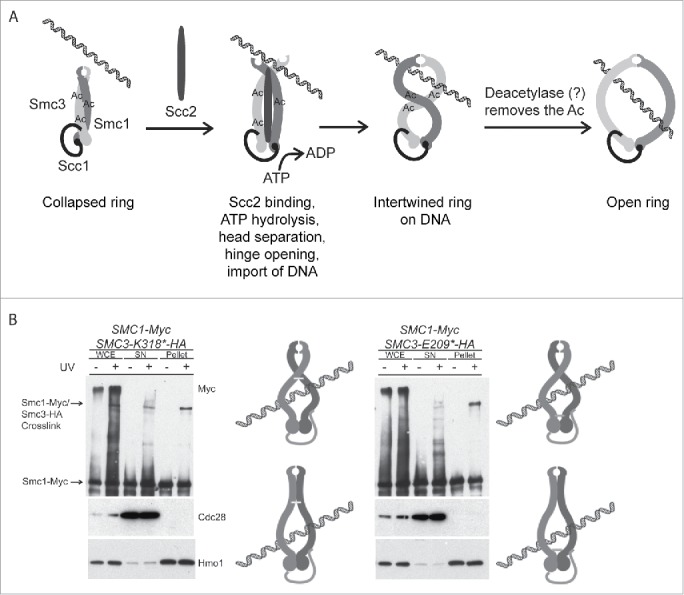
Collapsed conformation of cohesin ring is involved in cohesin complex assembly and its loading on the DNA. (A) Schematic illustrating a hypothetical mechanism of cohesin loading on the DNA. Smc1 and Smc3 arms are likely to be acetylated during or after protein synthesis. The post-translationally modified arms intertwine, bringing the head domains together and facilitating their tethering via Scc1. ATP hydrolysis by the heads leads to their separation from each other while still being connected by Scc1. Head separation results in the unraveling of the coiled coils, which might be facilitated by the binding of the cohesin loader Scc2 and the opening of the cohesin ring at the hinge. The opened hinge binds to DNA, which leads to DNA being imported into the ring. After the cohesin ring embraces the DNA, the Smc arms can still interact with each other, restricting the ring opening and inhibiting its ability to slide along the DNA. The interaction between the arms might be regulated by chromatin-associated acetylases/deacetylases. (B) The Smc arms can be cross-linked on DNA. Strains expressing a photo-crosslinkable version of Smc3 with K318 (mid-coiled coil) or E209 (head-proximal) substituted for p-benzoylphenylalanine (BPA) were UV-irradiated, cell lysates were fractionated into the supernatant and chromatin-containing pellet and Smc1-Myc was detected by western blot. Cdc28 and Hmo1 were detected as the markers of the soluble and chromatin-associated fractions, respectively.
Conformational transitions in Rad50/Mre11 vs cohesin
Rod-like forms were reported for cohesin-related bacterial Smc complexes and eukaryotic condensin.16,24-26 Thus, it is likely that all protein complexes containing Smc-like subunits are able to transition between the “arms together” and “arms apart” forms and this transition is important for their function. The long-range allosteric regulation involving coiled coils was studied in the cohesin-related Rad50/Mre11 complex which tethers the ends of broken DNA molecules to facilitate DNA repair. Similar to cohesin's Smc proteins, Rad50 folds into a long intramolecular coiled coil with globular N- and C-terminal domains. However, instead of a hinge domain, Rad50 contains a central CXXC motif which is called a “zinc hook” and, like the hinge, is also capable of dimerizing. Similar to Scc1, which binds to the Smc3 head-proximal coiled coil via its N-terminal region27 and to the Smc1 head via its C-terminus,28 Mre11 interacts with the Rad50 head and the head-proximal region of the coiled coil.29-33 However, mutations of the hook disrupted Rad50 interaction with Mre11.34 Truncation of the hook-proximal Rad50 coiled coil was reported to have a modest effect on sister chromatid recombination but, surprisingly, severely impaired non-homologous end-joining which is thought to be executed by the Rad50 globular domain. It was proposed that changes in the distal regions of the coiled coils might perturb the mutual orientation of the globular head domains.35 These results parallel our observation that amino acid substitutions in the Smc coiled coils abolished the recruitment of Scc1, although the sites of Scc1 binding remained unchanged.9 The most likely explanation is that altered interaction between the Smc1 and Smc3 coiled coils resulted in an incorrect head orientation which precluded them from being connected by Scc1. Conversely, crystal structures of the Rad50 head/Mre11 complex demonstrated that conformational changes accompanying ATP binding by the globular domain reposition the coiled coils, changing the angle at their base.29-33 This is consistent with our hypothesis that the cycle of ATP binding/ATP hydrolysis might be coupled to the unraveling of the Smc arms. When human Rad50/Mre11 complex is bound to DNA via its globular domain, the coiled coils are in the “parallel” conformation and move synchronously. This orientation of the coiled coils promotes tethering of the DNA ends by Rad50/Mre11 complexes bound to different DNA molecules. Upon release from the DNA, the Rad50/Mre11 complex assumes an open conformation in which the coiled coils bend independently of each other in either an open or a closed ring-like structure depending on the intra-complex hook dimerization.36 The conformational cross-talk between the globular and coiled coil domains is likely to be critical for the function of both Rad50/Mre11 and cohesin.
“Collapsed” rings on DNA
An interesting question is whether the collapsed rod-like cohesin rings exist only before loading on the DNA or whether the Smc arms can associate with each other even after the DNA had been captured inside the ring. We were able to crosslink the arms of chromatin-bound cohesins in vivo (Fig. 1B), which suggests that at least some of the chromosomal cohesins have their Smc arms contacting each other and might be in the partially collapsed conformation. This observation is in agreement with the recent reports from Koshland's and Peters' groups that fission yeast and human cohesins, which are loaded on the DNA in vitro, can diffuse past DNA-bound proteins smaller than approximately 11 nm but are blocked by the particles of approximately 21 nm.22,23 Both groups concluded that the diameters of the cohesin rings, which had been loaded on the DNA in vitro, appear to be smaller than the diameter of an O-form of cohesin ring, which was estimated to be approximately 35 nm.10,24 In the in vitro assays, the presumably partially collapsed rings were pushed by the T7 RNA polymerase and the FtsK motor protein. A collapsed ring on the DNA is likely to present an obstacle to transcription, replication, and repair in vivo and might contribute to the reported roles of cohesin in regulating transcriptional elongation37,38 and blocking DNA synthesis in the presence of radiation-induced damage.39-41
Role of post-translational modifications
We have demonstrated that the ability of the Smc arms to associate with each other depends on acetylation and possibly other post-translational modifications of the coiled coil lysines. A coiled coil is composed of 2 intertwined α-helices which display a 7 amino acid periodicity in their pattern of hydrophobicity and are held together by a hydrophobic core of amino acids at positions a and d. Side chains of positively charged lysines mostly face the solvent and can be potentially involved in interaction with other proteins, e.g., the coiled coil of the neighboring Smc. Notably, mass-spectrometry identified multiple acetylated and ubiquitylated lysines in the Smc arms. The conservative substitution of lysines for arginines would be expected to have little or no effect on the coiled coil fold but would abolish the post-translational modifications. Remarkably, we were able to show that substitution of an increasing number of lysines for arginines in the Smc1 and Smc3 coiled coils resulted in incremental cohesion defects and eventually in lethality. At the molecular level, mutant cohesins were defective in loading on the DNA and, as more lysines were mutated, lost the ability to recruit Scc1 and connect the Smc heads. Arginine mutants were observed by scanning force microscopy to display altered packing of the Smc arms and treatment of recombinant wild-type Smc1/Smc3 dimers with deacetylase facilitated the arms' separation.9 A known example when amino acid charge neutralization by post-translational modifications affects the interaction interface between the long coiled coils is the bacterial chemotactic receptor.42 In this system, methylation of 4 glutamate residues is thought to increase the packing of the 4-helix bundle generating a conformational adaptation signal which can be transmitted along the coiled coils.43
The identity of the acetyltransferases involved in modifying the Smc arms remains to be established. We have discovered that deletions of certain N-terminal acetyltransferases, nat3 (naa20) and ard1 (naa10), result in synthetic sickness when combined with the smc arginine mutants.9 The N-terminal acetyltransferases are known to associate with the ribosomes44 and could potentially modify Smc1 and Smc3 proteins co-translationally, which would facilitate their heterodimerisation, binding of Scc1 and transport into the nucleus. In addition, human Ard1 was found to translocate into the nucleus.45 However, the reports on the ability of N-terminal acetyltransferases to modify internal lysines46-49 remain controversial,50,51 and our attempts to acetylate Smc proteins in vitro using recombinant Nat3/Mdm20 and Ard1/Nat1 N-terminal acetyltransferases were unsuccessful. It is conceivable that it would be necessary to couple an acetylation assay to an in vitro translation system.
Remarkably, collapsed rings are also found in association with the chromosomes. Since the transition between the collapsed rod-like and the O-forms of cohesin rings is likely to be regulated by post-translational modifications, chromatin-associated lysine acetyltransferases and deacetylases might be able to regulate the extent of Smc arms' interaction and hence the diameter of the cohesin ring and its ability to slide along the DNA which would in turn affect transcription and repair. Modifying enzymes might also either facilitate or impair cohesin loading at certain genomic loci. This exciting possibility awaits further investigation.
Perspectives
The collapsed rod-like form of the cohesin ring is a functionally important conformation shared by the related protein complexes containing Smc-like proteins. Understanding the role of ATP hydrolysis in the conformational transitions of the cohesin complex will be critical for delineating the molecular mechanism of its loading on the DNA. The combination of the recently established in vitro DNA loading assays and advanced microscopy should provide important insights. The identification of modifying enzymes, which stabilize certain conformations of cohesin is another exciting field of discovery.
Rephrasing the quote from the English playwright Colley Cibber “Oh! How many torments lie in the small circle of a wedding ring!” the “torments” of the cohesin ring bring about unexpected twisted conformations, which are inseparable from its function.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
The work described in the original paper was supported by the Biomedical Research Council of A*STAR (Agency for Science, Technology and Research), Singapore Ministry of Education Academic Research Fund Tier 3 (MOE2012-T3–1–001) to Jie Yan, and the National Research Foundation of Singapore through the Mechanobiology Institute at National University of Singapore to Jie Yan.
References
- [1].Marston AL. Chromosome segregation in budding yeast: sister chromatid cohesion and related mechanisms. Genetics 2014; 196:31-63; PMID:24395824; https://doi.org/ 10.1534/genetics.112.145144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dorsett D, Strom L. The ancient and evolving roles of cohesin in gene expression and DNA repair. Curr Biol: CB 2012; 22:R240-50; PMID:22497943; https://doi.org/ 10.1016/j.cub.2012.02.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Merkenschlager M, Nora EP. CTCF and Cohesin in Genome Folding and Transcriptional Gene Regulation. Annu Rev Genomics Hum Genet 2016; 17:17-43; PMID:27089971; https://doi.org/ 10.1146/annurev-genom-083115-022339 [DOI] [PubMed] [Google Scholar]
- [4].Ivanov D, Nasmyth K. A topological interaction between cohesin rings and a circular minichromosome. Cell 2005; 122:849-60; PMID:16179255; https://doi.org/ 10.1016/j.cell.2005.07.018 [DOI] [PubMed] [Google Scholar]
- [5].Haering CH, Farcas AM, Arumugam P, Metson J, Nasmyth K. The cohesin ring concatenates sister DNA molecules. Nature 2008; 454:297-301; PMID:18596691; https://doi.org/ 10.1038/nature07098 [DOI] [PubMed] [Google Scholar]
- [6].Gligoris T, Lowe J. Structural Insights into Ring Formation of Cohesin and Related Smc Complexes. Trends Cell Biol 2016; 26:680-93; PMID:27134029; https://doi.org/ 10.1016/j.tcb.2016.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kurze A, Michie KA, Dixon SE, Mishra A, Itoh T, Khalid S, Strmecki L, Shirahige K, Haering CH, Löwe J, et al.. A positively charged channel within the Smc1/Smc3 hinge required for sister chromatid cohesion. EMBO J 2011; 30:364-78; PMID:21139566; https://doi.org/ 10.1038/emboj.2010.315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hu B, Itoh T, Mishra A, Katoh Y, Chan KL, Upcher W, Godlee C, Roig MB, Shirahige K, Nasmyth K. ATP hydrolysis is required for relocating cohesin from sites occupied by its Scc2/4 loading complex. Curr Biol : CB 2011; 21:12-24; PMID:21185190; https://doi.org/ 10.1016/j.cub.2010.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kulemzina I, Ang K, Zhao X, Teh JT, Verma V, Suranthran S, Chavda AP, Huber RG, Eisenhaber B, Eisenhaber F, et al.. A Reversible Association between Smc Coiled Coils Is Regulated by Lysine Acetylation and Is Required for Cohesin Association with the DNA. Mol Cell 2016; 63:1044-54; PMID:27618487; https://doi.org/ 10.1016/j.molcel.2016.08.008 [DOI] [PubMed] [Google Scholar]
- [10].Huis in 't Veld PJ, Herzog F, Ladurner R, Davidson IF, Piric S, Kreidl E, Bhaskara V, Aebersold R, Peters JM. Characterization of a DNA exit gate in the human cohesin ring. Science 2014; 346:968-72; PMID:25414306; https://doi.org/ 10.1126/science.1256904 [DOI] [PubMed] [Google Scholar]
- [11].Hons MT, Huis In 't Veld PJ, Kaesler J, Rombaut P, Schleiffer A, Herzog F, Stark H, Peters JM. Topology and structure of an engineered human cohesin complex bound to Pds5B. Nat Commun 2016; 7:12523; PMID:27549742; https://doi.org/ 10.1038/ncomms12523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gruber S, Arumugam P, Katou Y, Kuglitsch D, Helmhart W, Shirahige K, Nasmyth K. Evidence that loading of cohesin onto chromosomes involves opening of its SMC hinge. Cell 2006; 127:523-37; PMID:17081975; https://doi.org/ 10.1016/j.cell.2006.08.048 [DOI] [PubMed] [Google Scholar]
- [13].Arumugam P, Gruber S, Tanaka K, Haering CH, Mechtler K, Nasmyth K. ATP hydrolysis is required for cohesin's association with chromosomes. Curr Biol : CB 2003; 13:1941-53; PMID:14614819; https://doi.org/ 10.1016/j.cub.2003.10.036 [DOI] [PubMed] [Google Scholar]
- [14].Weitzer S, Lehane C, Uhlmann F. A model for ATP hydrolysis-dependent binding of cohesin to DNA. Curr Biol: CB 2003; 13:1930-40; PMID:14614818; https://doi.org/ 10.1016/j.cub.2003.10.030 [DOI] [PubMed] [Google Scholar]
- [15].Huber RG, Kulemzina I, Ang K, Chavda AP, Suranthran S, Teh JT, Kenanov D, Liu G, Rancati G, Szmyd R, et al.. Impairing Cohesin Smc1/3 Head Engagement Compensates for the Lack of Eco1 Function. Structure 2016; 24:1991-9; PMID:27692962; https://doi.org/ 10.1016/j.str.2016.09.001 [DOI] [PubMed] [Google Scholar]
- [16].Soh YM, Burmann F, Shin HC, Oda T, Jin KS, Toseland CP, Kim C, Lee H, Kim SJ, Kong MS, et al.. Molecular basis for SMC rod formation and its dissolution upon DNA binding. Mol Cell 2015; 57:290-303; PMID:25557547; https://doi.org/ 10.1016/j.molcel.2014.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Minnen A, Burmann F, Wilhelm L, Anchimiuk A, Diebold-Durand ML, Gruber S. Control of Smc Coiled Coil Architecture by the ATPase Heads Facilitates Targeting to Chromosomal ParB/parS and Release onto Flanking DNA. Cell Rep 2016; 14:2003-16; PMID:26904953; https://doi.org/ 10.1016/j.celrep.2016.01.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bermudez VP, Farina A, Higashi TL, Du F, Tappin I, Takahashi TS, Hurwitz J. In vitro loading of human cohesin on DNA by the human Scc2-Scc4 loader complex. Proc Natl Acad Sci U S A 2012; 109:9366-71; PMID:22628566; https://doi.org/ 10.1073/pnas.1206840109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Murayama Y, Uhlmann F. Biochemical reconstitution of topological DNA binding by the cohesin ring. Nature 2014; 505:367-71; PMID:24291789; https://doi.org/ 10.1038/nature12867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chao WC, Murayama Y, Munoz S, Jones AW, Wade BO, Purkiss AG, Hu XW, Borg A, Snijders AP, Uhlmann F, et al.. Structure of the cohesin loader Scc2. Nat Commun 2017; 8:13952; PMID:28059076; https://doi.org/ 10.1038/ncomms13952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kikuchi S, Borek DM, Otwinowski Z, Tomchick DR, Yu H. Crystal structure of the cohesin loader Scc2 and insight into cohesinopathy. Proc Natl Acad Sci U S A 2016; 113:12444-9; PMID:27791135; https://doi.org/ 10.1073/pnas.1611333113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Davidson IF, Goetz D, Zaczek MP, Molodtsov MI, Huis In 't Veld PJ, Weissmann F, Litos G, Cisneros DA, Ocampo-Hafalla M, Ladurner R, et al.. Rapid movement and transcriptional re-localization of human cohesin on DNA. The EMBO J 2016; 35:2671-85; PMID:27799150; https://doi.org/ 10.15252/embj.201695402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Stigler J, Camdere GO, Koshland DE, Greene EC. Single-Molecule Imaging Reveals a Collapsed Conformational State for DNA-Bound Cohesin. Cell Rep 2016; 15:988-98; PMID:27117417; https://doi.org/ 10.1016/j.celrep.2016.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Anderson DE, Losada A, Erickson HP, Hirano T. Condensin and cohesin display different arm conformations with characteristic hinge angles. J Cell Biol 2002; 156:419-24; PMID:11815634; https://doi.org/ 10.1083/jcb.200111002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Barysz H, Kim JH, Chen ZA, Hudson DF, Rappsilber J, Gerloff DL, Earnshaw WC. Three-dimensional topology of the SMC2/SMC4 subcomplex from chicken condensin I revealed by cross-linking and molecular modelling. Open Biol 2015; 5:150005; PMID:25716199; https://doi.org/ 10.1098/rsob.150005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yoshimura SH, Hizume K, Murakami A, Sutani T, Takeyasu K, Yanagida M. Condensin architecture and interaction with DNA: regulatory non-SMC subunits bind to the head of SMC heterodimer. Curr Biol: CB 2002; 12:508-13; PMID:11909539; https://doi.org/ 10.1016/S0960-9822(02)00719-4 [DOI] [PubMed] [Google Scholar]
- [27].Gligoris TG, Scheinost JC, Burmann F, Petela N, Chan KL, Uluocak P, Beckouët F, Gruber S, Nasmyth K, Löwe J. Closing the cohesin ring: structure and function of its Smc3-kleisin interface. Science 2014; 346:963-7; PMID:25414305; https://doi.org/ 10.1126/science.1256917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Haering CH, Schoffnegger D, Nishino T, Helmhart W, Nasmyth K, Lowe J. Structure and stability of cohesin's Smc1-kleisin interaction. Mol Cell 2004; 15:951-64; PMID:15383284; https://doi.org/ 10.1016/j.molcel.2004.08.030 [DOI] [PubMed] [Google Scholar]
- [29].Lammens K, Bemeleit DJ, Mockel C, Clausing E, Schele A, Hartung S, Schiller CB, Lucas M, Angermüller C, Söding J, et al.. The Mre11:Rad50 structure shows an ATP-dependent molecular clamp in DNA double-strand break repair. Cell 2011; 145:54-66; PMID:21458667; https://doi.org/ 10.1016/j.cell.2011.02.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mockel C, Lammens K, Schele A, Hopfner KP. ATP driven structural changes of the bacterial Mre11:Rad50 catalytic head complex. Nucleic Acids Res 2012; 40:914-27; PMID:21937514; https://doi.org/ 10.1093/nar/gkr749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Williams GJ, Williams RS, Williams JS, Moncalian G, Arvai AS, Limbo O, Guenther G, SilDas S, Hammel M, Russell P, et al.. ABC ATPase signature helices in Rad50 link nucleotide state to Mre11 interface for DNA repair. Nat Struct Mol Biol 2011; 18:423-31; PMID:21441914; https://doi.org/ 10.1038/nsmb.2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lim HS, Kim JS, Park YB, Gwon GH, Cho Y. Crystal structure of the Mre11-Rad50-ATPgammaS complex: understanding the interplay between Mre11 and Rad50. Genes Dev 2011; 25:1091-104; PMID:21511873; https://doi.org/ 10.1101/gad.2037811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Deshpande RA, Williams GJ, Limbo O, Williams RS, Kuhnlein J, Lee JH, Classen S, Guenther G, Russell P, Tainer JA, et al.. ATP-driven Rad50 conformations regulate DNA tethering, end resection, and ATM checkpoint signaling. The EMBO J 2014; 33:482-500; PMID:24493214; https://doi.org/ 10.1002/embj.201386100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hopfner KP, Craig L, Moncalian G, Zinkel RA, Usui T, Owen BA, Karcher A, Henderson B, Bodmer JL, McMurray CT, et al.. The Rad50 zinc-hook is a structure joining Mre11 complexes in DNA recombination and repair. Nature 2002; 418:562-6; PMID:12152085; https://doi.org/ 10.1038/nature00922 [DOI] [PubMed] [Google Scholar]
- [35].Hohl M, Kwon Y, Galvan SM, Xue X, Tous C, Aguilera A, Sung P, Petrini JH. The Rad50 coiled-coil domain is indispensable for Mre11 complex functions. Nat Struct Mol Biol 2011; 18:1124-31; PMID: 21892167; https://doi.org/ 10.1038/nsmb.2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Moreno-Herrero F, de Jager M, Dekker NH, Kanaar R, Wyman C, Dekker C. Mesoscale conformational changes in the DNA-repair complex Rad50/Mre11/Nbs1 upon binding DNA. Nature 2005; 437:440-3; PMID:16163361; https://doi.org/ 10.1038/nature03927 [DOI] [PubMed] [Google Scholar]
- [37].Fay A, Misulovin Z, Li J, Schaaf CA, Gause M, Gilmour DS, Dorsett D. Cohesin selectively binds and regulates genes with paused RNA polymerase. Curr Biol: CB 2011; 21:1624-34; PMID: 21962715; https://doi.org/ 10.1016/j.cub.2011.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Schaaf CA, Kwak H, Koenig A, Misulovin Z, Gohara DW, Watson A, Zhou Y, Lis JT, Dorsett D. Genome-wide control of RNA polymerase II activity by cohesin. PLoS Genet 2013; 9:e1003382; PMID:23555293; https://doi.org/ 10.1371/journal.pgen.1003382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kitagawa R, Bakkenist CJ, McKinnon PJ, Kastan MB. Phosphorylation of SMC1 is a critical downstream event in the ATM-NBS1-BRCA1 pathway. Genes Dev 2004; 18:1423-38; PMID:15175241; https://doi.org/ 10.1101/gad.1200304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kim ST, Xu B, Kastan MB. Involvement of the cohesin protein, Smc1, in Atm-dependent and independent responses to DNA damage. Genes Dev 2002; 16:560-70; PMID:11877376; https://doi.org/ 10.1101/gad.970602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Yazdi PT, Wang Y, Zhao S, Patel N, Lee EY, Qin J. SMC1 is a downstream effector in the ATM/NBS1 branch of the human S-phase checkpoint. Genes Dev 2002; 16:571-82; PMID:11877377; https://doi.org/ 10.1101/gad.970702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hazelbauer GL, Lai WC. Bacterial chemoreceptors: providing enhanced features to two-component signaling. Curr Opin Microbiol 2010; 13:124-32; PMID:20122866; https://doi.org/ 10.1016/j.mib.2009.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Starrett DJ, Falke JJ. Adaptation mechanism of the aspartate receptor: electrostatics of the adaptation subdomain play a key role in modulating kinase activity. Biochemistry 2005; 44:1550-60; PMID:15683239; https://doi.org/ 10.1021/bi048089z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gautschi M, Just S, Mun A, Ross S, Rucknagel P, Dubaquie Y, Ehrenhofer-Murray A, Rospert S. The yeast N(alpha)-acetyltransferase NatA is quantitatively anchored to the ribosome and interacts with nascent polypeptides. Mol Cell Biol 2003; 23:7403-14; PMID:14517307; https://doi.org/ 10.1128/MCB.23.20.7403-7414.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Park JH, Seo JH, Wee HJ, Vo TT, Lee EJ, Choi H, Cha JH, Ahn BJ, Shin MW, Bae SJ, et al.. Nuclear translocation of hARD1 contributes to proper cell cycle progression. PloS one 2014; 9:e105185; PMID:25133627; https://doi.org/ 10.1371/journal.pone.0105185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Jeong JW, Bae MK, Ahn MY, Kim SH, Sohn TK, Bae MH, Yoo MA, Song EJ, Lee KJ, Kim KW. Regulation and destabilization of HIF-1alpha by ARD1-mediated acetylation. Cell 2002; 111:709-20; PMID:12464182; https://doi.org/ 10.1016/S0092-8674(02)01085-1 [DOI] [PubMed] [Google Scholar]
- [47].Lim JH, Park JW, Chun YS. Human arrest defective 1 acetylates and activates beta-catenin, promoting lung cancer cell proliferation. Cancer Res 2006; 66:10677-82; PMID:17108104; https://doi.org/ 10.1158/0008-5472.CAN-06-3171 [DOI] [PubMed] [Google Scholar]
- [48].Evjenth R, Hole K, Karlsen OA, Ziegler M, Arnesen T, Lillehaug JR. Human Naa50p (Nat5/San) displays both protein N alpha- and N epsilon-acetyltransferase activity. J Biol Chem 2009; 284:31122-9; PMID:19744929; https://doi.org/ 10.1074/jbc.M109.001347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Chu CW, Hou F, Zhang J, Phu L, Loktev AV, Kirkpatrick DS, Jackson PK, Zhao Y, Zou H. A novel acetylation of beta-tubulin by San modulates microtubule polymerization via down-regulating tubulin incorporation. Mol Biol Cell 2011; 22:448-56; PMID:21177827; https://doi.org/ 10.1091/mbc.E10-03-0203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Arnesen T, Kong X, Evjenth R, Gromyko D, Varhaug JE, Lin Z, Sang N, Caro J, Lillehaug JR. Interaction between HIF-1 alpha (ODD) and hARD1 does not induce acetylation and destabilization of HIF-1 alpha. FEBS Lett 2005; 579:6428-32; PMID:16288748; https://doi.org/ 10.1016/j.febslet.2005.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Liszczak G, Arnesen T, Marmorstein R. Structure of a ternary Naa50p (NAT5/SAN) N-terminal acetyltransferase complex reveals the molecular basis for substrate-specific acetylation. J Biol Chem 2011; 286:37002-10; PMID:21900231; https://doi.org/ 10.1074/jbc.M111.282863 [DOI] [PMC free article] [PubMed] [Google Scholar]


