Supplemental digital content is available in the text.
Key Words: Cervical cancer, Prognosis, Stromal estrogen receptor α, Stromal progesterone receptor B
Abstract
Objectives
The aim of this study was to investigate the expression of estrogen receptor α (ERα) and progesterone receptor B (PRB) in the stroma and carcinoma tissues of cervical cancer and their relationship to clinical characteristics and the status of human papillomavirus (HPV) infection.
Methods
Expressional levels of ERα and PRB in tissue blocks of 95 cervical carcinomas were independently scored by 2 pathologists. Human papillomavirus DNA, viral load, and genotypes were determined by polymerase chain reaction. Clinical characteristics were reviewed from chart and cancer registry.
Results
Estrogen receptor α and PRB were mainly expressed in the stroma but not in the carcinoma tissues of the cervical cancer, and their expressions were highly correlated. More stromal ERαs were found in early-stage tumors than in advanced-stage tumors. Greater stromal expressions of ERα and PRB were associated with a more favorable prognosis (P = 0.018 and P = 0.004, respectively). The expressions were not related to the differentiation of cancer, the status of HPV infection, the HPV load, or the genotype. In multivariate analysis, stromal ERα and PRB expressions were independently associated with a lower risk of mortality. The adjusted hazard ratios of mortality for low and high expressions of ERα were 0.19 (95% confidential interval [95% CI], 0.04–0.87) and 0.15 (95% CI, 0.03–0.81), respectively, whereas for low and high expressions of PRB hazard ratios were 0.46 (95% CI, 0.19–1.16) and 0.24 (95% CI, 0.06–0.96), respectively.
Conclusions
This study showed that stromal ERα and PRB expressions are independent prognostic indicators of cervical squamous cell carcinoma.
The uterine cervix is a hormone-dependent organ that expresses both estrogen and progesterone receptors (ERs and PRs, respectively) in the epithelium and stroma.1–3 Large-scale epidemiologic studies have suggested that long-term exposure to endogenous and exogenous sex hormones, such as full-term pregnancies or oral contraceptives use, is an independent risk factor for cervical cancer and human papillomavirus (HPV) infection.4,5
Estrogen receptors are highly expressed in the endocervical mucosa irrespective of the phases of menstrual cycle and are moderately expressed by the basal and parabasal cells of the squamous epithelium in the exocervix, mainly during the proliferative phase of the menstrual cycle.6 Estrogen receptors are also expressed by metaplastic cells at the squamous-columnar junction.1 The expression pattern of PRs in the cervix is similar to that of ERs because it depends on the transactivation of estradiol-bound ER on the gene promoter. The only difference between PR and ER expression is that PRs are expressed by parabasal cells at the luteal phase, whereas ERs are not expressed during this phase.2 The expression of both ERs and PRs is down-regulated in cervical intraepithelial neoplasia and progressively decreases as lesion severity increases. Moreover, these receptors have been rarely detected in invasive squamous cell carcinoma1,3 and are less expressed in adenocarcinoma.7,8
Estrogen receptors are of 2 isoforms, namely, ERα and ERβ, with each being encoded by a different gene. Both ERα and ERβ form homodimers or heterodimers for interacting with estradiol and performing multiple functions.9 Estrogen receptor α is the most studied ER and is routinely examined in patients with breast and endometrial cancer. Progesterone receptors are also of 2 isoforms, namely, PR isoform B (PRB) and PR isoform A (PRA), which are encoded by a single gene and differ with respect to the presence and absence of an additional 5, exon, respectively. Although sharing many structural domains, the 2 isoforms are in fact 2 functionally distinct transcription factors, mediating their own response genes and physiological effects.10 Many studies and routine clinical examinations have detected both PRA and PRB in tissue by using nonspecific antibodies (Table S1, http://links.lww.com/IGC/A486).
Researchers have become increasingly aware of the crosstalk between cancer cells and neoplastic stromal cells.11 Tumor cells crosstalk with stroma cells to develop alterations in their shape and promote their attachment to other cells and extracellular matrix for invasion and metastasis.12–14 However, ER and PR expression status in tumor stromal cells during cervical cancer development is unknown. Previous studies have focused on the expression of PRs, especially on the expression of PRA (Table S2, http://links.lww.com/IGC/A487). Most importantly, all of the studies have focused on the expression of these receptors in neoplastic epithelium (Table S2).7,8,15–19 However, the expression of these receptors in tumor stromal cells has been rarely reported. The only study to examine ER and PR expressions in cervical cancer stroma reported frequent expression of ERα and PRA in stromal cells but not in carcinoma; however, in this study, no prognosis analysis correlated to ERα and PRA was reported.3
Recent transgenic studies on the HPV E6/E7 transformation of the mouse uterine cervix have reported that estradiol and ERα are required throughout the course of the carcinogenesis of the cervical epithelium, from the early formation of an atypical metaplastic lesion to the formation of low- and high-grade dysplasias and invasive carcinomas.20,21 Additional studies have clarified the specific location of ERα expression in the stroma, which is essential for the transformation in transgenic mice.22,23
The present study investigated ERα and PRB expressions in both the epithelium and stromal cells of cervical cancer and analyzed the association of their expressions with the clinicopathologic characteristics, HPV infection status, and disease outcomes.
MATERIALS AND METHODS
Patients and Methods
This study examined 95 patients who received a diagnosis of invasive cervical carcinoma at Buddhist Tzu Chi General Hospital in Hualien, Taiwan, during 2000–2006. Archived paraffin blocks of cervical cancer tissues were retrieved from surgical specimens obtained during radical hysterectomies, cervical conization, or cervical biopsies. These paraffin blocks were resectioned by the director of the pathology department (Y.H.H.) to confirm the presence of cervical cancer and adequate cancer stroma. If cervical cancer or adequate cancer stroma was not observed, the case was excluded. Two senior gynecological pathologists (C.C.S. and M.H.L.) from 2 different hospitals independently confirmed the histology of tumor tissues and the sufficiency of tumor stroma. Clinical characteristics, including patient age, International Federation of Gynecology and Obstetrics (FIGO) stage, cancer differentiation, and HPV infection status, were reviewed. All patients were retrospectively followed up for 5 years or until death. Because squamous cell carcinoma (SCC) and non-SCC may have quite different clinical characteristics, and there were only 13 patients with non-SCC in this study, we performed the correlational analysis base on the SCC patients. The study protocol was approved by the research ethics committee of Buddhist Tzu Chi General Hospital, Hualien, Taiwan (IRB102-129 and IRB103-139-A).
Immunohistochemistry
Estrogen receptor α and PRB expressions in formalin-fixed, paraffin-embedded primary cervical cancer tissues were analyzed by performing immunohistochemistry (IHC). The specimens were cut into 5-μm sections and mounted on slides. After deparaffinization in xylene, the slides were rehydrated in a graded alcohol series and placed in running water. The Novolink Polymer Detection System (Novocastra Laboratories, United Kingdom) was used for immunohistochemical detection. Briefly, antigens were retrieved by heating the slides in 10 mM citrate buffer (pH 6.0). Next, the slides were incubated with a peroxidase block to neutralize endogenous peroxidase activity. The slides were then treated with anti-ERα monoclonal antibody (dilution, 1:250; ab108398; Abcam) or anti-PR antibody (PRB-specific YR85; dilution, 1:100; ab32085; Abcam) for 30 minutes. Next, the slides were reacted with a Novolink polymer, followed by incubation with DAB chromogen solution, to induce peroxidase activity for visualization. Each IHC assay was performed in duplicate or triplicate, depending on the amount of tissue available.
IHC Scoring
Immunohistochemistry evaluation of ERα and PRB expressions was conducted independently by 2 experienced histopathologists (C.C.S. and M.H.L.) from 2 different hospitals. The staining of ERα and PRB in tumor cells and adjacent stroma was performed. Although polymorphonuclear cell infiltration on ER/PR evaluation is uncommon, this interference was considered by evaluating areas with low polymorphonuclear cell infiltration. The results were evaluated and scored using an immunoreactive scoring system.24 The percentage of positively stained cells was scored as 0 (0%), 1 (1%–10%), 2 (11%–50%), 3 (51%–80%), or 4 (>80%), and the intensity of staining was scored as 0 (none), 1 (weak), 2 (moderate), or 3 (strong). Multiplying these 2 scores yielded an immunoreactive score (IRS) of 0 to 12. Stratified comparison was performed by defining the following 3 categories (Fig. 1): no expression (IRS 0), low expression (IRS 1–5), and high expression (IRS ≥6).
FIGURE 1.
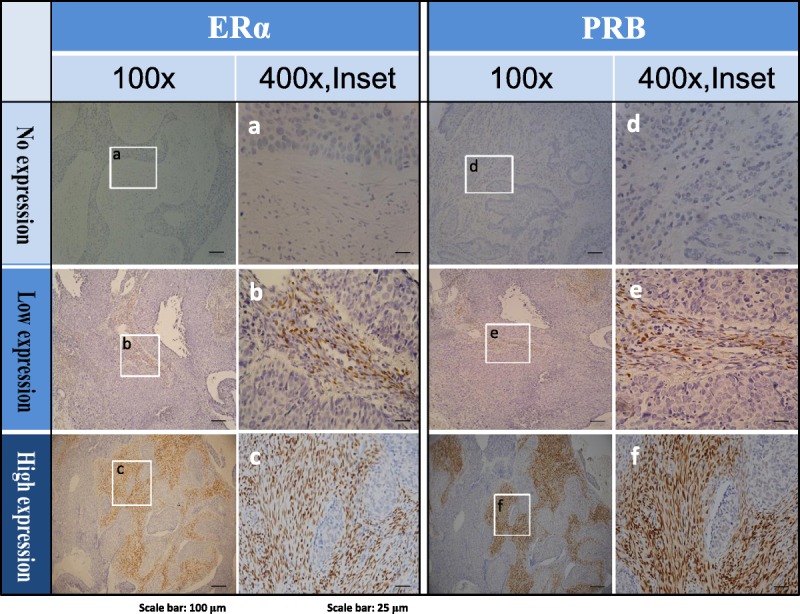
Representative IHC of ERα and PRB in the tumor stromal of cervical cancer with no (A, D), low (B, E), and high (C, F) expression levels. For both ERα and PRB, left panel: original magnification ×100, right panel (inset): original magnification ×400.
DNA Extraction
DNA was extracted from 10-μm sections of the paraffin-embedded tissues. The specimens were deparaffinized with xylene and washed twice with alcohol. Air-dried pellet was digested in 200 μL solution containing proteinase K (1 mg/mL) at 56°C for 16 to 20 hours. The samples were further purified using iStat nucleic acid extraction kit (iStat Biomedical, Taiwan, Republic of China). Finally, 100 μL DNA solution was eluted, and 2.5 μL of the aliquot was used for performing polymerase chain reaction (PCR).
HPV DNA Detection and Genotyping
The HPV-D Speed Gel Kit (iStat Biomedical) was used for amplifying a 190-bp fragment in the L1 open reading frame of HPV DNA.25 To ensure PCR quality, primers against the housekeeping gene Col2A (producing a 104-bp fragment) were used as an internal control. Polymerase chain reaction was performed as follows: preheating at 94°C for 5 minutes; 42 cycles of denaturation at 94°C for 30 seconds, annealing at 45°C for 1 minute, and extension at 72°C for 30 seconds; and final extension at 72°C for 7 minutes. Polymerase chain reaction products were analyzed by performing capillary gel electrophoresis. The amount of HPV (viral load) was semiqualitatively classified into 5 levels according to the ratio–to–copy number control of HPV standards: ≤500: 1+, 500–103: 2+, 103–104: 3+, 104–105:4+, and >105: 5+.26 For genotyping HPV DNA, PCR products were isolated from agarose gels and sequenced using Rhodamine Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems). The DNA sequences obtained were compared with sequences in GenBank by using the BLAST Program from the Web site of the National Center for Biotechnology Information.
Statistical Analyses
The values of continuous variables were compared using an independent 2-sample t test or 1-way analysis of variance. The Pearson correlation coefficient was used to measure linear correlations between pairs of continuous variables. Categorical variables were compared using χ2 or Fisher exact test. The Cochran-Armitage trend test was used to evaluate underlying trends. Univariate Kaplan-Meier analysis and multivariate Cox proportional hazard models corrected for demographic and clinical covariates were used to investigate 5-year mortality risk. P < 0.05 was considered statistically significant. All the statistical analyses were performed using SPSS software (version 17.0; SPSS Inc, Chicago, IL).
RESULTS
Patients and Clinical Characteristics
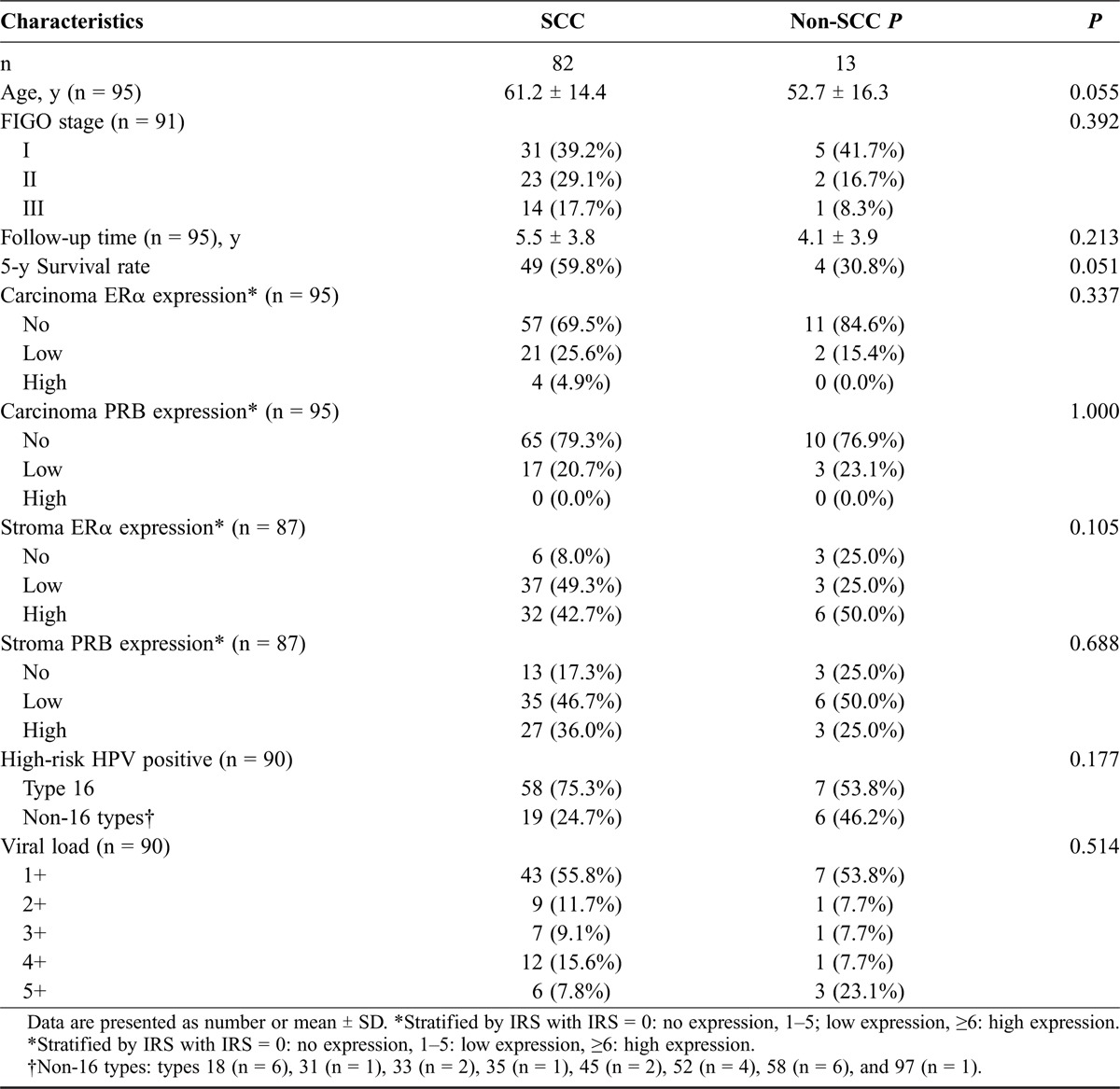
Table 1 presents the clinical characteristics, ERα/PRB expression, and HPV infection status of the 95 patients with the 2 major histological subtypes of cervical cancer. Of them, 82 patients (86.3%) had SCCs, and 13 (13.7%) had non-SCC tumors. Among the 13 patients with non-SCC tumors, 10 had adenocarcinomas, 2 had mixed tumors containing adenocarcinoma and SCC, and 1 patient had mixed tumors containing SCC and small cell carcinoma. Two-thirds of the patients had FIGO stage II tumors or less, and 69% of these patients underwent radical or modified radical hysterectomy. Most patients with advanced tumors underwent concurrent chemoradiotherapy. Other patients underwent radiotherapy and/or chemotherapy. All the patients were followed up for 5 years or until death. At diagnosis, the patients with non-SCC tumors were younger than those with SCCs (52.7 ± 16.3 years vs 61.2 ± 14.4 years) and showed a poorer prognosis; however, this difference was not statistically significant. Human papillomavirus DNA was detected in 95% of the patients with SCCs and 100% of the patients with non-SCC tumors. As expected, the patients with SCC tumors exhibited a higher incidence of HPV type 16 infection than did the patients with non-SCCs. However, viral loads did not differ between the 2 groups.
TABLE 1.
Demographics, survival, ERα/PRB expression, and HPV status in different histological types of cervical cancer
ERα and PRB Are Majorly Expressed in the Stroma of Cervical Cancer
Estrogen receptor α and PRB were not expressed (71.6% and 78.9%, respectively) or were lowly expressed (23.2% and 21.1%, respectively) in the carcinoma portion of all the cervical specimens, irrespective of the histological subtype (Table 1). However, both ERα and PRB were majorly expressed in the cancer-associated stroma, with lowly expressed (46.0% and 47.1%, respectively) and highly expressed (43.7% and 34.5%, respectively) (Table 1).
Stromal PRB Expression Is Inversely Correlated With Patient Age
Estrogen receptor α and PRB expression levels were strongly correlated with each other (r = 0.598, P < 0.001; Fig. 2A). Stromal PRB expression, but not stromal ERα expression, was inversely correlated with patient age (r = −0.409, P < 0.001; Fig. 2B and Table 2).
FIGURE 2.
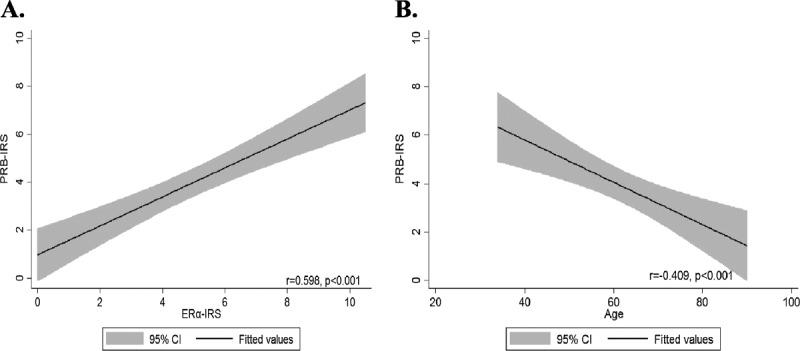
A, Relationship of IRS scores of stromal ERα and stromal PRB expressions in the studied SCC. B, Level of stromal PRB expression was inversely correlated with patient’s age.
TABLE 2.
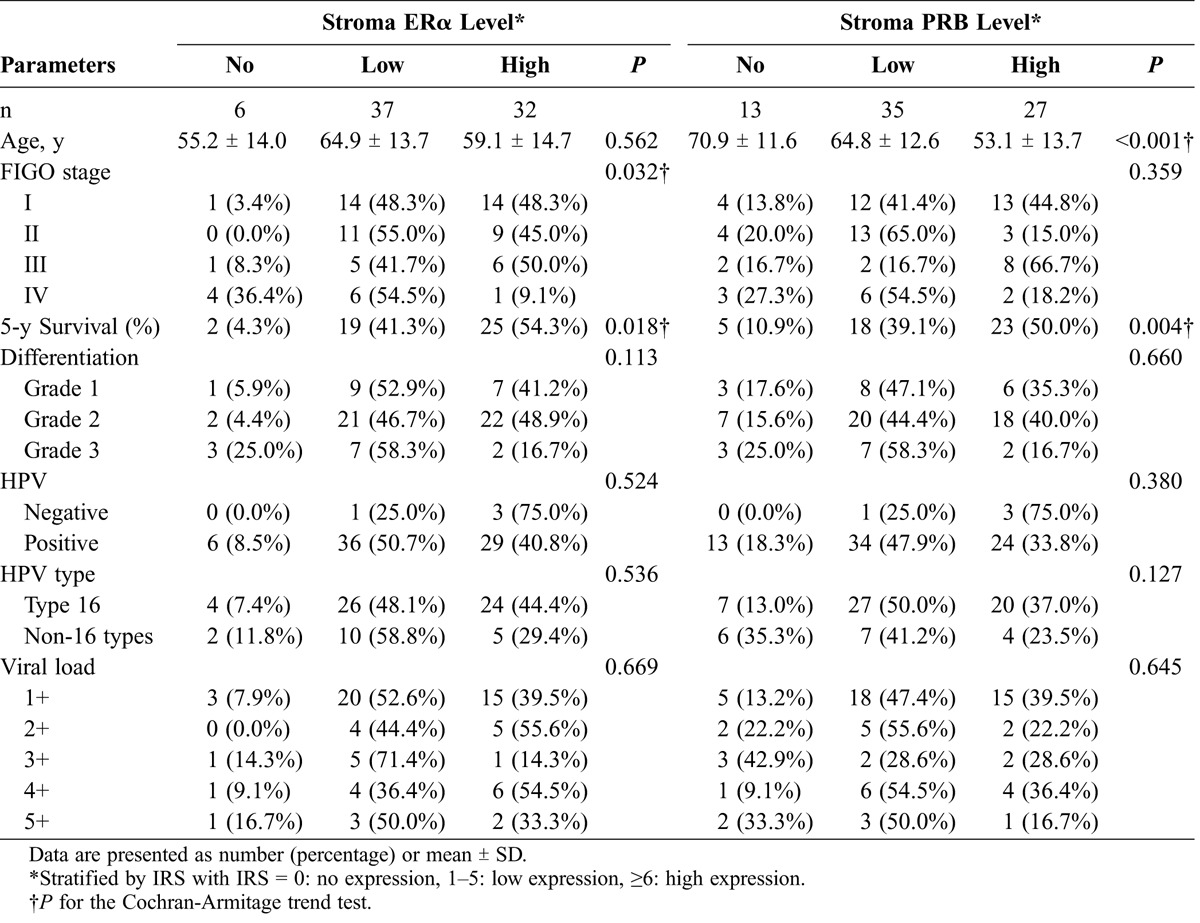
Levels of stroma ERα/PRB expression in relation to clinicopathologic parameters and status of HPV in SCC (n = 75)
Down-regulation of Stromal ERα Expression Is Correlated With Cancer Stage
Stromal ERα expression was lower in the patients with advanced cancer (P for trend = 0.032; Table 2). The patients with no or low stromal ERα expression more commonly had stages III and IV disease, whereas those with high stromal ERα expression more commonly had stages I and II disease. However, this down-regulation with the advancement of cancer was not significant for stromal PR (P for trend = 0.359). Moreover, stromal ERα or PRB expression was not associated with cancer differentiation, HPV infection status, or HPV load or genotype.
Stromal ERα and PRB Expressions Are Associated With Favorable Prognosis of SCC
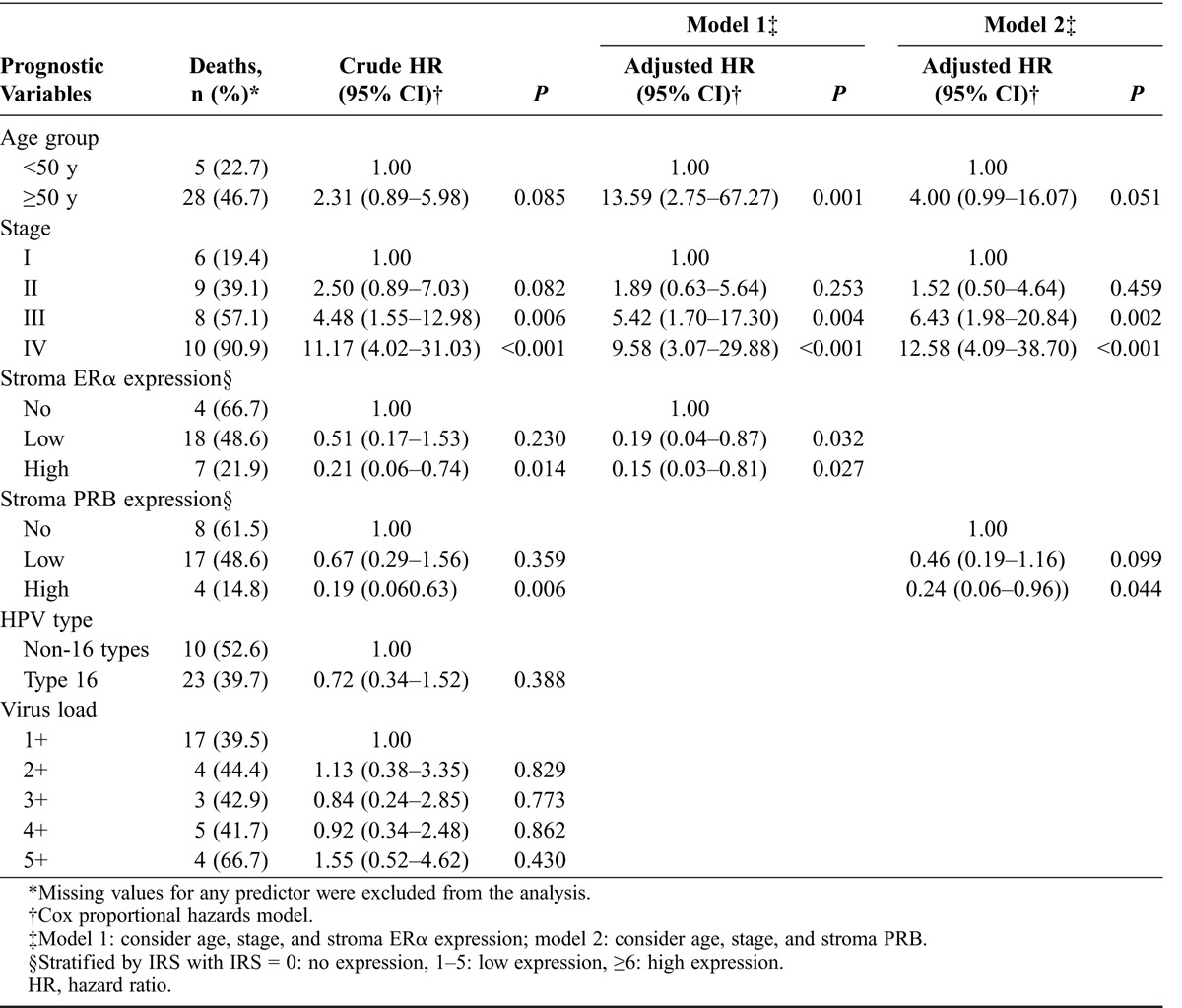
Stromal ERα and PRB expressions were positively correlated with patient survival (Table 2; Figs. 3A and B). Five-year survival rates for no, low, and high stromal ERα expression were 4.3%, 41.3%, and 54.3%, respectively (P = 0.018), and those for no, low, and high stromal PRB expression were 10.9%, 39.1%, and 50.0%, respectively (P = 0.004). Multivariate analysis showed that stromal ERα and PRB expressions both were independent good prognostic factors of SCC besides FIGO stage (Table 3). Compared with the patients with no stromal ERα or PRB expression, the patients with low and high stromal ERα expression had adjusted 5-year mortality hazard ratios of 0.19 (95% confidence interval [CI], 0.04–0.87) and 0.15 (95% CI, 0.03–0.81), respectively, whereas for low and high expressions of PRB hazard ratios were 0.46 (95% CI, 0.19–1.16) and 0.24 (95% CI, 0.06–0.96), respectively (Table 3).
FIGURE 3.
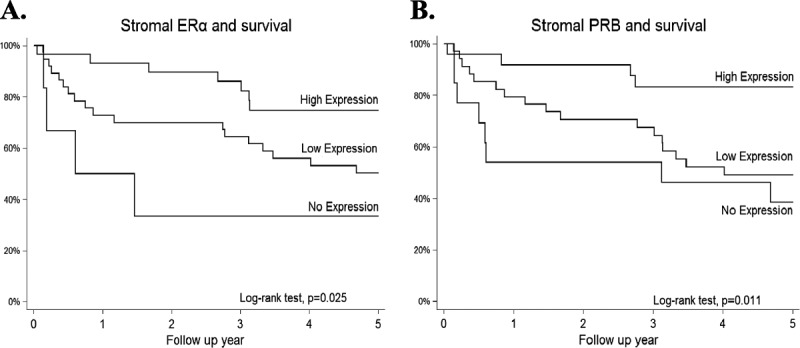
Kaplan-Meier survival analysis of different levels of ERα and PRB expressions in the stroma of SCC. Both ERα (A) and PRB (B) expressions correlated with good prognosis; it was noted that the higher the expression of ERα or PRB, the better the prognosis.
TABLE 3.
Hazard ratios of 5-year mortality according to different prognostic factors in SCC patients (n = 82)
DISCUSSION
In the epithelium of adult uterine cervix, ER and PR are expressed at the basal and parabasal cells but are rarely expressed in SCC.1,3 Zhai et al27 found that expression of ERα is progressively down-regulated in the transformation of cervical epithelium, and loss of ERα expression plays a major role in mediating the invasion and progression of cervical cancer. A recent extensive analysis of the development of neoplasia in cervical epithelium also revealed a progressive depletion of ERα accompanied with extensive, coordinated alterations in expression of estrogen-responsive genes.28 These findings are, however, inconsistent with the epidemiologic findings that estrogen exposure is risky for cervical carcinogenesis.29 The present study examined both epithelial and stromal expressions of ERα and PRB and found that these sex hormone receptors were mainly expressed in the stroma. Most importantly, their expressions are significantly associated with favorable survival, indicating that these sex hormones in the cervical stroma may play a role in invasion and/or metastasis of SCC.
Results of the present study are consistent with the crucial role of stroma in the development of the female genital tract. During the early postnatal period, the epithelium of the fused müllerian ducts undergoes region-specific differentiation under the effect of the underlying mesenchyme.28 Cunha30 performed heterotypic recombination transplantation of isolated epithelium and stroma from the vagina or uterus of neonatal mice and observed that the stroma exerted a paracrine effect on the differentiation of the supported epithelium; this stroma-induced differentiation was maintained until adulthood. Later studies have also established the importance of stromal signals for regulating epithelial proliferation in the adult mouse vagina.31 The regulation of epithelial proliferation by estrogen and progesterone involves paracrine mechanisms, which require appropriate receptors in the stroma but not in the epithelium.32 Thus, the development of cervical carcinoma may involve the same paracrine control by sex hormones, as well as sex hormone receptors located in the stroma.
Notably, our results showed that besides FIGO stage ERα and PRB expressions were independent prognostic factors for SCC. Higher stromal ERα and PRB expressions were associated with superior 5-year survival. Hence, stromal ERα and PRB expressions may be used to predict clinical outcome and stratify diseases for investigating hormone therapy in cervical cancer, similar to breast and endometrial cancers. However, the mechanisms of stromal ERα and/or PRB in invasion or metastasis of SCC are poorly understood.
To the best of our knowledge, this is the first study specifically looking at PRB in cervical cancer. Previous studies on PR expression in cervical cancer have used antibodies nonspecific to the PR isoforms8,18,19 or the PRA-specific antibodies3,17 and mostly focused on PR expression in neoplastic epithelium.15,16 Using nonspecific antibodies in detecting PR could not tell which isoforms were related to survival and may overestimate the actual expression of any of the PR isoforms with unknown proportion and thus could not yield results obtained in the present study. Tables S1 and S2 summarize the isoform specificity of commonly used antibodies for detecting ERs and PRs and previous studies of ER/PR expression and prognosis of cervical cancer. These supplementary tables explain the unique findings of this study.
Epidemiologic studies looking for associations between progesterone use and risk of cervical neoplasia have been inconclusive,33–35 mainly because of the uncontrolled variables that change the hormone status or function. These include the menstrual cycle, use of oral contraceptives, and exposure to endocrine disruptors.35 The results of the present study suggest that stromal PR may exert a tumor-suppressive or tumor-static effect on cervical cancer. The mechanism underlying stromal PRB-mediated suppression of SCC is poorly understood. Recently, Yoo et al36 used an HPV E6/E7-overexpressing mouse model of cervical cancer to show that progesterone inhibited estradiol-induced physiological hyperplasia in the cervical epithelium in a PR-dependent manner. Also, the use of progesterone promoted the regression of cervical and vaginal neoplasias. They further conducted the transgenic ablation of PR in these transgenic mice and observed that progesterone inhibited cervical or vaginal epithelial cell proliferation in a ligand-dependent manner.35,36 However, the PR they observed, unlike the status in humans, was in the neoplastic epithelium. More human studies are needed to elucidate the role and mechanism of stromal PR in the development of cervical cancer.
In summary, this study demonstrated that ERα and PRB were predominantly expressed in the stroma but not the carcinoma tissue of cervical cancer, and both were independent prognostic factors for SCC irrespective of patient age, cancer stage, and HPV infection status.
Supplementary Material
Footnotes
This work was supported by grants from National Science Council, Taiwan (103-2314-B-303-011-MY2) and Buddhist Tzu Chi General Hospital, Hualien (TCRD103-45 and TCRD104-06).
The authors declare no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citation appears in the printed text and is provided in the HTML and PDF versions of this article on the journal’s Web site (www.ijgc.net).
REFERENCES
- 1.Nonogaki H, Fujii S, Konishi I, et al. Estrogen receptor localization in normal and neoplastic epithelium of the uterine cervix. Cancer. 1990;66:2620–2627. [DOI] [PubMed] [Google Scholar]
- 2.Konishi I, Fujii S, Nonogaki H, et al. Immunohistochemical analysis of estrogen receptors, progesterone receptors, Ki-67 antigen, and human papillomavirus DNA in normal and neoplastic epithelium of the uterine cervix. Cancer. 1991;68:1340–1350. [DOI] [PubMed] [Google Scholar]
- 3.Kwasniewska A, Postawski K, Gozdzicka-Jozefiak A, et al. Estrogen and progesterone receptor expression in HPV-positive and HPV-negative cervical carcinomas. Oncol Rep. 2011;26:153–160. [DOI] [PubMed] [Google Scholar]
- 4.International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical carcinoma and reproductive factors: collaborative reanalysis of individual data on 16,563 women with cervical carcinoma and 33,542 women without cervical carcinoma from 25 epidemiological studies. Int J Cancer. 2006;119:1108–1124. [DOI] [PubMed] [Google Scholar]
- 5.International Collaboration of Epidemiological Studies of Cervical Cancer; Appleby P, Beral V, Berrington de Gonzalez A, et al. Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16,573 women with cervical cancer and 35,509 women without cervical cancer from 24 epidemiological studies. Lancet. 2007;370:1609–1621. [DOI] [PubMed] [Google Scholar]
- 6.Scharl A, Vierbuchen M, Graupner J, et al. Immunohistochemical study of distribution of estrogen receptors in corpus and cervix uteri. Arch Gynecol Obstet. 1988;241:221–233. [DOI] [PubMed] [Google Scholar]
- 7.Masood S, Rhatigan RM, Wilkinson EW, et al. Expression and prognostic significance of estrogen and progesterone receptors in adenocarcinoma of the uterine cervix. An immunocytochemical study. Cancer. 1993;72:511–518. [DOI] [PubMed] [Google Scholar]
- 8.Fujiwara H, Tortolero-Luna G, Mitchell MF, et al. Adenocarcinoma of the cervix. Expression and clinical significance of estrogen and progesterone receptors. Cancer. 1997;79:505–512. [PubMed] [Google Scholar]
- 9.Li X, Huang J, Yi P, et al. Single-chain estrogen receptors (ERs) reveal that the ERalpha/beta heterodimer emulates functions of the ERalpha dimer in genomic estrogen signaling pathways. Mol Cell Biol. 2004;24:7681–7694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kastner P, Krust A, Turcotte B, et al. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990;9:1603–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. [DOI] [PubMed] [Google Scholar]
- 12.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. [DOI] [PubMed] [Google Scholar]
- 13.Egeblad M, Nakasone ES, Werb Z. Tumors as organs: complex tissues that interface with the entire organism. Dev Cell. 2010;18:884–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao YL, Twiggs LB, Leung BS, et al. Cytoplasmic estrogen and progesterone receptors in primary cervical carcinoma: clinical and histopathologic correlates. Am J Obstet Gynecol. 1983;146:299–306. [DOI] [PubMed] [Google Scholar]
- 16.Potish RA, Twiggs LB, Adcock LL, et al. Prognostic importance of progesterone and estrogen receptors in cancer of the uterine cervix. Cancer. 1986;58:1709–1713. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki Y, Nakano T, Arai T, et al. Progesterone receptor is a favorable prognostic factor of radiation therapy for adenocarcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys. 2000;47:1229–1234. [DOI] [PubMed] [Google Scholar]
- 18.Baalbergen A, Ewing-Graham PC, Eijkemans MJ, et al. Prognosis of adenocarcinoma of the uterine cervix: p53 expression correlates with higher incidence of mortality. Int J Cancer. 2007;121:106–110. [DOI] [PubMed] [Google Scholar]
- 19.Bodner K, Laubichler P, Kimberger O, et al. Oestrogen and progesterone receptor expression in patients with adenocarcinoma of the uterine cervix and correlation with various clinicopathological parameters. Anticancer Res. 2010;30:1341–1345. [PubMed] [Google Scholar]
- 20.Chung SH, Wiedmeyer K, Shai A, et al. Requirement for estrogen receptor alpha in a mouse model for human papillomavirus–associated cervical cancer. Cancer Res. 2008;68:9928–9934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung SH, Lambert PF. Prevention and treatment of cervical cancer in mice using estrogen receptor antagonists. Proc Natl Acad Sci U S A. 2009;106:19467–19472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung SH, Shin MK, Korach KS, et al. Requirement for stromal estrogen receptor alpha in cervical neoplasia. Horm Cancer. 2013;4:50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar MM, Davuluri S, Poojar S, et al. Role of estrogen receptor alpha in human cervical cancer–associated fibroblasts: a transcriptomic study. Tumour Biol. 2016;37:4409–4420. [DOI] [PubMed] [Google Scholar]
- 24.Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue [in German]. Pathologe. 1987;8:138–140. [PubMed] [Google Scholar]
- 25.Sotlar K, Diemer D, Dethleffs A, et al. Detection and typing of human papillomavirus by e6 nested multiplex PCR. J Clin Microbiol. 2004;42:3176–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin CY, Chen HC, Lin RW, et al. Quality assurance of genotyping array for detection and typing of human papillomavirus. J Virol Methods. 2007;140:1–9. [DOI] [PubMed] [Google Scholar]
- 27.Zhai Y, Bommer GT, Feng Y, et al. Loss of estrogen receptor 1 enhances cervical cancer invasion. Am J Pathol. 2010;177:884–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.den Boon JA, Pyeon D, Wang SS, et al. Molecular transitions from papillomavirus infection to cervical precancer and cancer: role of stromal estrogen receptor signaling. Proc Natl Acad Sci U S A. 2015;112:E3255–E3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsieh CJ, Hong MK, Chen PC, et al. Antiestrogen use reduces risk of cervical neoplasia in breast cancer patients: a population-based study [published online ahead of print October 27, 2016]. Oncotarget. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cunha GR. Stromal induction and specification of morphogenesis and cytodifferentiation of the epithelia of the mullerian ducts and urogenital sinus during development of the uterus and vagina in mice. J Exp Zool. 1976;196:361–370. [DOI] [PubMed] [Google Scholar]
- 31.Kurita T, Cooke PS, Cunha GR. Epithelial-stromal tissue interaction in paramesonephric (müllerian) epithelial differentiation. Dev Biol. 2001;240:194–211. [DOI] [PubMed] [Google Scholar]
- 32.Cunha GR, Cooke PS, Kurita T. Role of stromal-epithelial interactions in hormonal responses. Arch Histol Cytol. 2004;67:417–434. [DOI] [PubMed] [Google Scholar]
- 33.Shapiro S, Rosenberg L, Hoffman M, et al. Risk of invasive cancer of the cervix in relation to the use of injectable progestogen contraceptives and combined estrogen/progestogen oral contraceptives (South Africa). Cancer Causes Control. 2003;14:485–495. [DOI] [PubMed] [Google Scholar]
- 34.Castle PE, Walker JL, Schiffman M, et al. Hormonal contraceptive use, pregnancy and parity, and the risk of cervical intraepithelial neoplasia 3 among oncogenic HPV DNA-positive women with equivocal or mildly abnormal cytology. Int J Cancer. 2005;117:1007–1012. [DOI] [PubMed] [Google Scholar]
- 35.Harris TG, Miller L, Kulasingam SL, et al. Depot-medroxyprogesterone acetate and combined oral contraceptive use and cervical neoplasia among women with oncogenic human papillomavirus infection. Am J Obstet Gynecol. 2009;200:489e1–489e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoo YA, Son J, Mehta FF, et al. Progesterone signaling inhibits cervical carcinogenesis in mice. Am J Pathol. 2013;183:1679–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


