Abstract
Objective
Ovarian cancer recurs in most patients, with a 5-year survival rate less than 30%. Quality of life is an increasingly important issue in patients with cancer, but there are limited data in women with recurrent ovarian cancer in this regard.
Materials and Methods
We used an ad hoc questionnaire to compare changes in health perceptions, burden of disease, and expectations for the future quality of life in women with and without recurrence of ovarian cancer. A total of 173 women were included, 116 with relapse and 57 without, undergoing follow-up in a routine clinical setting.
Results
Substantial differences were seen in self-assessed health status between women with and without recurrence; 33.6% and 82.4% of women with and without recurrence rated their health as good to excellent, respectively. More patients with recurrence of disease reported limitations in moderate activity than those without. Furthermore, 79.0% of women without recurrence reported that pain did not affect or only slightly affected daily activities, compared with 28.2% with recurrence. Most women with recurrence (59.5%) reported that they were able to do less than they wanted to because of their emotional status compared with only 15.8% of women without recurrence. In addition, 66.4% of women with recurrence referred that they had problems concentrating at work and home versus 26.3% of women without recurrence.
Conclusions
From this survey, it is clear that relapse of disease has a negative psychological and physical impact, highlighting the importance of time without recurrence and the need for effective treatment in the long term.
Key Words: Ovarian cancer, Chemotherapy, Quality of life, Daily activities, Outlook for future
Despite progress in therapy, ovarian cancer remains a major cause of death, with an estimated 225,000 new cases per year worldwide.1 Survival rates depend on many factors including tumor stage and patient age at diagnosis2,3; in patients with early stage disease, 5-year survival is 80% to 90%, while it is approximately 25% for those with advanced stage disease, which is diagnosed in approximately two thirds of cases.4 Despite improvements in surgery and chemotherapy, most patients will present with recurrent disease within 12 to 18 months.4 Survival rates are worse in platinum-refractory disease, not exceeding 5 months.5
Quality of life (QoL) is an important patient-related outcome. However, there are limited data on the QoL in patients with ovarian cancer and on the impact that treatment may have on it, especially in women with relapsed disease. It is unsurprising that a diagnosis of ovarian cancer is associated with increased stress and diminished QoL. More than half of women refer that such a diagnosis would lead to changes in their life course, whereas most also reported that the surgical scar, weight gain, and hair loss were among their primary concerns.6 Patients with distress also have significantly worse functioning, more problems, and lower QoL than patients without distress.7
Previous studies have suggested that it may be important for patients to maintain hope for cure.8,9 Notwithstanding, others have questioned the utility of providing hope to patients, because if hopes are raised and not fulfilled, the false hope created may increase the risk for depression and other negative effects on well-being.10 This suggests that additional interventions and strategies are needed to improve communication and peer support for women with ovarian cancer.11
In patients undergoing palliative chemotherapy, it has been suggested that patient expectations are often unrealistic, even if they may be associated with benefits in terms of emotional function and overall QoL.12 In general, women with recurrent ovarian cancer seem to have high expectations of treatment, despite a complex array of symptoms and substantial symptom burden.13 In addition, it has also been reported that there are no significant differences in the severity of distress and self-reported problems in patients with and without recurrence.7 Nonetheless, when present, distress causes patients to experience more overall problems in both physical and emotional functioning, with a lower QoL.7
Due to the paucity of data on the QoL in women with ovarian cancer, especially those with recurrent disease, and to further explore the potential impact of recurrence on QoL, we used an ad hoc survey to investigate the perspectives of the patient relative to daily life and outlook for the future.
MATERIALS AND METHODS
Study Design
The pilot study involved 50 oncologists located in different centers throughout Italy. Two or three patients were involved per center. Inclusion criteria were between the ages of 18 and 80 years and histologically confirmed ovarian carcinoma International Federation of Gynecology and Obstetrics stage IIIB/IIIC/IV at diagnosis. Absence of recurrence was defined as no detectable symptoms of relapsed disease either clinically or by imaging after 1 or more lines of chemotherapy for a minimum of 3 years after the last cycle of chemotherapy. Elevated CA125 levels were not considered in the present study design, and recurrence was defined as clinical or radiological evidence of disease within 6 months of the last line of chemotherapy. There were no exclusion criteria, such as that related to extent of disease. During routine follow-up visits, patients were explained the nature of the study and asked to participate by their oncologist. Those willing to participate were given the ad hoc questionnaire, who compiled it privately and returned it in a closed envelope. The patient questionnaires contained no personal information. Participating oncologists also compiled a dedicated questionnaire. These questionnaires did not contain any personal or other information that could be used to identify the patient. Because this was not a clinical investigation and because questionnaires were self-administered, the study did not fall under the realm of the respective ethics committees. The data collection period lasted from August 5 to 23, 2013.
Questionnaires
Patient Questionnaire
The questionnaire covered many aspects of QoL, including health perceptions, burden of disease, and expectations for the future. The questionnaire was divided into 2 parts. The first part contained basic demographic and clinical information (age, number of children, date of first diagnosis, surgical interventions, chemotherapy, and adverse effects of therapy) along with several questions regarding daily life and future expectations. The second part was composed of 6 questions aimed at understanding the patient’s general health status and QoL as follows: (1) “Rate your health status”; (2) “Does the disease cause limitations in physical activity such as moving a table or climbing stairs?”; (3) “During the last 4 weeks, did you have problems in work or daily life because of your physical health?”; (4) “During the last 4 weeks, have you been able to do less than you would have liked due to emotional problems?”; (5) “During the past 4 weeks, have you felt calm, full of energy, or discouraged/sad?”; and (6) “During the last 4 weeks, how much has your physical or mental health negatively impacted your social activities?” It was also of interest to determine how women perceived their future. To evaluate this aspect, women were asked to rate their response to 10 statements on a scale from 1 to 7, where 1 was total disagreement and 7 was complete agreement. Women were lastly asked whether they agreed with several statements about their daily life and how the disease has affected it.
Physician Questionnaire
The oncologists proposing the study were asked to compile a questionnaire containing information on the patient and disease characteristics and on their impressions about the impact of various aspects such as diagnosis and discovery of recurrence on the patient. The questionnaire for the physicians included 11 questions on clinical information and treatment (age, tumor size, surgical data, Eastern Cooperative Oncology Group (ECOG) score, and comorbidities), along with 6 questions (plus 5 additional questions in case of recurrent disease) asking how difficult the oncologist believed the various stages of the disease/treatment were for the patient (scored from 1 [no difficulty] to 10 [extremely difficult]). The same set of questions was also present in the patient questionnaire.
Data Analysis
Continuous variables are expressed as mean and standard error of the mean (SEM), whereas categorical variables are reported as percentages. The significance of differences between raw/processed data was determined using a Student t test (2 samples). A P value of less than 0.05 was considered statistically significant. Statistical analyses were performed using Microsoft Excel for Windows (Microsoft Corporation, Seattle, Wash).
RESULTS
A total of 173 patients agreed to compile the ad hoc questionnaire during the 4-week data collection period, 116 with recurrence and 57 without. Demographic and patient data are shown in Table 1. The mean patient age was 59.8 years. Substantial differences were seen in self-assessed health status between groups. Among those with recurrence, only 33.6% of patients rated their health as good to excellent, whereas in those with no recurrence, it was 82.4% (P < 0.05). This difference was also reflected in physician-referred ECOG scores, where 91.1% of patients without recurrence had an ECOG score of 0 to 1 compared with 50.9% in those with recurrence (P < 0.05). Of note, patient and physician responses to the impact of different phases of the disease were highly correlated (data not shown). In particular, with the exception of time postintervention, most oncologists and patients, respectively, responded with a score of 8 to 10, indicating very difficult, the moment of first diagnosis (59.2% and 83.9%), the time before the first surgical intervention (71.8% and 68.7%), the period after intervention (42.9% and 49.2%), and chemotherapy after intervention (69.4% and 61.3%). This indicates that oncologists accurately judged the difficulties faced by the patient. Routine follow-up visits were not rated as being particularly stressful by either oncologists or patients.
TABLE 1.
Characteristics of women with and without recurrence of ovarian cancer
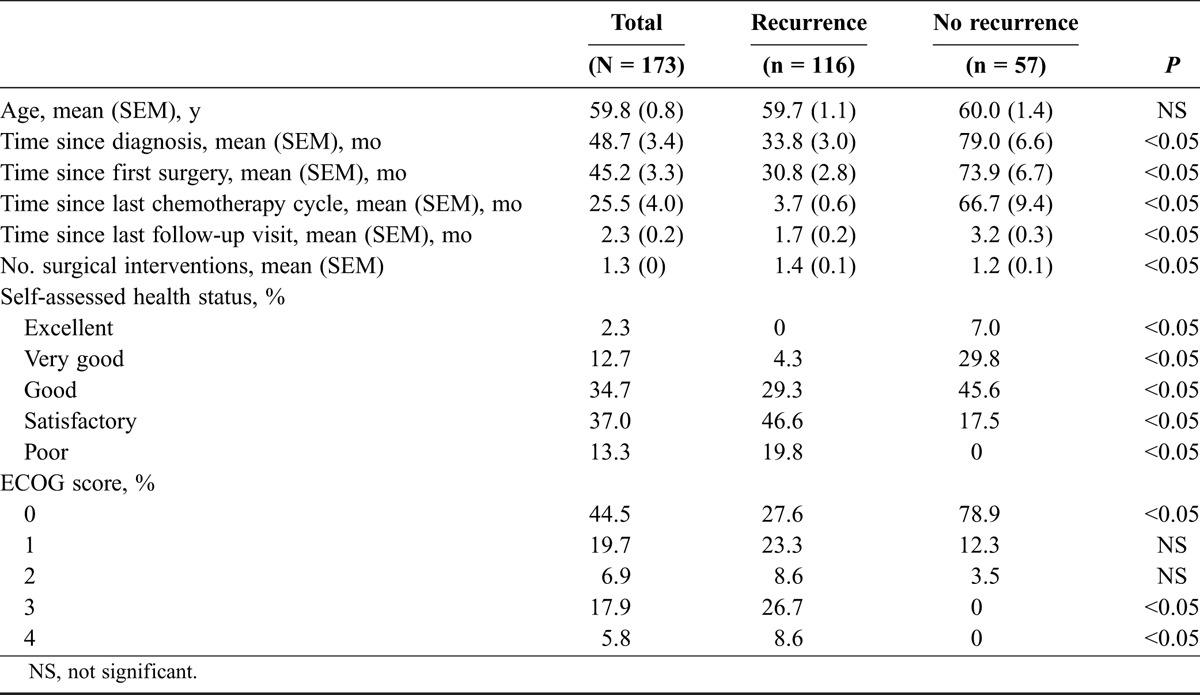
Effects of Physical Health on Daily Activities
Figure 1 show patient responses regarding limitations in daily activities due to physical health. Substantially more patients with recurrence of disease reported limitations in moderate activity (such as moving a table, using a vacuum cleaner, going for a walk, or a bicycle ride) and a limited ability to climb several flights of stairs compared with those without recurrence. Most women with recurrence also reported that they were able to do less than desired (64.7%) and that they were limited in work and in routine daily activities (71.6%) in comparison with women without recurrence (14% and 21.1%, respectively). When asked if pain affected their daily activities, 79.0% of women without recurrence referred “no” or “very little,” compared with only 28.2% of those with recurrence. All the differences between groups were statistically significant.
FIGURE 1.
Limitations in daily activities between patients with and without recurrence due to physical problems. The percentage of women responding “no” or “very little” is shown. *P < 0.05 between groups. †The questions refer to the past 4 weeks due to physical health.
Effects of Emotional Status on Daily Activities
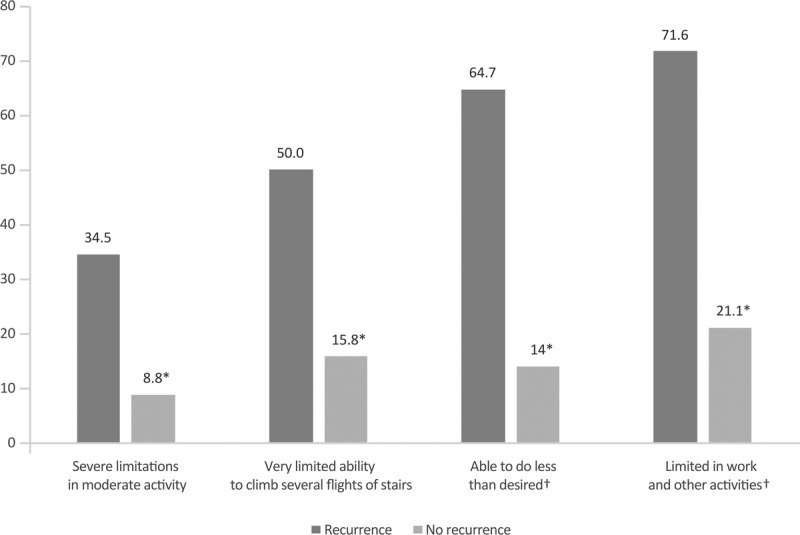
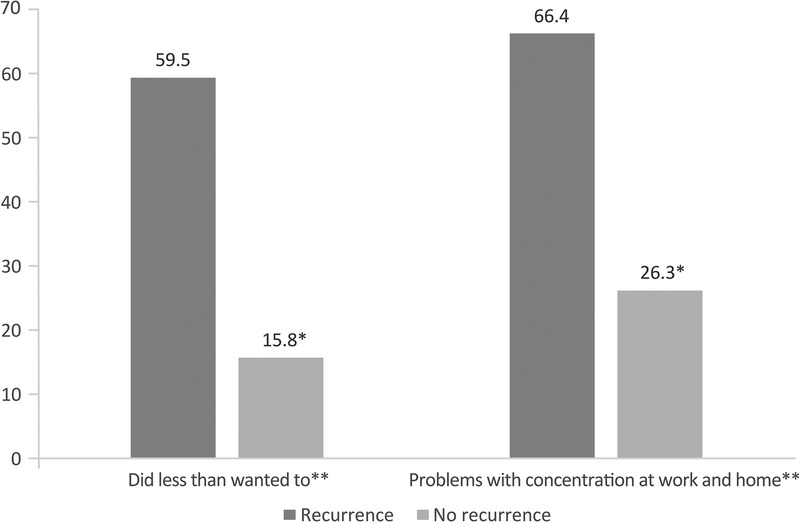
Patients were also asked if they had limitations in daily activities due to emotional status (eg, depression, anxiety, agitation, sadness) (Fig. 2). Although most women with recurrence (59.5%) responded that they were able to do less than they wanted to, only 15.8% of women without recurrence referred the same limitation. In addition, when asked if they had problems concentrating at work and home because of their emotional status, 66.4% of women with recurrence referred that they did, compared with 26.3% of women without.
FIGURE 2.
Limitations in daily activities between patients with and without recurrence due to emotional problems. The percentage of women responding “yes” is shown. *P < 0.05 between groups. **The questions refer to the past 4 weeks due to physical health.
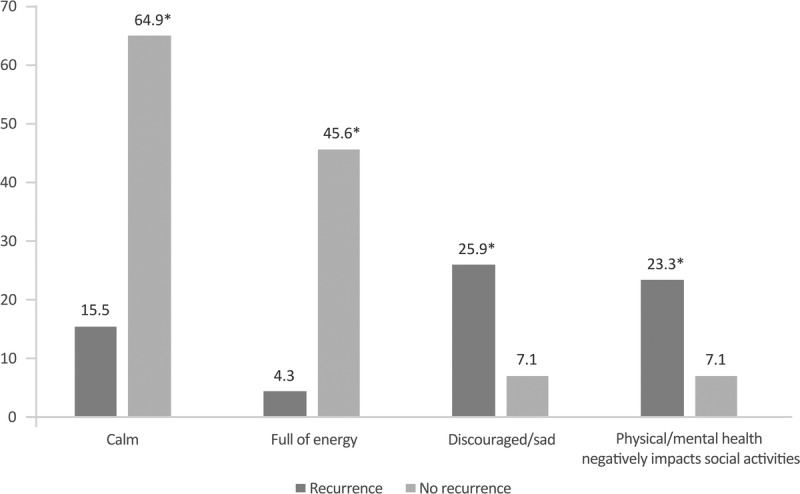
Most women without recurrence responded that they felt calm (64.9%) and almost half “full of energy” (Fig. 3). In contrast, only 15.5% and 4.3%, respectively, of those with recurrence referred feeling calm and full of energy. Similarly, more women with recurrence reported feeling sad/discouraged and referred that their physical and/or mental health negatively affected social activities. All the differences between groups were statistically significant.
FIGURE 3.
General feelings during the last 4 weeks between patients with and without recurrence. The percentage of women responding “always” or “almost always” is shown. *P < 0.05 between groups.
Outlook for the Future
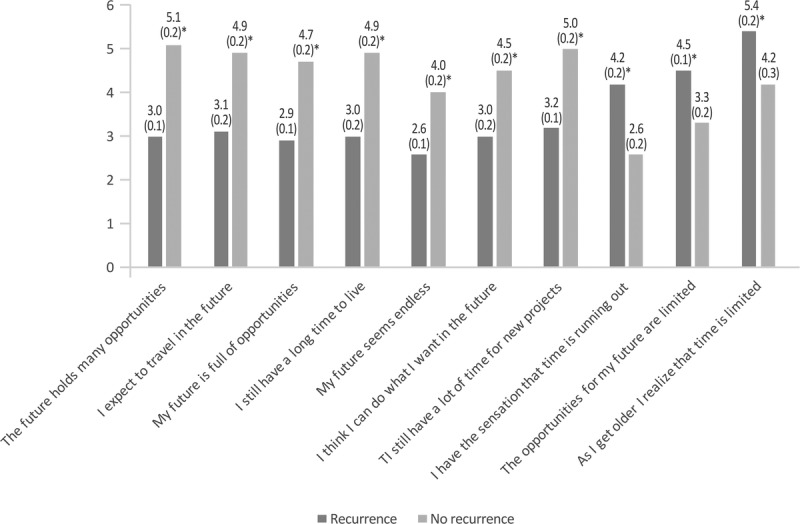
As shown in Figure 4, the perception of the future was substantially different between women with and without recurrence. For all positive attitudes, women without recurrence consistently referred a higher level of agreement with the statement than women with recurrence. For example, more women without recurrence felt that “the future still holds many opportunities” and that “they have time for new projects” compared with those with recurrence. For statements with a negative outlook, that is, “time is running out” and the “opportunities for the future are limited,” women with recurrence referred a higher level of agreement. All the differences between groups were statistically significant.
FIGURE 4.
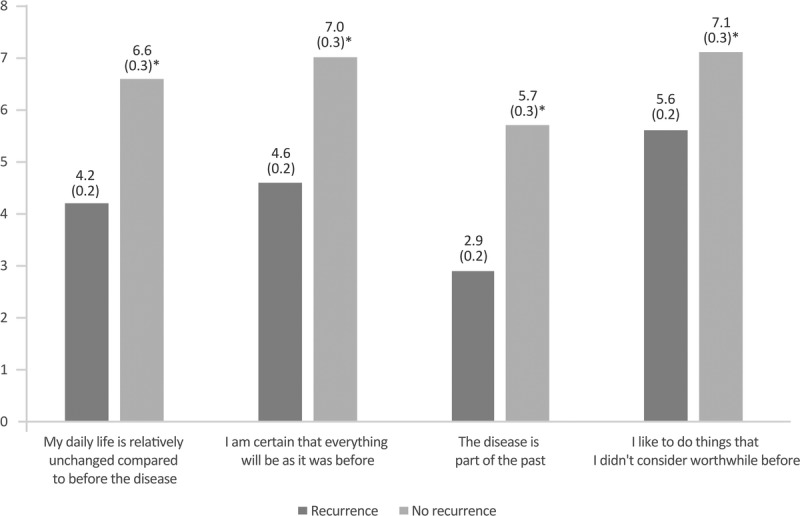
Outlook for the future in patients with and without recurrence. The mean score of responses is shown (possible score 1–7; 1, total disagreement; 7, complete agreement). Values in parentheses indicate the SEM. *P < 0.05 between groups.
Compared with women with recurrence, those without recurrence had consistently higher scores regarding how the disease affected daily life, believing that things will go back to the way they were before, thinking the disease was part of the past, and thinking they valued doing things that were valued less before the disease (Fig. 5). All the differences between groups were statistically significant.
FIGURE 5.
Differences in how the disease has affected daily life and future prospects in women with and without recurrence. The mean score of responses is shown (possible score 1–10; 1, total disagreement; 10, complete agreement). Values in parentheses indicate the SEM. *P < 0.05 between groups.
DISCUSSION
To our knowledge, this is the first study to directly investigate the outlook for future perspectives in women with ovarian cancer. Using an ad hoc questionnaire to explore patient perspectives in women with or without recurrence of ovarian cancer undergoing routine follow-up visits, it is clear that relapse of disease has a negative impact on the QoL, physical activities, and work. Of interest, the survey data found that women with recurrent ovarian cancer viewed the future differently than those considered to be free of relapse for at least 3 years. In general, women without recurrence had a more positive outlook for the future considering all statements included in the survey, thinking that the future still holds possibilities and new projects. Along these lines, a previous study examined end-of-life concerns in ambulatory ovarian cancer patients, reporting that 55% acknowledged fear of dying and 31.6% acknowledged loss of hope in the fight against their illness.14 Such fears and loss of hope are likely to be increased in women with recurrence.
Pain was reported to be of substantial burden by most women with recurrence (79%), but in only approximately one third of those without recurrence. In addition, emotional health, such as the presence of depression, anxiety, agitation, and sadness, had important negative effects on daily activities, causing problems in concentration, work, and social activities.
It is clear that a diagnosis of recurrence has a devastating impact on an individual. In ovarian cancer, QoL issues are complex because treatment usually involves a combination of surgery and chemotherapy, which may influence physical, social, and emotional well-being. As previously noted by other authors, QoL is affected by disease site, as well as by treatment- and patient-specific factors.15 Changes in the QoL are affected by changes in physical functioning due to adverse effects of treatment and the disease itself, psychological distress that may be caused by fear and anxiety, sexual dysfunction associated with anatomic and physiologic changes of treatment, and even loss of childbearing potential. In a study of hospitalized patients with ovarian cancer undergoing chemotherapy, the following 7 symptom clusters were identified: psychological distress, fatigue-pain, abdominal discomfort, flu-like symptoms, fluid accumulation, and peripheral neuropathy, highlighting that treatment and cancer itself affect a wide range of components of emotional status and daily life.16
The lack of energy documented herein, which was greater among women with recurrence, has been previously reported in recurrent ovarian cancer, where this was the most prevalent symptom during the last year of life.17 In the study by Price et al,17 higher optimism, higher minimization (P = 0.003), and lower helplessness/hopelessness at baseline were significant predictors of subsequent higher QoL. Differences were also seen in self-rated health: 33.6% of women without recurrence rated their health as good to excellent compared with 82.4% among those with no recurrence. The study by Liavaag et al18 reported that self-rated health assessments is strongly related to common somatic complaints, impairment, and fatigue, but not to cancer-related variables. Moreover, women with poor self-rated health had higher levels of somatic symptoms, anxiety, depression, and fatigue than those with better self-rated health. This is in agreement with our findings that more women with recurrence referred feeling depressed and sad that those without recurrence. The prevalence of depression and anxiety in women with ovarian cancer, across the treatment spectrum, is significantly greater than in the healthy female population.19
A previous study has compared the QoL in early and advanced ovarian cancer survivors and found that overall QoL, fatigue, hopelessness, spirituality, social support, degree to which unmet needs were met, and use of complementary therapy did not differ between those with early or advanced cancers.20 In another study on disease status and self-reported distress, the authors reported that there were no differences in distress severity and self-reported problems between patients with and without recurrence, although distressed patients did experience greater problems in physical and emotional functioning and had a lower QoL.7 Our results using an ad hoc questionnaire are somewhat in contrast with the previous results, showing significant differences in many aspects of QoL and outlook for the future between women with ovarian cancer with and without recurrence. It is clear that, however, QoL and health-related QoL are complex concepts that include domains related to physical, mental, emotional, and social functioning and perceptions. Moreover, all these aspects are prone to substantial variability, which may also be related to the instruments used for its evaluation. In a study from 2016 in women surviving ovarian cancer for at least 1 year, lower health-related QoL was associated with disease recurrence, treatment status, symptom burden, age, and number of comorbidities.21 A recent review noted that ovarian cancer survivors experience a wide range of sequelae that may persist for a lengthy time and negatively impact QoL.22
One limitation of the present survey is that nonvalidated questionnaires were used to evaluate QoL and not validated systems such as European Organization for Research and Treatment of Cancer or Functional Assessment of Cancer Therapy. We are aware that our ad hoc questionnaire did not address all the factors contributing to QoL and its variability. However, in addition to assessing QoL through aspects related to daily living and activities, it was also our intention to focus on how ovarian cancer affects women’s outlook for the future. As already noted, a diagnosis of ovarian cancer or its recurrence has a very stressful effect on the QoL, and thus, early assessment of psychological problems must be an integral part of the therapeutic pathway.6 Healthcare providers should aim to provide clear and useful information regarding the disease itself, prospects for treatment, and symptom relief. In this regard, it has been reported that on occasion, treatment expectations may be unrealistic,12 which may lead to a sense of false hope. Another limitation of the present study was that it involved a large number of centers, each with a very small sample size, which may be a source of selection bias.
The results using this ad hoc questionnaire indicate that compared with women without recurrence of ovarian cancer, those with recurrence have poorer self-assessed QoL, more limitations in daily activities, work, and social activities due to physical and emotional health. They have less energy overall and have a poorer outlook for the future. This underlines the relevance of providing patients with as much time as possible without physical and psychological burden of disease and treatment-related toxicity and reinforces the need for effective long-term treatments for recurrent disease.
ACKNOWLEDGMENTS
This survey was realized by Doxapharma and supported by Roche. Editorial assistance was provided by Health Publishing & Services Srl, Milan, with the unconditional grant of Roche.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. [DOI] [PubMed] [Google Scholar]
- 2.Chen T, Jansen L, Gondos A, et al. Survival of ovarian cancer patients in Germany in the early 21st century: a period analysis by age, histology, laterality, and stage. Eur J Cancer Prev. 2013;22:59–67. [DOI] [PubMed] [Google Scholar]
- 3.Diab SG, Elledge RM, Clark GM. Tumor characteristics and clinical outcome of elderly women with breast cancer. J Natl Cancer Inst. 2000;92:550–556. [DOI] [PubMed] [Google Scholar]
- 4.Colombo N, Van Gorp T, Parma G, et al. Ovarian cancer. Crit Rev Oncol Hematol. 2006;60:159–179. [DOI] [PubMed] [Google Scholar]
- 5.Fu S, Hennessy BT, Ng CS, et al. Perifosine plus docetaxel in patients with platinum and taxane resistant or refractory high-grade epithelial ovarian cancer. Gynecol Oncol. 2012;126:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mangone L, Mandato VD, Gandolfi R, et al. The impact of epithelial ovarian cancer diagnosis on women’s life: a qualitative study. Eur J Gynaecol Oncol. 2014;35:32–38. [PubMed] [Google Scholar]
- 7.Ploos van Amstel FK, van Ham MA, Peters EJ, et al. Self-reported distress in patients with ovarian cancer: is it related to disease status? Int J Gynecol Cancer. 2015;25:229–235. [DOI] [PubMed] [Google Scholar]
- 8.von Gruenigen VE, Daly BJ. Treating ovarian cancer patients at the end of life: when should we stop? Gynecol Oncol. 2005;99:255–256. [DOI] [PubMed] [Google Scholar]
- 9.von Gruenigen VE, Daly BJ. Futility: clinical decisions at the end-of-life in women with ovarian cancer. Gynecol Oncol. 2005;97:638–644. [DOI] [PubMed] [Google Scholar]
- 10.Sjoquist KM, Friedlander ML, O’Connell RL, et al. Hope, quality of life, and benefit from treatment in women having chemotherapy for platinum-resistant/refractory recurrent ovarian cancer: the gynecologic cancer intergroup symptom benefit study. Oncologist. 2013;18:1221–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reb AM. Transforming the death sentence: elements of hope in women with advanced ovarian cancer. Oncol Nurs Forum. 2007;34:E70–E81. [DOI] [PubMed] [Google Scholar]
- 12.Doyle C, Crump M, Pintilie M, et al. Does palliative chemotherapy palliate? Evaluation of expectations, outcomes, and costs in women receiving chemotherapy for advanced ovarian cancer. J Clin Oncol. 2001;19:1266–1274. [DOI] [PubMed] [Google Scholar]
- 13.Friedlander ML, Stockler M, O’Connell R, et al. Symptom burden and outcomes of patients with platinum resistant/refractory recurrent ovarian cancer: a reality check: results of stage 1 of the gynecologic cancer intergroup symptom benefit study. Int J Gynecol Cancer. 2014;24:857–864. [DOI] [PubMed] [Google Scholar]
- 14.Shinn EH, Taylor CL, Kilgore K, et al. Associations with worry about dying and hopelessness in ambulatory ovarian cancer patients. Palliat Support Care. 2009;7:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun CC, Ramirez PT, Bodurka DC. Quality of life for patients with epithelial ovarian cancer. Nat Clin Pract Oncol. 2007;4:18–29. [DOI] [PubMed] [Google Scholar]
- 16.Hwang KH, Cho OH, Yoo YS. Symptom clusters of ovarian cancer patients undergoing chemotherapy, and their emotional status and quality of life. Eur J Oncol Nurs. 2016;21:215–222. [DOI] [PubMed] [Google Scholar]
- 17.Price MA, Bell ML, Sommeijer DW, et al. Physical symptoms, coping styles and quality of life in recurrent ovarian cancer: a prospective population-based study over the last year of life. Gynecol Oncol. 2013;130:162–168. [DOI] [PubMed] [Google Scholar]
- 18.Liavaag AH, Dørum A, Fosså SD, et al. Morbidity associated with "self-rated health" in epithelial ovarian cancer survivors. BMC Cancer. 2009;9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watts S, Prescott P, Mason J, et al. Depression and anxiety in ovarian cancer: a systematic review and meta-analysis of prevalence rates. BMJ Open. 2015;5:e007618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirabeau-Beale KL, Kornblith AB, Penson RT, et al. Comparison of the quality of life of early and advanced stage ovarian cancer survivors. Gynecol Oncol. 2009;114:353–359. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Y, Irwin ML, Ferrucci LM, et al. Health-related quality of life in ovarian cancer survivors: results from the American Cancer Society’s Study of Cancer Survivors - I. Gynecol Oncol. 2016;141:543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed-Lecheheb D, Joly F. Ovarian cancer survivors’ quality of life: a systematic review. J Cancer Surviv. 2016;10:789–801. [DOI] [PubMed] [Google Scholar]


