Supplemental digital content is available in the text.
Key Words: Ovarian cancer, CA125, Monitoring, Screening, Counseling, Tumor markers
Abstract
Aims
The Medical Research Council OVO5/EORTC 55955 trial showed that patients in remission after first-line therapy for ovarian cancer did not benefit from routine measurement of CA125 during follow-up. Since the presentation of these results, we have counseled patients about the options for follow-up and provided them with an information leaflet about the trial results and the symptoms that should prompt an early appointment and CA125 measurement. We present an audit of practice after the presentation of those results.
Methods
The medical records of 143 consecutive patients completing first-line therapy for epithelial ovarian, fallopian tube, or primary peritoneal cancer in our unit between July 2009 and December 2013 were analyzed.
Results
An agreed plan of CA125 follow-up was recorded in 69 (79%) of 87 eligible patients on completion of first-line therapy. No routine CA125 follow-up was selected by 55 (80%) patients, and routine CA125 follow-up was selected by 14 (20%), of whom 3 wished not to be informed of the results. CA125 levels were checked in 28 (51%) patients in the no routine CA125 follow-up group, in 26 cases because of the development of symptoms. Relapse was confirmed in 22. Median follow-up was 360 days (range, 100–836). CA125 levels were checked in all 14 patients who had requested routine CA125 follow-up. Relapse has been confirmed in 2 patients. Median follow-up was 560 days (range, 500–620).
Conclusions
If patients are given sufficient information about the role of routine CA125 measurements during follow-up, the majority decide against CA125 monitoring and hence, avoid these blood tests.
Epithelial ovarian cancer is newly diagnosed in 220,000 women worldwide every year and is the fourth commonest cause of female cancer death in the developed world.1 The disease is often widely disseminated within the abdomen at diagnosis; however, a significant proportion of patients achieve a complete response after first-line surgery and chemotherapy. The majority of patients who present with advanced disease will, however, recur within 2 years of treatment.2 The early detection and management of recurrent cancer is generally perceived to be critical in improving outcome but lacks an evidence base for efficacy. This concept has resulted in the regular follow-up of patients who have completed potentially curative treatment for ovarian cancer. The appropriate schedule of follow-up after primary therapy, however, has an insufficient evidence base, and consequently, practice varies. Typically, clinical evaluation and measurement of the serum tumor marker CA125 is performed every 3 months for 2 years, then every 6 months up to 5 years.
CA125 is a valuable marker in the initial diagnosis and monitoring of response to chemotherapy in epithelial ovarian cancer, and regular CA125 measurement after completion of primary therapy can detect cancer recurrence several months before a patient experiences symptoms or exhibits signs of disease.3–6 Indeed, the Gynecologic Cancer InterGroup defines recurrence on the basis of a doubling in serum CA125 levels to more than twice the upper limit of normal or to more than twice the nadir if never within the normal range,3 with an elevated value being confirmed by 2 separate measurements obtained at least 1 week apart. However, although the benefit of CA125 monitoring during therapy and its role in diagnosing relapsing disease is not disputed, there are questions as to the value of routine CA125 monitoring after completion of treatment.
The value of routine CA125 monitoring was examined in the OVO5/EORTC 55955 study of women who had achieved complete remission after first-line platinum-based chemotherapy. This large randomized trial compared the earlier intervention of second-line treatment based on increased CA125 levels with delaying treatment until clinical evidence of relapse. The study demonstrated no overall survival benefit of early CA125-driven retreatment.7 Indeed, women assigned to delayed treatment started chemotherapy 4.8 months later than those assigned to early treatment, with no detriment to overall survival, whereas early treatment was detrimental to quality of life.7 The counterintuitive results of the OVO5/EORTC 55955 study led to a variety of criticisms about the trial and its conclusions, which have been vigorously defended.8
Since the presentation of the OVO5/EORTC 55955 results, we have counseled patients at our institution about their follow-up at completion of their first-line chemotherapy. We have offered them 3 options for follow-up: (1) not to have routine CA125 measurements providing they are well and have no symptoms suggesting relapse, (2) to continue having routine CA125 measurements but not be told the result unless requested by the patient (particularly suitable for patients on clinical trials, where regular CA125 measurements are mandated), and (3) to have routine CA125 measurements and be told the results. We provided them with an information leaflet outlining the rationale for follow-up, why we recommend not having routine CA125 measurements, and the symptoms that should prompt an early appointment and CA125 measurement (appendix 1, http://links.lww.com/IGC/A456). We present an audit of practice at our institution since the presentation of the OVO5/EORTC 55955 results.
METHODS
Patients who had completed first-line therapy for epithelial ovarian, fallopian tube, or primary peritoneal cancer in our unit between July 2009 and December 2013 were identified from a preexisting database. The medical records of 143 patients who met these criteria were then analyzed (Fig. 1). Six (4%) of these patients did not receive first-line chemotherapy, 23 (16%) did not achieve remission (residual disease on computed tomography (CT) or persistently elevated CA125), and 27 (19%) were not followed up in our unit after completion of first-line therapy. These patients were excluded from further analysis. For the remaining 87 (61%) patients, data were collected in respect of the patient’s age, occupation, and social situation as well as the stage, grade and histology of disease, and CA125 level at diagnosis. Details of first-line surgery and chemotherapy received, the date of last chemotherapy received, and the presence or absence of residual disease after first-line therapy were documented as was the end-of-treatment CA125 level for each patient.
FIGURE 1.
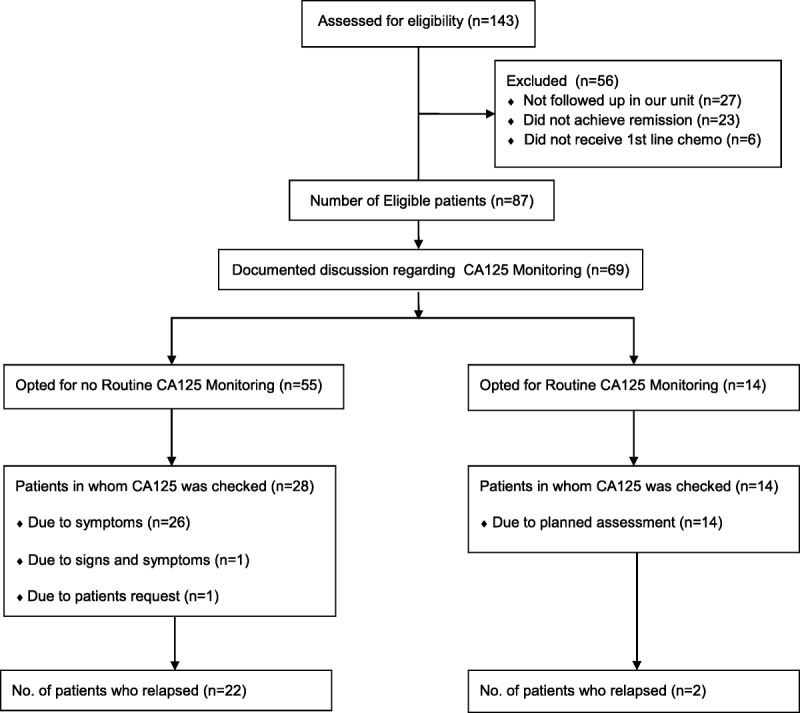
Consort flow diagram of patients included in the study.
Evidence of a discussion between the treating oncologist and the patient regarding CA125 monitoring at the end of treatment was recorded, and the outcome of that discussion was documented. The outcome was categorized as follows: no discussion documented; discussion documented, and the patient requested no routine CA125 monitoring; discussion documented, and the patient requested routine CA125 monitoring and to be informed of the result; discussion documented, and the patient requested routine CA125 monitoring but not to be informed of the result.
The number of CA125 tests performed during follow-up in each group was recorded as was the indication for each test, namely: planned; developed new signs; or developed new symptoms.
The number of CT scans performed during follow-up in each group was also recorded as was the indication for the scan and the scan result (recurrence vs no recurrence).
The number of patients who relapsed in each group was established, and the first indicator of relapse was recorded, namely: rising CA125 levels; development of new signs; or development of new symptoms.
Finally, the start date of second-line chemotherapy or salvage surgery was documented, and the patient’s status (alive or deceased) at the time of data collection was recorded.
RESULTS
A total of 87 (61%) of the 143 patients analyzed were eligible for inclusion in the study. Evidence of a discussion about the pros and cons of CA125 monitoring and an agreed CA125 follow-up plan were recorded in 69 (80%) of the 87 eligible patients on completion of first-line therapy. Of these 69 patients, no routine CA125 follow-up was selected by 55 (80%), and routine CA125 follow-up was selected by 14 (20%). Eleven (79%) patients who opted for routine CA125 follow-up requested that they be informed of the results, and 3 (21%) wished not to be informed.
The “No Routine CA125” Monitoring Group
The average age of patients in the “no routine CA125” monitoring group at completion of first-line chemotherapy was 65.3 years (range, 48–85) (Table 1). The average end-of-treatment CA125 level was 16.9 (range, 7–35). Forty-three (78%) patients lived with another family member, and 12 (22%) patients lived alone. Fifteen (27%) were employed at diagnosis, 12 (22%) were retired, 23 (42%) were not employed, and in 5 (9%) cases, the employment history was not documented.
TABLE 1.
Patient characteristics, CA125 testing, and number of relapses in each patient group
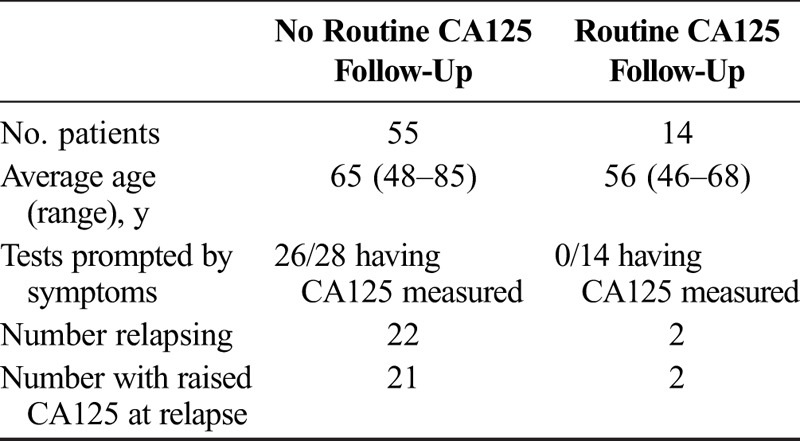
In total, CA125 levels were checked in 28 (51%) patients in the no routine CA125 monitoring group during follow-up after completion of first-line therapy. In 26 (93%) cases, CA125 testing was instigated because of the development of symptoms, in 1 (3.5%) case because of the development of clinical signs, and in 1 (3.5%) case, specifically at the patient’s request. Relapse was detected in 21 (75%) of those who had a CA125 test performed. Relapse was also detected by CT scan in 1 patient without a CA125 level being checked in advance.
Hence, overall, 22 (40%) patients in the no routine CA125 follow-up group relapsed after completion of first-line therapy. The first indicator of relapse was symptoms in 21 (95%) and signs in 1 (5%). The average CA125 level at relapse was 346 (range, 41–1656).
Twenty (90%) of the patients who relapsed subsequently underwent second-line chemotherapy, and 1 (5%) patient underwent salvage surgery. The mean treatment-free interval was 395 days. At the time of data collection, 12 (55%) of 22 relapsed patients remained alive and under follow-up, 9 (40%) of 22 had died, and 1 (5%) of 22 was lost to follow-up at completion of second-line therapy.
The “Routine CA125” Monitoring Group
The average age of patients in the “routine CA125” monitoring group at completion of first-line chemotherapy was 56 years (range, 46–68). The average end-of-treatment CA125 level was 13 (range, 7–24). Twelve (86%) patients lived with another family member, and 2 (14%) patients lived alone. Eight (58%) patients were employed at diagnosis, 3 (21%) were retired, and 3 (21%) were not employed.
CA125 levels were checked in all 14 (100%) patients who had requested routine CA125 follow-up. The indication for CA125 measurement was planned follow-up in all cases, although 1 patient had also developed symptoms at the time of routine CA125 monitoring. Relapse was detected in 2 (14%) of those who requested routine CA125 measurements. Both relapsed patients underwent second-line chemotherapy. The mean treatment-free interval was 585 days, and the average CA125 level at relapse was 39 (range, 35–43).
At the time of data collection, both patients remain alive and under follow-up.
CONCLUSIONS
This audit shows that if patients who have completed first-line chemotherapy for ovarian cancer are appropriately educated and counseled about follow-up, the majority opt not to have routine CA125 measurements. Since the results of the MRC OVO5/EORTC 55955 trial were presented, it has been our recommendation to patients not to have routine CA125 measurements. At the same consultation that we discuss follow-up options, we also give patients a leaflet that provides information on why we carry out follow-up, the different follow-up options, and a list of symptoms that should prompt an earlier appointment (Appendix 1, http://links.lww.com/IGC/A456). Aspects that are frequently discussed include the management dilemma of a woman with a rising serum CA125 concentration who remains well and asymptomatic.9 The results of the MRC OVO5/EORTC55955 trial suggest that as long as the patient is well and there is no evidence of current or impending compromised organ function, it is reasonable to delay chemotherapy. Although patients are reassured by the finding of a normal CA125 reading, many find routine CA125 measurement to be a major source of anxiety.10,11 The guidelines produced by various oncological organizations at least now suggest that patients should be offered a choice, although some are more in favor of routine CA125 measurements than others.12,13
It seemed that those who accepted our advice were older and had a higher relapse rate than those who opted for routine CA125 measurements. No association could be demonstrated between tumor stage, grade and histological subtype, and choice of CA125 monitoring in this audit. However, the study may have been underpowered to detect such an association. We had insufficient numbers to determine whether factors such as future planning for work, family events, or holidays influenced patients’ decisions related to follow-up options.
Since the OVO5/55955 trial was completed, bevacizumab, given with first-line carboplatin and paclitaxel and then as maintenance therapy, has been shown to improve survival in a high-risk subgroup of patients with ovarian cancer.14 It is standard practice to monitor for tumor progression with 3 or 6 weekly CA125 measurements while on bevacizumab maintenance therapy, even though a rising level should not on its own lead to discontinuation of bevacizumab. Once patients complete bevacizumab maintenance with maintained remission, they can continue follow-up without the need for routine CA125 measurements. Majority of women are currently not receiving maintenance therapy so do not require routine CA125 monitoring.
If there was therapy that could improve survival of patients with relapsed ovarian cancer and the improvement in survival was dependent on the tumor volume being as small as possible, it would then be advisable to perform routine CA125 monitoring. Unfortunately, although several therapies have been shown to improve progression-free survival, no therapy for relapsed ovarian cancer has so far been shown to significantly improve survival. Retrospective data from the AGO-DESKTOP trial demonstrated that cytoreductive surgery offered a survival benefit in patients if complete resection was achieved,15 highlighting the importance of being able to predict those patients in whom complete resection is a realistic goal. The first prospective surgical trial in recurrent ovarian cancer, AGO-DESKTOPII, validated a predictive score for complete resection, the “AGO score”, which may be a useful tool in selecting patients for secondary cytoreductive surgery. DESKTOP II showed that patients with an Eastern Cooperative Oncology Group PS0, no residual disease after surgery for primary ovarian cancer, and an absence of ascites preoperatively had a 76% likelihood of undergoing complete resection.16 The randomized prospective AGO-DESKTOP III trial is currently underway investigating the role of surgery and chemotherapy versus chemotherapy alone in patients with recurrent platinum-sensitive ovarian cancer, the results of which may help to clarify whether cytoreductive surgery significantly improves survival in this patient group and identify which patients would benefit from this intervention. These results may in turn have an impact on the role of CA125 monitoring in follow-up.
We conclude that, when given sufficient information about the role of routine CA125 measurements during follow-up, the majority decide against CA125 monitoring and, hence, avoid these routine blood tests.
Supplementary Material
Footnotes
The authors declare no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citation appears in the printed text and is provided in the HTML and PDF versions of this article on the journal’s Web site (www.ijgc.net).
REFERENCES
- 1.International Agency for Research on Cancer. Global cancer statistics. 2008. Available at: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx. Accessed November 12, 2016.
- 2.Jayson GC, Kohn EC, Kitchener HC, et al. Ovarian cancer. Lancet. 2014;384:1376–1388. [DOI] [PubMed] [Google Scholar]
- 3.Rustin GJ, Marples M, Nelstrop AE, et al. Use of CA-125 to define progression of ovarian cancer in patients with persistently elevated levels. J Clin Oncol. 2001;19:4054–4057. [DOI] [PubMed] [Google Scholar]
- 4.Rustin GJ, Nelstrop AE, Tuxen MK, et al. Defining progression of ovarian carcinoma during follow-up according to CA 125: a North Thames Ovary Group Study. Ann Oncol. 1996;7:361–364. [DOI] [PubMed] [Google Scholar]
- 5.van der Burg ME, Lammes FB, Verweij J. The role of CA 125 in the early diagnosis of progressive disease in ovarian cancer. Ann Oncol. 1990;1:301–302. [DOI] [PubMed] [Google Scholar]
- 6.Vergote I, Rustin GJ, Eisenhauer EA, et al. Re: new guidelines to evaluate the response to treatment in solid tumors [ovarian cancer]. Gynecologic Cancer Intergroup. J Natl Cancer Inst. 2000;92:1534–1535. [DOI] [PubMed] [Google Scholar]
- 7.Rustin GJ, van der Burg ME, Griffin CL, et al. Early versus delayed treatment of relapsed ovarian cancer (MRC OV05/EORTC 55955): a randomised trial. Lancet. 2010;376:1155–1163. [DOI] [PubMed] [Google Scholar]
- 8.Roux R, Romero I, Zweifel M, et al. Recurrent ovarian cancer: when to treat and how to assess. In: Ledermann JA, Creutzberg CL, Quinn MA, eds. Controversies in the Management of Gynaecological Malignancies. London, England: Springer Verlag; 2014:17–27. [Google Scholar]
- 9.Goonewardene TI, Hall MR, Rustin GJ. Management of asymptomatic patients on follow-up for ovarian cancer with rising CA-125 concentrations. Lancet Oncol. 2007;8:813–821. [DOI] [PubMed] [Google Scholar]
- 10.Parker PA, Kudelka A, Basen-Engquist K, et al. The associations between knowledge, CA125 preoccupation, and distress in women with epithelial ovarian cancer. Gynecol Oncol. 2006;100:495–500. [DOI] [PubMed] [Google Scholar]
- 11.Reid A, Ercolano E, Schwartz P, et al. The management of anxiety and knowledge of serum CA-125 after an ovarian cancer diagnosis. Clin J Oncol Nurs. 2011;15:625–632. [DOI] [PubMed] [Google Scholar]
- 12.Ledermann JA, Raja FA, Fotopoulou C, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi24–vi32. [DOI] [PubMed] [Google Scholar]
- 13.van der Zee AG, Colombo N, Gitsch G, et al. ESGO statement on the role of CA-125 measurement in follow-up of epithelial ovarian cancer. Int J Gynecol Cancer. 2012;22:175. [DOI] [PubMed] [Google Scholar]
- 14.Oza AM, Cook AD, Pfisterer J, et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015;16:928–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harter P, du Bois A, Hahmann M, et al. Surgery in recurrent ovarian cancer: the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) DESKTOP OVAR trial. Ann Surg Oncol. 2006;13:1702–1710. [DOI] [PubMed] [Google Scholar]
- 16.Harter P, Sehouli J, Reuss A, et al. Prospective validation study of a predictive score for operability of recurrent ovarian cancer: the Multicenter Intergroup Study DESKTOP II. A project of the AGO Kommission OVAR, AGO Study Group, NOGGO, AGO-Austria, and MITO. Int J Gynecol Cancer. 2011;21:289–295. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


