Case report.
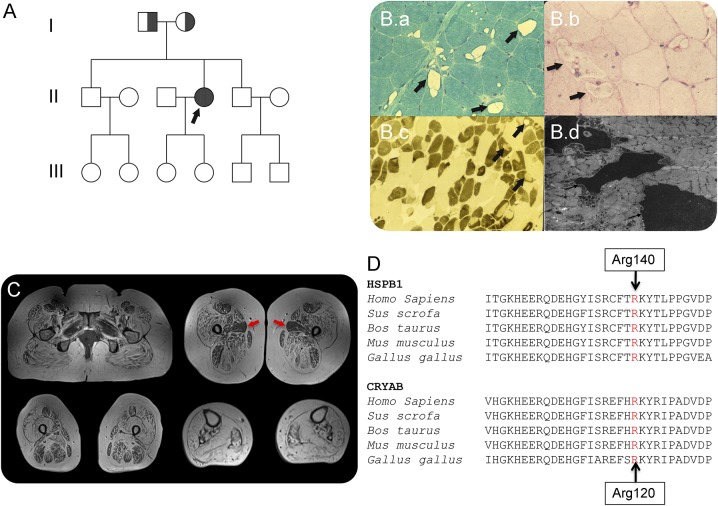
A 57-year-old woman, born to parents of Gujarati Indian descent (figure, A), presented at age 19 with pain and stiffness in her calves and a tendency to trip. In her 20s, a formal neurologic examination demonstrated predominantly distal lower limb weakness and normal upper limb muscle strength.1 Motor and sensory nerve conduction studies were normal with the exception that no motor response was elicited from the extensor digitorum brevis. Fibrillations and polyphasic action potentials were present on EMG. The creatine kinase (CK) level was 1,452 IU/L. A quadriceps muscle biopsy was performed at age 27 from which images were available in the records.1 At that time, muscle fiber diameters were large ranging from 50 to 80 μm. Many of the fibers contained single or multiple unrimmed vacuoles that appeared empty in the modified Gomori trichrome preparation (figure, B.a). There was no increase in endomysial connective tissue or evidence of inflammation, necrosis, or regeneration. There was no evidence of glycogen, increased lipid, or acid phosphatase staining in the vacuoles (figure, B.b). The ATPase at pH 9.5 demonstrated that most of the vacuolated fibers were of type 2, and electron microscopy showed electron-dense material within vacuoles (figure, B.c and d).1 The overall appearances were those of a vacuolar myopathy without any features suggesting neurogenic change, and she was diagnosed with a distal myopathy.1
Figure. Clinical-pathologic features of patient homozygous for the HSPB1 p.Arg140Gly mutation and protein conservation between species.
(A) Family pedigree: an arrow indicates the proband; half-filled indicates distal weakness in parents who were heterozygous for p.Arg140Gly mutation. (B) Biopsy of the quadriceps muscle performed at age 27; (B.a) modified Gomori trichrome staining shows variation in fiber diameter and prominent vacuoles within many muscle fibers, arrows; (B.b) Periodic acid–Schiff preparation showed no evidence of glycogen accumulation within vacuoles (arrows); (B.c) ATPase pH 9.5 demonstrates that vacuoles are predominantly in darkly stained type 2 fibers, arrows; and (B.d) ultrastructural examination of the muscle revealed electron-dense material within vacuoles (arrows). (C) Muscle MRI demonstrating severe widespread fatty infiltration of pelvic, thigh, and calf muscles with relative sparing of the adductor longus (red arrows). (D) Conservation of HSPB1 and HSPB5 (CRYAB) amino acid sequence between species. The Arg140 residue in HSPB1 is well conserved and corresponds to the position of Arg120 in the CRYAB gene.
She re-presented at age 57 following the development of slowly progressive severe upper and lower limb weaknesses. On examination at age 57, there was evidence of distal more than proximal upper limb weakness affecting wrist extension (Medical Research Council [MRC] grade 4+/5), finger extension (4 + 5), first dorsal interossei (1/5), abductor pollicis brevis (3/5 right and 4/5 left), and abductor digiti minimi (3/5 right and 4/5 left). In the lower limbs, she had severe proximal weakness (grade 2/3) with no movement at the ankles. Sensory modalities were preserved except for reduced vibration sense at the ankles. She was areflexic. Her CK level was 404 IU/L. Neurophysiologic studies suggested an axonal motor neuropathy. Sensory nerve action potentials were, nevertheless, at the lower limit of normal for amplitude in the lower limbs (right sural 6 μV, right superficial peroneal 7 μV, and normal range >5 μV), and distal lower limb motor responses were absent. Needle EMG showed prominent chronic neurogenic changes with large motor units recruiting in reduced numbers but at increased firing rates to a reduced interference pattern. This EMG pattern was most pronounced distally but evident proximally in the upper and lower limbs. No low amplitude or brief polyphasic motor units were seen on any occasion at re-presentation. Muscle MRI was performed, showing widespread severe muscle fatty replacement (figure, C).
There was no relevant family history. Her mother has diabetes and her father a right above knee amputation for peripheral vascular disease. Neither had neurologic complaints. However, clinical examination of both in their 80s revealed mild distal weakness (MRC grade 4/5) in the upper and lower limbs with areflexia. Pinprick sensation was reduced to the mid-forearm and foot in her mother. Her father had reduced vibration sense to the left ankle. Neurophysiologic testing was not possible in either.
Targeted exome sequencing of the proband's DNA using the Agilent Focused Exome kit identified a homozygous variant (c.418C>G, p.Arg140Gly) in HSPB1, which was confirmed by Sanger sequencing. Both parents were heterozygous. We have reported this variant previously in heterozygous form in individuals from 5 Indian Gujarati families with distal motor neuropathy.2
Discussion.
Our patient presented at age 19 with clinical and biopsy features consistent with a distal myopathy. Prominent vacuoles in type 2 fibers contained granular, electron-dense material (figure, B) that was interpreted to represent the product of myofibrillar degeneration.1 At presentation, neurophysiologic studies did show fibrillations and polyphasic action potentials. However, nerve conduction studies were normal with the exception of 1 absent motor nerve response, and overall, the clinical image was felt to represent a distal myopathy at this time. Subsequent neurophysiologic studies performed at age 57 following progressive limb weakness revealed an axonal motor neuropathy. Although chronic end-stage myopathy may have neurophysiologic features that can appear neurogenic, in this case, even the less affected proximal limb muscles failed to demonstrate any myopathic motor units or myopathic recruitment.
HSPB1 is a small heat-shock protein highly expressed in striated muscle with an important role in maintaining myofibrillar structure during stress conditions.3
Mutations in HSPB1, HSPB3, and HSPB8 are classically associated with motor neuropathy.4 HSPB5 (CRYAB) has been associated with a wide spectrum of clinical manifestations including desmin-related myofibrillar myopathy. The protein position of the Arg140Gly HSPB1 mutation in our case corresponds to the Arg120 HSPB5 residue mutated in this myopathy (figure, D).4 Heterozygous mutations in HSPB8 have recently been reported causing neuropathy and distal myopathy with rimmed vacuoles and fibrillar aggregates in 2 families.5 Subsequently, distal myopathy and neuronopathy have been attributed to an HSPB1 mutation in 1 family.6 We describe a patient with a homozygous HSPB1 mutation also presenting with a distal vacuolar myopathy, motor neuropathy, and minimal sensory involvement, supporting the association of HSPB1 mutations with this phenotype. This expands the genetic testing indicated in distal vacuolar myopathy.
Footnotes
Author contributions: Enrico Bugiardini: drafting and revising the manuscript, study concept, and interpretation of data. Alexander M. Rossor: interpretation of data and revising the manuscript. David S. Lynch: acquisition of data and analysis of data. Michael Swash: interpretation of data and revising the manuscript. Alan M. Pittman: acquisition of data and analysis of data. Julian C. Blake: interpretation of data and revising the manuscript. Michael G. Hanna: revising the manuscript and study concept. Henry Houlden: revising the manuscript and study concept. Janice L. Holton: interpretation of data and revising the manuscript. Mary M. Reilly: revising the manuscript and study concept. Emma Matthews: drafting and revising the manuscript, study concept, and interpretation of data.
Study funding: The research leading to these results has received funding from the European Community's Seventh Framework Programme (FP7/2007–2013) under grant agreement no. 2012-305121 “Integrated European–omics research project for diagnosis and therapy in rare neuromuscular and neurodegenerative diseases (NEUROMICS).” This work is also supported by a Medical Research Council Centre grant and Wellcome Trust grant on Synaptopathies. Part of this work was undertaken at University College London Hospitals/University College London, which received a proportion of funding from the Department of Health's National Institute for Health Research Biomedical Research Centres' funding scheme.
Disclosure: E. Bugiardini reports no disclosures. A.M. Rossor is funded by a Wellcome Trust Postdoctoral Fellowship for Clinicians (110043/Z/15/Z). D.S. Lynch reports no disclosures. M. Swash has received speaker honoraria for lectures and educational activities not funded by industry; has served on the editorial boards of various scientific and medical journals; has held patents regarding electronic measuring devices; receives royalties from the publication of several books; has served as Chief Medical Officer for Swiss Re Life and Health; serves on the Board of Directors for MedHand AB Stockholm and Malvern Arts Press; has served as an advisor to Best Doctors Inc., Europe (receives compensation); holds stock/stock options in MedHand AB Stockholm; and serves as an expert witness for legal proceedings. A.M. Pittman and J.C. Blake report no disclosures. M.G. Hanna is supported by a Medical Research Council Centre grant, the National Centre for Research Resources, the Myositis Support Group, and the National Highly Specialised Service (HSS) Department of Health UK and has been a consultant for Novartis. H. Houlden has received research support from the Medical Research Council (MRC) UK, The BRT, The MDA USA, Muscular Dystrophy UK, Ataxia UK, Muscular Dystrophy UK, Rosetrees Trust, The Wellcome Trust, and the National Institute for Health (NIHR) UCL/UCLH BRC. J.L. Holton has received travel funding from Merck Serono; has served on the editorial board of Neuropathology Applied Neurobiology; has been an employee of University College London; and has received research support from Alzheimer's Research Trust, the Margaret Watson Memorial Trust Grant from The Sarah Matheson Trust, Action Medical Research, Brain Net Europe: Support for the Queen Square Brain Bank for Neurological Disorders, the Sarah Matheson Trust, Myositis Support Group, the Multiple System Atrophy Trust, Michael J Fox Foundation for Parkinson's Research, Alzheimer's Research UK, MSA Coalition, King Baudouin Foundation Sophia Fund, and Medical Research Council. M.M. Reilly receives support from the Medical Research Council (MRC), MRC Centre grant (G0601943), and the National Institutes of Neurological Diseases and Stroke and office of Rare Diseases (U54NS065712); has served on the editorial boards of Brain, Neuromuscular Disorders; Journal of the Peripheral Nervous System; and JNNP; has been a consultant for Servier, Acceleron, and Alnylam; and has received research support from Wellcome Trust, UCL CBRC, Ipsen, Muscular Dystrophy Association (US), Muscular dystrophy campaign grants, CLH/UCL Comprehensive Biomedical Research Centre, and CRDC. E. Matthews is funded by a postdoctoral fellowship from the NIHR and has received research support from the Brain Research Trust and the Muscular Dystrophy Campaign. Go to Neurology.org/ng for full disclosure forms. The Article Processing Charge was funded by the authors.
References
- 1.Swash M, Schwarz MS, Thompson A, Cox E, Gray A. Distal myopathy with focal granular degenerative change in vacuolated type 2 fibers. Clin Neuropathol 1988;7:249–253. [PubMed] [Google Scholar]
- 2.Rossor AM, Morrow JM, Polke JM, et al. . Pilot phenotype and natural history study of hereditary neuropathies caused by mutations in the HSPB1 gene. Neuromuscul Disord 2017;27:50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sugiyama Y, Suzuki A, Kishikawa M, et al. . Muscle develops a specific form of small heat shock protein complex composed of MKBP/HSPB2 and HSPB3 during myogenic differentiation. J Biol Chem 2000;275:1095–1104. [DOI] [PubMed] [Google Scholar]
- 4.Benndorf R, Martin JL, Kosakovsky Pond SL, Wertheim JO. Neuropathy- and myopathy-associated mutations in human small heat shock proteins: characteristics and evolutionary history of the mutation sites. Mutat Res Rev Mutat Res 2014;76:15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghaoui R, Palmio J, Brewer J, et al. . Mutations in HSPB8 causing a new phenotype of distal myopathy and motor neuropathy. Neurology 2016;86:391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis-Smith DJ, Duff J, Pyle A, et al. . Novel HSPB1 mutation causes both motor neuronopathy and distal myopathy. Neurol Genet 2016;2:e110 doi: 10.1212/NXG.0000000000000110. [DOI] [PMC free article] [PubMed] [Google Scholar]