Abstract
Background
Previous studies suggest that cannabidiol (CBD) may potentiate or antagonize Δ9-tetrahydrocannabinol’s (THC) effects. The current study examined sex differences in CBD-THC interactions on antinociception, locomotion, and THC metabolism.
Methods
In Experiment 1, CBD (0, 10 or 30 mg/kg) was administered 15 min before THC (0, 1.8, 3.2, 5.6 or10 mg/kg), and rats were tested for antinociception and locomotion 15–360 min post-THC injection. In Experiments 2 and 3, CBD (30 mg/kg) was administered 13 hr or 15 min before THC (1.8 mg/kg); rats were tested for antinociception and locomotion 30–480 min post-THC injection (Experiment 2), or serum samples were taken 30–360 min post-THC injection to examine CBD modulation of THC metabolism (Experiment 3).
Results
In Experiment 1, CBD alone produced no antinociceptive effects, while enhancing THC-induced paw pressure but not tail withdrawal antinociception 4–6 hr post-THC injection. CBD alone increased locomotor activity at 6 hr post-injection, but enhanced THC-induced hypolocomotion 4–6 hr post-THC injection, at lower THC doses. There were no sex differences in CBD-THC interactions. In Experiments 2 and 3, CBD did not significantly enhance THC’s effects when CBD was administered 13 hr or 15 min before THC; however, CBD inhibited THC metabolism, and this effect was greater in females than males.
Conclusions
These results suggest that CBD may enhance THC’s antinociceptive and hypolocomotive effects, primarily prolonging THC’s duration of action; however, these effects were small and inconsistent across experiments. CBD inhibition of THC metabolism as well other mechanisms likely contribute to CBD-THC interactions on behavior.
Keywords: Sex Differences, Cannabinoids, Gender, Pain, Sedation
1. Introduction
Cannabis has been used to treat pain for centuries (reviewed in Aggarwal et al., 2008). The main psychoactive constituent of cannabis, Δ9-tetrahydrocannabinol (THC), produces antinociception in studies of acute pain in animals (Smith et al., 1998; Tseng and Craft, 2001; Varvel et al., 2005) and in humans (Greenwald et al., 2000). Cannabinoids such as THC also produce antinociception against chronic pain in animals (Cox and Welch, 2004; Mao et al., 2000; Schley et al., 2006) and in humans (reviewed in Lynch and Ware, 2015). Studies on the pain-relieving effects of cannabidiol (CBD), another cannabis constituent that may have therapeutic potential, are limited. CBD did not produce antinociception using acute pain tests in mice (Booker et al., 2009; Sanders et al., 1979; Sofia et al., 1975; Varvel et al., 2006) or rats (Sofia et al., 1975), although it did prevent the development of allodynia in paclitaxel-treated mice (Ward et al., 2014). However, CBD and THC have been shown to synergize on several outcomes, including inhibition of glioblastoma cell proliferation, in vitro (Marcu et al., 2010), and THC-induced place preference (Klein et al., 2011), hypolocomotion (Fernandes et al., 1974; Hayakawa et al.,2008) and antinociception (Karniol and Carlini, 1973) in male rodents. In humans, a 1:1 CBD-THC combination produced greater pain relief than THC alone (Johnson et al., 2010).
Several studies have shown that THC has greater effects in females than males. For instance, female rats given THC show greater catalepsy (Craft et al., 2012; Tseng and Craft, 2001), anxiety-like behavior (Harte-Hargrove et al., 2012), hypothermia (Wiley et al., 2007), hypolocomotion (Craft et al., 2012; Tseng and Craft, 2001), and antinociception (e.g., Romero et al., 2002; Tseng and Craft, 2001) compared to males given the same dose of THC. Although several studies have found sex differences in THC’s behavioral effects, to our knowledge no previous studies have compared CBD alone or CBD-THC interactions on behavior in males and females of any species.
One potential mechanism underlying CBD-THC interactions is CBD-induced inhibition of THC metabolism. THC is metabolized primarily by the liver enzyme cytochrome P450 3A (Watanabe et al., 2007) into the active metabolite 11-OH-THC and inactive metabolite THC-COOH (Wall et al., 1983), but also may be converted into cannabinol (CBN), a less potent psychoactive (Perez-Reyes et al., 1973) and antinociceptive (Sofia et al., 1975; Sanders et al., 1979; Booker et al., 2009) cannabinoid in comparison to THC. Female rats produce more 11-OH-THC than males do (Narimatsu et al., 1991; Wiley and Burston, 2014), and this appears to contribute to greater THC-induced behavioral effects in female compared to male rats (Tseng et al., 2004). CBD has been shown to inhibit metabolism of THC by inactivating cytochrome P450 3A (Bornheim and Grillo, 1998). For example, in an in vitro assay, CBD reduced THC metabolism by 60% (Jaeger, Benet and Bornheim, 1996). Further, when CBD was given to mice 15–60 min prior to THC administration, increased THC levels were found in the brain (Jones and Pertwee, 1972; Reid and Bornheim 2001).
The interval between CBD and THC administration has been suggested to affect CBD-THC interactions (Zuardi et al., 2012), but no systematic investigation of the effects of CBD pretreatment time has been conducted. Thus, the purpose of the present study was three-fold: to examine sex differences in CBD-THC interactions on nociception and locomotion, to determine whether CBD pretreatment time alters the drug interaction, and to determine whether CBD alters THC metabolism similarly in both sexes. In the first experiment, multiple CBD-THC dose combinations were examined using a 15-min interval between CBD and THC injections. Zuardi et al. (2012) estimated that a 1.8:1 CBD:THC ratio is optimal for observing CBD enhancement of THC’s effect. Therefore, we examined a wide range of CBD:THC dose ratios that included this dose ratio. Based on the results of the first experiment, the dose combination of CBD 30 mg/kg + THC 1.8 mg/kg was chosen to compare two pretreatment times for CBD, one short (15 min) and one long (13 hr), to determine if CBD pretreatment time would alter CBD-THC interactions (Experiment 2). One previous study using a 24-hr CBD pretreatment time reported that CBD enhanced THC’s effect on corticosterone levels, suggesting that CBD can alter THC’s effects even when CBD is administered long before THC (Zuardi et al., 1984). The same dose combination was examined in Experiment 3, to determine the effects of CBD given at the two pretreatment times on THC metabolism.
2. Methods
2.1 Animals
All experiments were completed in accordance with the NIH Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 2011). Gonadally intact, male and female Sprague-Dawley rats aged 60–100 days were used (bred in-house from Harlan stock, Livermore, CA). They were housed in same-sex groups of 2–3, under a 12:12 hr light:dark cycle (lights on at 0700 h). The room was maintained at 21±2°C. Food and water were available ad libitum except during testing. Rats were randomly assigned to treatment groups with the exception that same-sex siblings generally were not assigned to the same treatment group.
2.2 Drugs
Drugs were obtained from the National Institute on Drug Abuse (Bethesda, MD). THC and CBD were dissolved in a 1:1:18 ethanol:cremophor:saline solution, which also served as the vehicle. Drugs were administered by i.p. injection.
2.3 Apparatus
Tail withdrawal antinociception was measured using a 2.5-liter warm water bath (Precision Scientific Inc., Winchester, VA) set to 50 ± 0.5°C; latency to withdraw the tail was measured using a hand-held stopwatch with a 15-s cutoff. Paw pressure antinociception was measured using an Analgesy meter (Ugo-Basile, Varese, Italy). The pressure on the paw began at 30 g and increased at a constant rate of 48 g/s to a maximum of 750 g (15-s cutoff). Horizontal locomotor activity was measured in a 20 × 40 × 23 cm clear Plexiglas rodent cage placed within a photo-beam apparatus (Opto-Varimex, Columbus Instruments, Columbus, OH) that has 15 photo-beams spaced 2.5 cm apart, and the number of photobeam breaks in 10 min was recorded.
2.4 Behavioral Procedures
All testing was completed during the light phase (between 0730 – 1700 h). In Experiments 1 and 2, baseline data on the tail withdrawal and paw pressure tests were collected 3 times over 30 min just prior to the first injection. In Experiment 1, rats were given vehicle or CBD (10 or 30 mg/kg) i.p. followed 15 min later by vehicle or THC (1.8, 3.2, 5.6, or 10 mg/kg) i.p. Rats were then tested on tail withdrawal and paw pressure tests at 15, 30, 60, 120, 240 and 360 min post-THC injection. Locomotor activity was tested for 10 min at each time point, after completion of nociceptive testing. In Experiments 2 and 3, rats were given vehicle or CBD 30 mg/kg i.p. either 13 hr or 15 min before vehicle or THC 1.8 mg/kg i.p. In Experiment 2 rats were then tested on tail withdrawal and paw pressure tests at 30, 60, 120, 240, 360 and 480 min post-THC injection. Again, locomotor activity was tested for 10 min at each time point, after completion of nociceptive testing. In Experiment 3, trunk blood was collected at 30, 120, or 360 min post-THC injection (separate rats at each time point); blood samples were centrifuged for 20 min at 2000 rpm at −4 °C, and serum samples were stored at −80°C for later determination of cannabinoid concentrations.
2.5 Determination of Estrous Cycle
Vaginal lavage samples were collected from all females after completion of behavioral testing. Vaginal cytology was scored as follows: the proestrus stage was identified by a predominance (75% or more of cells in the sample) of nucleated epithelial cells; estrus by dense sheets of cornified epithelial cells; diestrus by scattered, nucleated and cornified epithelial cells, and leukocytes (Freeman, 1994).
2.6 Serum Cannabinoid Analysis
Quantitation of THC, THC metabolites, CBD and CBN in rat blood was achieved using an ultra performance liquid chromatography system (Waters Acquity I-Class UPLC, Milford, MA, USA) coupled with a quadrupole time of flight mass spectrometer (QTOF, Waters Xevo G2, Manchester, UK). The first step of sample preparation was sample centrifugation at 8000 rpm for 10 min to remove any remaining cells. 185 uL of the resulting supernatant was spiked with 15 uL of solution containing 200 ppb each of the deuterated standards (THC-d3, OH-THC-d3, COOH-THC-d3, CBD-d3 and CBN-d3, Cerilliant, Round Rock, TX). Combined with the high resolution and accurate mass of the QTOF platform, these internal standards can minimize contributions from non-ideal metabolite extraction and instrumental variability and allow for direct quantitation of each targeted analyte. Following the internal standard addition, protein precipitation was promoted by adding 400 uL of cold acetonitrile (ACN) dropwise while vortexing. Immediately the samples experienced centrifugation at 4000 g for 10 min at 25°C. 0.6 mL of 1% ammonium hydroxide was added to the sample and vortexed before solid phase extraction (SPE). A mixed mode SPE cartridge (OAXIS Max 1 cc, Waters, Ireland) was used for cannabinoid isolation. Each SPE cartridge was conditioned with 1 mL of methanol followed by 1 mL of 1% ammonium hydroxide. After the cartridge conditioning, the newly prepared sample was loaded onto the SPE cartridge and pulled through the system using a light vacuum (~1–2 psi). Then 0.5 mL of 35% ACN was added and allowed to dry under full vacuum for 10 min. 1.5 mL of a hexane/ethyl acetate/acetic acid (49:49:2, v/v/v) mixture was used to elute the samples. The eluent was then evaporated under nitrogen at room temperature and 100 uL of a methanol:water solution (80:20, v/v) was used for final sample and transferred to an autosampler vial. Analyte separation was achieved using a 50-mm C18 BEH UPLC column (Waters, Milford, MA, USA) kept at 40°C. The mobile phases were high purity water (Fisher Scientific Co., Fair Lawn, NJ) with 0.1% formic acid (A) and pure acetonitrile (Fisher Scientific Co., Fair Lawn, NJ) with 0.1% formic acid (B), respectively. Initially mobile phase B was increased from 5% to 60% in 0.2 min, and kept increasing to 90% at 3.5 min. This level was kept for an additional 0.5 min. At 4 min, mobile B was decreased to its initial condition of 5% within 0.1 min and was held static for 0.9 min for column re-equilibration. With an operational flow rate of 0.3 mL/min, a total of 10 uL of each prepared sample was injected onto the column. The obtained experimental data was analyzed by TargetLynx (Waters, Milford, MA), a software used to generate quantitative results. Briefly, the parameters used for TargetLynx were listed below: Retention time window: ± 0.2 min, Response use: integrated area, Polynomial Type: linear, Weighting function: 1/X.
2.7 Data Analysis
Baseline nociceptive latencies for each rat on the tail withdrawal and paw pressure tests were calculated as the mean of the last two baseline trials. Due to baseline sex differences (see Results) and individual differences among rats, latency to respond on the tail withdrawal and paw pressure tests (in s) was converted to percentage of maximum possible effect (%MPE) for each rat at each time point: (drug latency - baseline latency)/(cutoff latency- baseline latency) × 100. To adjust for a baseline sex difference in locomotor activity (see Results) and habituation over time, locomotor data (photobeam breaks) were converted to percentage of the same-sex vehicle control group (% control) at each time point: (number of photobeam breaks in drug-treated rat/mean number of photobeam breaks in same-sex, vehicle control group) × 100.
In Experiment 1, time course data (%MPE and % control) were analyzed using a repeated measures 4-way ANOVA to determine whether the effects produced by CBD (3 levels) and THC (5 levels) in each sex (2 levels) changed over time, with time as the repeated measure (6 levels). Additionally, estrous stage modulation of drug effects was assessed in females by a 3-way ANOVA with factors of CBD (3 levels), THC (5 levels) and time (6 levels, repeated), with estrous stage entered as a covariate. In Experiment 2, time course data (%MPE and % control) were analyzed using a repeated measures 4-way ANOVA to determine whether CBD given at either pretreatment time (3 levels) and THC (2 levels) produced effects in each sex (2 levels) over time, with time as the repeated measure (6 levels). In Experiment 3, time course data were analyzed using a 3-way ANOVA to determine whether CBD given at either pretreatment time (3 levels) in each sex (2 levels) altered serum concentrations of THC, 11-OH-THC, THC-COOH, CBD and CBN over time (3 levels). Tukey’s HSD and Dunnett’s t-test were used for post-hoc determination of significance. Significance level was p≤ 0.05 for all statistical tests.
3. Results
3.1 Experiment 1: Time Course of CBD-THC Interactions
Analysis of baseline data showed sex differences in response latency on both nociceptive tests (tail withdrawal: Sex, F1,273=15.56, p<0.001; paw pressure: Sex, F1,273=4.92, p=0.027). On the tail withdrawal test, females had a longer latency to withdraw the tail compared to males (6.22 ± 0.15 vs. 5.38 ± 0.15 sec, respectively). On paw pressure test, males had a longer latency to withdraw the paw compared to females (7.21 ± 0.17 vs. 6.67 ± 0.17 sec, respectively). On the locomotor activity test, sex differences were time-dependent (Sex × Time, F5,1365=2.66, p=0.021); however, post-hoc analysis at each time point revealed no specific time points at which males and females were significantly different. The mean of all 10-min samples showed that females locomoted slightly more than males (367 ± 15 vs. 356 ± 15 photobeam breaks, respectively). Given the various sex differences in baseline measures, data were converted to %MPE (tail withdrawal and paw pressure) or % control (locomotor activity) before analysis of drug effects.
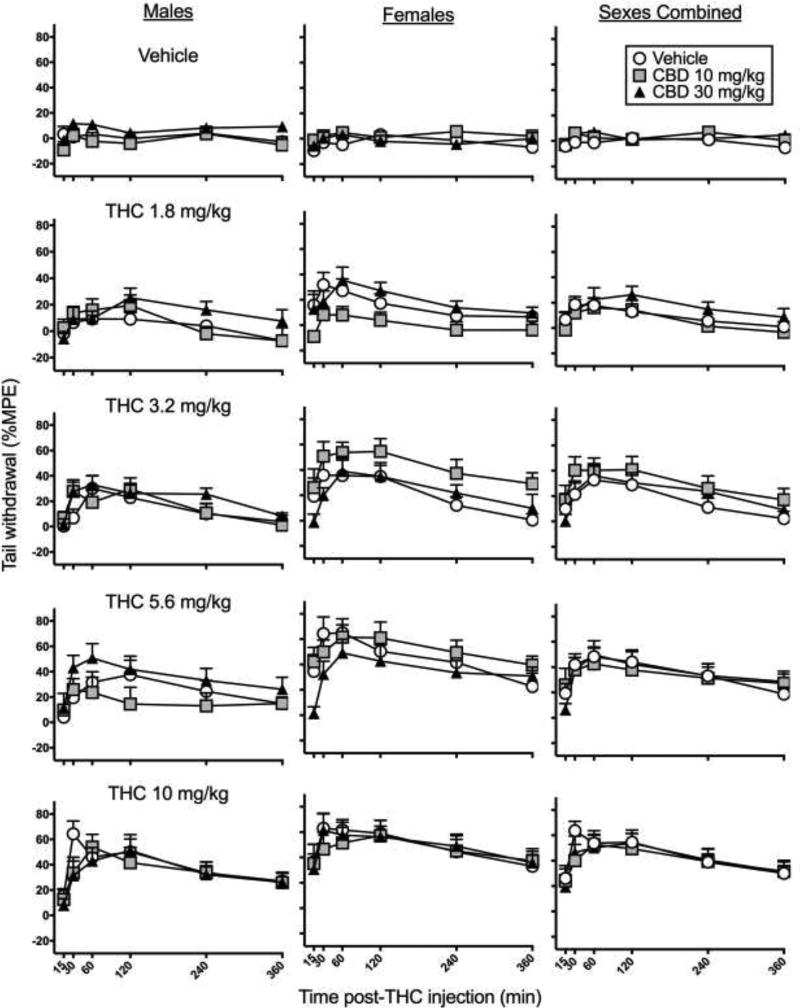
Figure 1 shows time-response curves on the tail withdrawal test in males, females and both sexes combined. THC produced dose- and time-dependent increases in tail withdrawal antinociception in both males and females, with peak effects occurring at 30 to 60 min post-injection (THC dose × Time, F10,1365=4.19 p<0.001). THC produced greater tail withdrawal antinociception in females compared to males (Sex × THC dose, F4,273=2.62, p=0.035). CBD produced some changes in tail withdrawal antinociception (CBD dose × Time, F10,1365=2.62, p=0.004); however, subsequent analysis of the CBD effect at each time point revealed no significant effects. There was no significant interaction between CBD and THC, or between CBD and sex.
Figure 1.
Experiment 1. Time-response curves on the tail withdrawal test in male rats (left), female rats (center) and both sexes combined (right). CBD was administered 15 min before THC. THC-induced antinociception was greater in females than males, with no significant CBD enhancement of THC’s effect in either sex. Each point is the mean ± 1 S.E.M of 10 male or female rats (left and center panels), or 20 total rats (right panel).
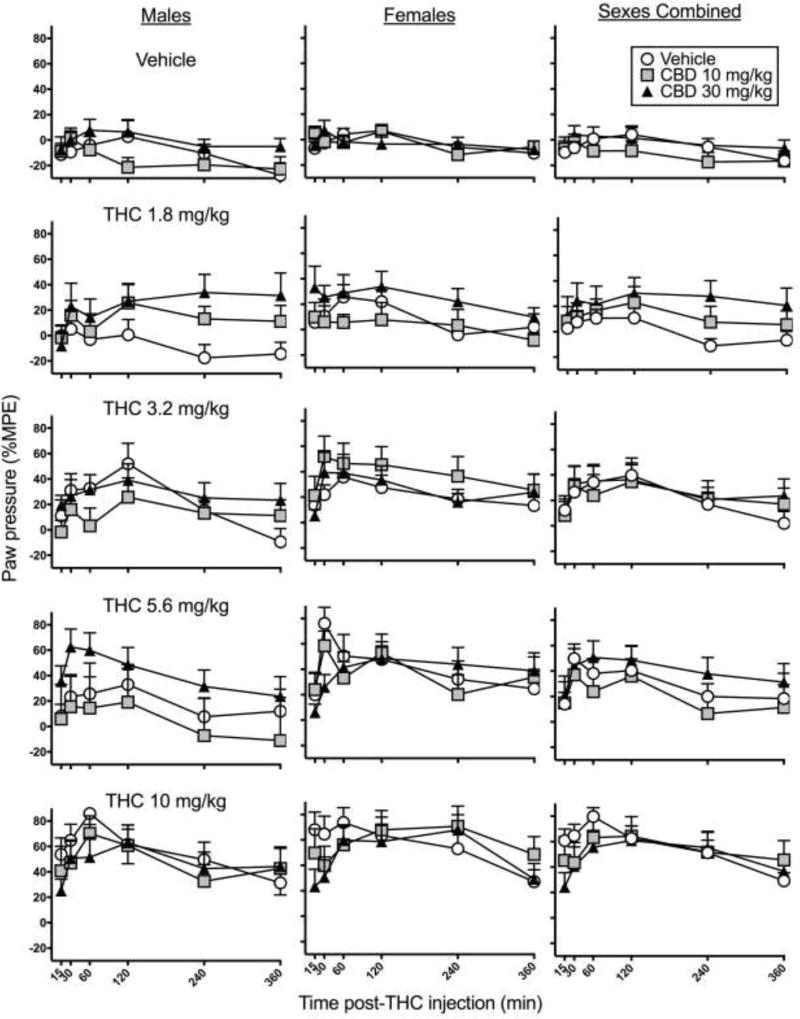
Figure 2 shows time-response curves for paw pressure antinociception in males, females, and both sexes combined. THC dose- and time-dependently increased paw pressure antinociception (THC dose × Time, F20,1365=1.60, p=0.044), and this effect was greater in females than males (Sex, F1,273=4.40, p=0.037). CBD altered paw pressure antinociception at some time points (CBD dose × Time, F10,1365=2.24, p=0.014). Subsequent analysis conducted at each time point showed that CBD increased paw pressure antinociception at 240 and 360 min post-THC injection (240 min, F2,300=3.51, p=0.031; 360 min, F2,300=3.61, p=0.028). Specifically, CBD 30 mg/kg increased paw pressure antinociception at 240 and 360 min post-THC injection (240 min, p=0.040; 360 min, p=0.014). Although CBD also appeared to decrease THC’s effect at early time points, there was no significant interaction between CBD dose and THC dose, or between CBD dose, THC dose and Time or Sex in the overall ANOVA.
Figure 2.
Experiment 1. Time-response curves on the paw pressure test in male rats (left), female rats (center) and both sexes combined (right). CBD was administered 15 min before THC. THC-induced antinociception was greater in females than males, and CBD enhanced THC’s effect at 240 and 360 min post-THC injection. Each point is the mean ± 1 S.E.M of 10 male or female rats (left and center panels), or 20 total rats (right panel).
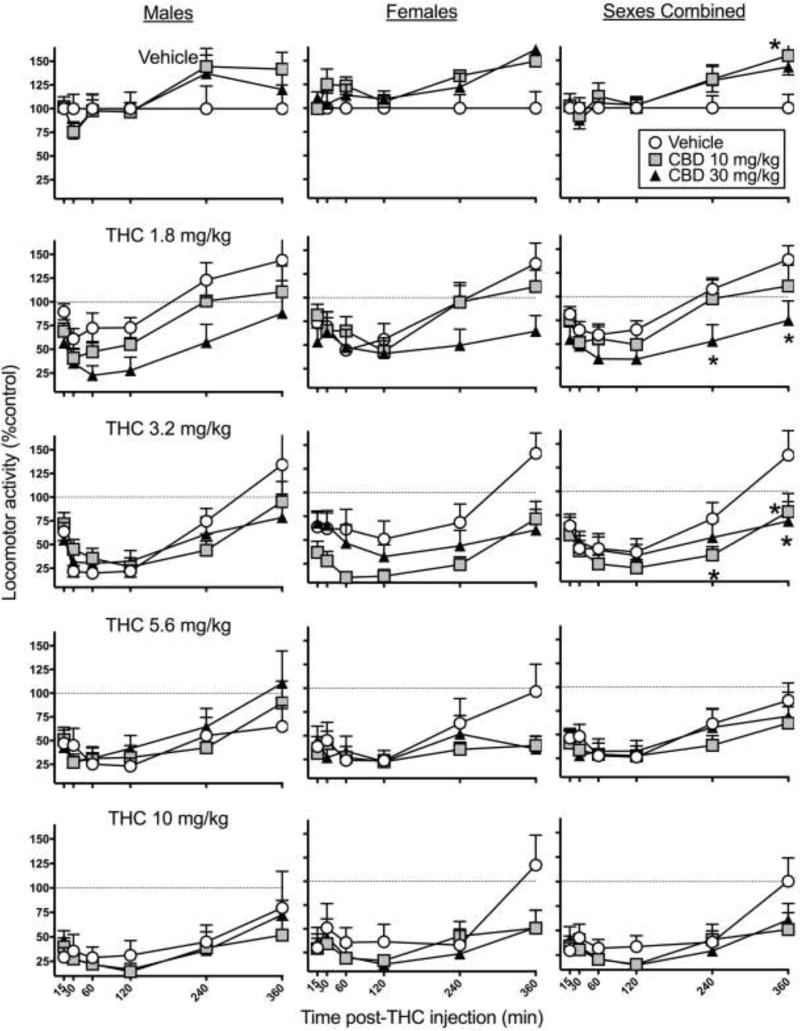
Figure 3 shows time-response curves for locomotor activity. THC caused dose- and time-dependent decreases in locomotor activity (THC dose × Time, F12,788=2.53, p=0.003). THC decreased locomotion for 1–2 hr and thereafter locomotion gradually recovered; this pattern was sex-dependent (Sex × Time, F5,1365=5.11, p<0.001). Further analysis showed that the only time point at which sex differences were significant was at 30 min, when males were less active than females (Sex, F1,273=10.43, p=0.001). CBD enhanced THC-induced hypolocomotion at the low to moderate THC doses, especially at later time points (CBD dose × THC dose × Time, F40,1365= 2.13, p<0.001), with no sex differences. Subsequent analysis at each time point showed that CBD altered THC’s effect at 240 and 360 min post-THC injection (240 min, CBD dose × THC dose, F8,288= 3.15, p=0.002; 360 min, CBD dose × THC dose, F8,288= 3.13, p=0.002). Further analysis at these later time points was conducted for each dose of THC independently to determine at what THC doses CBD altered THC’s locomotor-decreasing effect. Analysis of 0.0 mg/kg THC data at 240 and 360 min showed that CBD alone increased locomotor activity at 360 min post THC-injection, compared to vehicle (CBD dose, F2,57=4.52, p=0.015). At 1.8 mg/kg THC, CBD significantly potentiated THC-induced hypolocomotion at both time points, compared to the same dose of THC alone (240 min, CBD dose, F2,58=5.86, p=0.005; 360 min, CBD dose, F2,58=4.85, p=0.011). Analysis of 3.2 mg/kg THC data showed that CBD again potentiated THC’s locomotor-suppressing effects at both time points, compared to the same dose of THC alone (240 min, CBD dose, F2,57=3.67, p=0.032; 360 min, CBD dose, F2,57=6.15, p=0.004). Analysis of 5.6 mg/kg and 10 mg/kg THC data showed that CBD did not significantly alter THC-induced decreases in locomotor activity at later time points, for these higher THC doses.
Figure 3.
Experiment 1. Time-response curves on the locomotor activity test in male rats (left), female rats (center) and both sexes combined (right). CBD was administered 15 min before THC. CBD potentiated THC’s effect at lower THC doses at later time points. Each point is the mean ± 1 S.E.M of 10 male or female rats (left and center panels), or 20 total rats (right panel). *significant difference, CBD-treated group compared to vehicle-treated group (p<0.05).
Analysis of estrous stage modulation of CBD and THC effects showed that estrous stage did not significantly alter antinociceptive or locomotor effects caused by CBD and/or THC (data not shown). However, estrous stage distribution varied considerably across groups, with 0–30% of females in each treatment group being in (early) proestrus, 0–40% in proestrus-estrus (late proestrus), 0–40% in estrus, and 30–70% in diestrus. That is, some groups did not have females in each estrous stage.
3.2 Experiment 2: Effect of CBD Pretreatment Time on CBD-THC Interactions
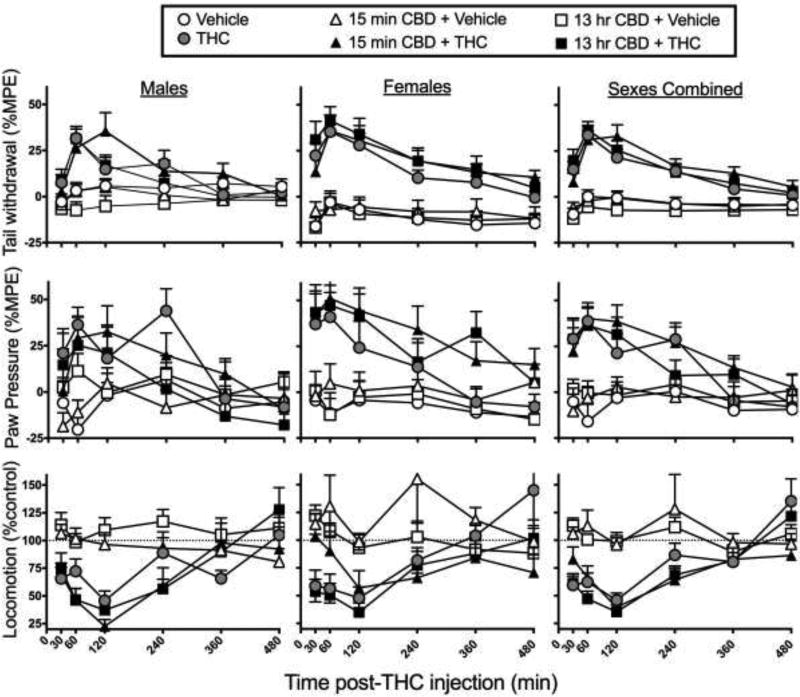
The highest CBD dose and lowest THC dose were chosen for the next experiment, and the time course was lengthened, since most interactions observed in the first experiment were at high CBD + low THC doses, and at 240–360 min post-THC injection. Figure 4 shows time-response curves in male vs. female rats treated with vehicle or 30 mg/kg CBD either 13 hr or 15 min before vehicle or 1.8 mg/kg THC. Similar to Experiment 1, THC time-dependently increased tail withdrawal antinociception (THC dose × Time, F5,760=25.91, p<0.001), and THC increased antinociception more in females than males (Sex × THC dose, F1,152=12.36, p=0.001). CBD tended to increase THC-induced tail withdrawal antinociception at some time points, but this effect was not statistically significant at either the 15-min or 13-hr CBD pretreatment time (CBD Pretreatment × THC dose × Time: F10,760=1.79, p=0.06). On the paw pressure test, THC increased paw pressure antinociception in a time-dependent manner (THC dose × Time, F5,760=10.92, p<0.001), and had a slightly but not significantly greater effect in females compared to males (Sex × THC dose: F1,151=3.43, p=0.07). CBD given at both pretreatment times appeared to increase THC-induced paw pressure antinociception in females, but this effect was not significant (Sex × CBD Pretreatment × THC dose: F2,151=1.07, n.s.). On the locomotor activity test, THC time-dependently decreased locomotor activity (THC dose × Time, F5,760=12.13, p<0.001), with no sex difference. CBD given 15 min or 13 hr before THC did not significantly alter THC’s effects on locomotor activity in either sex.
Figure 4.
Experiment 2. Time-response curves on the tail withdrawal test (top row), paw pressure test (middle row) and locomotor activity test (bottom row) in male rats (left column), female rats (middle column), and both sexes combined (right column). CBD 30 mg/kg was given either 13 hr or 15 min before THC 1.8 mg/kg. CBD pretreatment time did not significantly alter the CBD-THC interaction on any test. Each point is the mean ± 1 S.E.M of 12–14 male or female rats (left and center panels), or 24–28 total rats (right panels).
3.3 Experiment 3: Effect of CBD on THC Metabolism
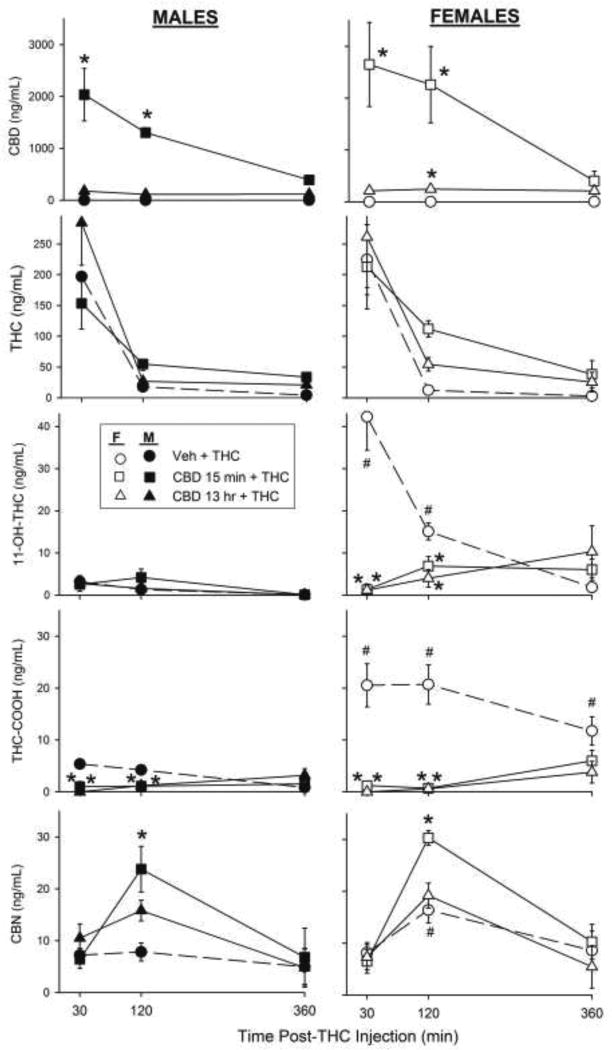
Figure 5 shows serum concentrations of CBD, THC, its major active metabolite 11-OH-THC, its major inactive metabolite THC-COOH, and a minor active metabolite, CBN, in male and female rats treated with 0 or 30 mg/kg CBD either 13 hr or 15 min before 1.8 mg/kg THC. As expected, both sexes had higher serum CBD levels when CBD was given 15 min compared to 13 hr before THC, and serum CBD decreased from 30 to 360 min (CBD pretreatment × Time, F4,80=5.75, p<0.001). CBD levels were slightly but not significantly higher in females than males (Sex, F1,80=1.77, p=0.19).
Figure 5.
Experiment 3. Serum CBD, THC, 11-OH-THC, THC-COOH and CBN levels in male and female rats. Vehicle or CBD 30 mg/kg was given 13 hr or 15 min before THC 1.8 mg/kg. Each point is the mean ± 1 S.E.M of 4–6 rats. *significant difference compared to THC-only control; #significant sex difference at same CBD-THC dose combination.
Serum THC levels in both sexes decreased from 30 to 360 min post-THC injection (Time, F2,80=45.24, p<0.001). CBD tended to increase serum THC levels at 120 and 360 min post-THC injection, but this effect was not statistically significant (CBD pretreatment × Time, F4,80=1.71, p=0.16).
CBD given either 13 hr or 15 min before THC decreased serum 11-OH-THC levels in females but not males, at 30 and 120 min post-THC injection (Sex × CBD pretreatment × Time, F4,80 =11.62, p<0.001). In rats that received only THC, serum 11-OH-THC levels were significantly higher in females than males at 30 (Sex, F1,10=23.66, p=0.001) and 120 min (Sex, F1,10=38.51, p<0.001) post-THC injection.
CBD given either 13 hr or 15 min before THC decreased serum THC-COOH levels in both males and females, at 30 and 120 min post-THC injection (CBD pretreatment × Time, F4,80=5.43, p=0.001). Additionally, serum THC-COOH levels were significantly higher in females than males that did not receive CBD (Sex, F1,31= 35.35, p<0.001), so CBD decreased THC-COOH more in females than males (Sex × CBD pretreatment, F2,80=28.06, p<0.001).
Lastly, CBD pretreatment increased serum CBN levels in a time-dependent manner (CBD pretreatment × Time, F4,79=5.07, p=0.001). At 120 min post-THC injection, CBD given 15 min but not 13 hr before THC significantly increased serum CBN (p<0.001). Serum CBN was higher in females than in males (Sex, F1,80=4.03, p=0.048), but there was no sex difference in CBD modulation of serum CBN.
4. Discussion
In the current study, THC caused dose- and time-dependent tail withdrawal and paw pressure antinociception that was generally greater in females than males, which is consistent with several previous studies examining sex differences in cannabinoid antinociception in rats (Craft et al., 2012; Romero et al., 2002; Tseng and Craft, 2001). The first experiment, in which multiple CBD-THC dose combinations were examined, showed that CBD enhanced THC-induced antinociception and hypolocomotion at 4–6 hr after THC injection, with no sex differences. However, in the second experiment, in which a single CBD-THC dose combination was examined using two different CBD pretreatment times, CBD did not significantly enhance any THC effects, suggesting that the interactions between these two drugs are not robust. In contrast to the weak CBD-THC interactions observed on behavioral tests, CBD significantly modulated THC metabolism in both sexes.
Two previous studies have reported CBD potentiation of THC-induced antinociception using acute pain tests. Varvel and colleagues (2006) found that at 20 min post-injection, CBD 30 mg/kg + THC 0.3 mg/kg and CBD 10 mg/kg + THC 3.0 mg/kg produced tail withdrawal antinociception greater than that produced by the same doses of THC alone, in male mice. Karniol and Carlini (1973) found that mice given CBD 10 mg/kg + THC 5 mg/kg had increased hot plate antinociception at 30 and 120 min post-injection compared to the same dose of THC alone, while CBD 20 mg/kg + THC 5 mg/kg increased hot plate antinociception at 60, 90 and 120 min post-injection compared to the same dose of THC alone. In contrast, Finn et al. (2004) found that CBD did not alter THC’s effects on a formalin-evoked acute pain test, when male rats were given CBD 5 mg/kg + THC 1 or 2.5 mg/kg; however, formalin-induced pain behaviors typically last approximately 60 min post-injection, so cannabinoid interactions at later time points could have been missed.
CBD potentiation of THC-induced hypolocomotion has also been reported in some but not all previous studies in rodents (Fernandes et al., 1974; Klein et al., 2011; Todd and Arnold, 2015; Varvel et al., 2006). For example, Fernandes and colleagues (1974) conducted a 6-hr dark phase locomotor activity study in male rats, and found that CBD 10 mg/kg + THC 5 mg/kg caused greater hypolocomotion at 210 and 270 min post-THC injection, compared to THC 5 mg/kg alone. Although our study shows CBD enhancement of THC-induced hypolocomotion at similar time points long after THC injection, we did not see this effect when a similar dose of THC (5.6 mg/kg) was given in combination with CBD 10 mg/kg. Differences in dose-specific results across studies could be due to light phase vs. dark phase testing, or a number of other variables, including strain of rat and experimenter handling.
Human studies have found that CBD-THC combinations are effective analgesics. Human participants who received a 1:1 ratio of CBD:THC for rheumatoid arthritis (Blake et al., 2006) or multiple sclerosis (Rog et al., 2007; Wade et al., 2004) reported significant relief of their pain. Additionally, one study found that a CBD-THC combination provided greater relief of cancer-related pain than THC alone (Johnson et al., 2010). However, a study in healthy cannabis smokers found that CBD did not alter the reinforcing, subjective or cardiovascular effects of smoked THC (Haney et al., 2016), suggesting that CBD-THC interactions are endpoint-specific. The mechanisms underlying CBD-THC interactions seen in human studies have not been investigated. However, animal studies suggest that increased TRPV1 and CB1 receptor co-localization in the DRG in response to chronic pain could contribute to CBD-THC interactions in chronic pain patients (Amaya et al., 2006). Thus, CBD-THC and their receptor interactions should be further investigated in male and female rats using chronic pain models.
Zuardi et al. (2012) suggested that a dose ratio of 1.8:1 is optimal for enhancement of THC’s effects, but in the present study, a dose ratio of 1.8:1 (CBD 10 mg/kg + THC 5.6 mg/kg) did not produce any interaction. In fact, dose ratios that produced interactions on the locomotor test ranged from approximately 17:1 (CBD 30 mg/kg + THC 1.8 mg/kg) and 9:1 (CBD 30 mg/kg + THC 3.2 mg/kg) to 3:1 (CBD 10 mg/kg + THC 3.2 mg/kg). Thus, the present results suggest that CBD enhancement of THC’s effects is more likely to be observed when a relatively low dose of THC is used, and that dose ratio per se is not crucial.
An unexpected finding in the first experiment was that CBD alone increased locomotor activity at later time points. In a previous study, in rats given CBD daily for 14 days, no changes in locomotor activity were observed (El Batsh et al., 2012). Another study conducted in mice found that 5 min after administration of 10 mg/kg CBD, locomotor activity increased, although 30 mg/kg CBD decreased locomotor activity (Varvel et al., 2006). Additionally, Hayakawa et al. (2008) found that 50 mg/kg CBD increased locomotor activity by approximately 60%, while 10 and 50 mg/kg CBD potentiated 1 mg/kg THC-induced decreases in locomotor activity, similar to the present study. In humans, one study found that CBD given to healthy young adults increased awake time during a sleeping session (Nicholson et al., 2004). Thus, although it is not a widely reported phenomenon, a few studies suggest that CBD alone may increase activity. The mechanism underlying this effect remains to be determined.
It has been suggested that CBD enhancement of THC’s effects may be due to CBD inhibition of liver enzymes involved in THC metabolism (Bornheim and Grillo, 1998; Watanabe et al., 2007). In a previous study showing that CBD potentiated THC-induced catalepsy, hypolocomotion and hypothermia, the authors concluded that prolongation of THC’s effects by CBD were likely related to a metabolic interaction (Fernandes et al., 1974). We examined this hypothesis by evaluating serum levels of THC and several metabolites at 30–360 min post-THC injection, in rats pretreated with vehicle or CBD given 15 min or 13 hr before THC. Our results demonstrate that CBD given 15 min before THC decreases production of the major active metabolite 11-OH-THC (females) and the major inactive metabolite THC-COOH (both sexes), as well as transiently increasing production of the minor active metabolite CBN (both sexes). These findings are consistent with previous studies showing that CBD decreased plasma levels of 11-OH-THC in mice given THC (Bornheim et al., 1995), and 11-OH-THC and THC-COOH in humans given THC (Nadulski et al., 2005). In the present study, even when CBD was given 13 hr before THC, serum levels of CBD were low but still detectable, and CBD still significantly reduced 11-OH-THC and THC-COOH production. This result indicates that CBD inhibition of THC metabolism is very long-lived, or it can occur even when circulating levels of CBD are relatively low.
Inhibition of THC metabolism by CBD would be expected to increase THC’s behavioral effects by increasing circulating THC levels. However, in the present study serum THC itself was not significantly increased by CBD in either sex. Moreover, decreased production of the active metabolite 11-OH-THC, which is similar to, or more potent than THC in its behavioral actions (Ford et al., 1977; Tseng et al., 2001), would be expected to decrease behavioral effects observed after THC injection. Thus, particularly in female rats, because they produce considerably more 11-OH-THC than males do (present study; Wiley and Burston, 2014), CBD would be expected to decrease THC’s behavioral effects by significantly reducing the production of 11-OH-THC. Possibly counteracting this effect would be CBD-induced increases in CBN, because CBN also has been shown to produce antinociception in (male) rats and mice, albeit with lower potency than THC (Sofia et al., 1975; Sanders et al., 1979; Booker et al., 2009). Overall, it is possible that despite its dramatic effects on THC metabolism, particularly in female rats, CBD did not consistently alter THC-induced behavioral effects because multiple active metabolites contribute to these effects, and although at least one active metabolite was decreased, another was increased.
Even in males, the time-dependent changes in THC metabolism observed in Experiment 3 suggest that CBD modulation of THC metabolism is not the primary mechanism underlying CBD enhancement of THC’s behavioral effects. CBD significantly altered serum levels of THC metabolites at 30 and 120 min post-THC injection, whereas significant drug interactions on behavior occurred at 240–360 min post-THC injection in Experiment 1 (and no drug interactions were significant at any time point in Experiment 2). Thus, other mechanisms of CBD-THC interaction must be considered. For instance, CBD inhibition of ATP-binding cassette (ABC) transporters, located on the blood brain barrier (BBB) (Zhu et al., 2006), could contribute to the development of drug interaction at later time points. The ABC transporter p-glycoprotein has been shown to assist in the efflux of THC across the BBB (Spiro et al., 2012), and p-glycoprotein is inhibited by CBD (Holland et al., 2006). Additionally, the multidrug ABC transporter, breast cancer resistance protein, located on the BBB, has been shown to be inhibited by CBD (Holland et al., 2007). Therefore, ABC transporter inhibition by CBD would lead to decreased efflux of THC across the BBB (Zhu et al., 2006), presumably prolonging THC’s effects in the brain. Another possible explanation for CBD-THC interactions is CB1 and TRPV1 receptor cross-talk (Di Marzo et al., 2008). For example, both receptor types have been identified in the striatum (Hohmann and Herkenham, 2000; Musella et al., 2009), and CB1-TRPV1 cross-talk via endocannabinoids has been demonstrated in the striatum (Maccarrone et al., 2006); thus, this mechanism may contribute to CBD-THC interactions on locomotor activity. Finally, it should be noted that although CBD’s known effect as a negative allosteric modulator of CB1 (Laprairie et al., 2015) could also influence CBD-THC interactions on behavior, this mechanism would be expected to result in CBD inhibiting THC’s behavioral effects. Although we did not observe CBD inhibition of THC’s behavioral effects in the present study, it is possible that negative allosteric modulation was also occurring, counteracting CBD enhancement of THC’s effects by other mechanisms.
In the present study, the interval between CBD and THC injection did not alter CBD-THC interactions on antinociception or locomotor activity; however, even the 15-min pretreatment time used in Experiment 2 did not produce the same result as in Experiment 1. One methodological difference between Experiments 1 and 2 is that rats in Experiment 2 were handled more times before testing than Experiment 1 rats. Changes in handling could lead to changes in the endocannabinoid system (Sciolino et al., 2010), which could theoretically change rats’ responses to exogenous cannabinoids. The smaller sample size in Experiment 2 compared to Experiment 1 also may have contributed to the lack of statistically significant CBD-THC interaction in the second experiment, given the relatively small effect size of the drug interaction in the first experiment.
In conclusion, CBD may enhance THC’s acute antinociceptive and locomotor-suppressing effects in rats, with enhancement more likely to be observed long (4–6 hr) after drug administration. CBD-THC interactions appear to be more likely when the THC dose is low, but do not appear to be sex-dependent. However, the drug interaction on behavior was not significant in a second, smaller experiment, despite the fact that CBD significantly altered THC metabolism. Further studies will be required to determine whether other mechanisms such as CBD inhibition of ABC transporters, negative allosteric modulation of CB1 receptors, and/or TRPV1-CB1 co-localization contribute to CBD-THC interactions on behavior.
Highlights.
CBD may prolong THC-induced antinociception and hypolocomotion in rats
CBD-THC interactions on behavior do not appear to be sex-dependent
CBD alters THC metabolite production to a greater extent in females than males
Acknowledgments
The authors thank H. Gogulski for technical assistance.
Role of Funding Source
This research was funded by grant DA-016644 from the National Institutes of Health, National Institute on Drug Abuse (NIH/NIDA), and by the State of Washington Initiative Measure 502. All drugs were purchased or were provided by the NIDA Drug Supply Program. NIH/NIDA did not have any other role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. The opinions, findings, and conclusions or recommendations expressed in this publication are those of the authors and do not necessarily reflect those of NIH or NIDA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Participated in research design: Britch, Wiley, Yu, Clowers, Craft
Performed data analysis: Britch
Wrote or contributed to the writing of the manuscript: Britch, Wiley, Yu, Clowers, Craft
All authors approved of the manuscript before submission.
Conflict of Interest
No conflict declared.
References
- Aggarwal SK, Carter GT, Sullivan MD, ZumBrunnen C, Morrill R, Mayer JD. Medicinal use of cannabis in the united states: Historical perspectives, current trends, and future directions. J. Opioid Manag. 2008;5:153–168. doi: 10.5055/jom.2009.0016. [DOI] [PubMed] [Google Scholar]
- Amaya F, Shimosato G, Kawasaki Y, Hashimoto S, Tanaka Y, Ji RR, Tanaka M. Induction of CB1 cannabinoid receptor by inflammation in primary afferent neurons facilitates antihyperalgesic effect of peripheral CB1 agonist. Pain. 2006;124:175–183. doi: 10.1016/j.pain.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Blake DR, Robson P, Ho M, Jubb RW, McCabe CS. Preliminary assessment of the efficacy, tolerability and safety of a cannabis-based medicine (Sativex) in the treatment of pain caused by rheumatoid arthritis. Rheumatology. 2006;45:50–52. doi: 10.1093/rheumatology/kei183. [DOI] [PubMed] [Google Scholar]
- Booker L, Naidu PS, Razdan RK, Mahadevan A, Lichtman AH. Evaluation of prevalent phytocannabinoids in the acetic acid model of visceral nociception. Drug Alcohol Depend. 2009;105:42–47. doi: 10.1016/j.drugalcdep.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornheim LM, Kim KY, Li J, Perotti BY, Benet LZ. Effect of cannabidiol pretreatment on the kinetics of tetrahydrocannabinol metabolites in mouse brain. Drug Metab. Dispos. 1995;23:825–831. [PubMed] [Google Scholar]
- Bornheim LM, Grillo MP. Characterization of cytochrome P450 3A inactivation by cannabidiol: Possible involvement of cannabidiol-hydroxyquinone as a P450 inactivator. Chem. Res. Toxicol. 1998;11:1209–1216. doi: 10.1021/tx9800598. [DOI] [PubMed] [Google Scholar]
- Craft RM, Wakley AA, Tsutsui KT, Laggart JD. Sex differences in cannabinoid 1 vs. cannabinoid 2 receptor-selective antagonism of antinociception produced by Δ9-tetrahydrocannabinol and CP55,940 in the rat. J. Pharmacol. Exp. Ther. 2012;340:787–800. doi: 10.1124/jpet.111.188540. [DOI] [PubMed] [Google Scholar]
- Cox ML, Welch SP. The antinociceptive effect of delta 9-tetrahydrocannabinol in the arthritic rat. Eur. J. Pharmacol. 2004;493:65–74. doi: 10.1016/j.ejphar.2004.04.022. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Cristino L. Why endocannabinoids are not all alike. Nat. Neurosci. 2008;11:124–126. doi: 10.1038/nn0208-124. [DOI] [PubMed] [Google Scholar]
- El Batsh MM, Assareh N, Marsden CA, Kendall DA. Anxiogenic-like effects of chronic cannabidiol administration in rats. Psychopharmacology. 2012;221:239–247. doi: 10.1007/s00213-011-2566-z. [DOI] [PubMed] [Google Scholar]
- Fernandes M, Schabarek A, Coper H, Hill R. Modification of Δ9-THC actions by cannabinol and cannabidiol in the rat. Psychopharmacologia. 1974;38:329–338. doi: 10.1007/BF00429130. [DOI] [PubMed] [Google Scholar]
- Finn DP, Beckett SRG, Roe CH, Madjd A, Fone KCF, Kendall DA, Marsden CA, Chapman V. Effects of coadministration of cannabinoids and morphine on nociceptive behaviour, brain monoamines and HPA axis activity in a rat model of persistent pain. Eur. J. Neurosci. 2004;19:678–686. doi: 10.1111/j.0953-816x.2004.03177.x. [DOI] [PubMed] [Google Scholar]
- Ford RD, Balster RL, Dewey WL, Beckner JS. Δ9-THC and 11-OH-Δ9-THC: behavioral effects and relationship to plasma and brain levels. Life Sci. 1977;20:1993–2004. doi: 10.1016/0024-3205(77)90178-3. [DOI] [PubMed] [Google Scholar]
- Freeman ME. The neuroendocrine control of the rat estrous cycle. The physiology of reproduction; New York: 1994. [Google Scholar]
- Greenwald MK, Stitzer ML. Antinociceptive, subjective and behavioral effects of smoked marijuana in humans. Drug Alcohol Depend. 2000;59:261–275. doi: 10.1016/s0376-8716(99)00128-3. [DOI] [PubMed] [Google Scholar]
- Haney M, Malcolm RJ, Babalonis S, Nuzzo PA, Cooper ZD, Bedi G, Gray KM, McRae-Clark A, Lofwall MR, Sparenborg S, Walsh SL. Oral cannabidiol does not alter the subjective, reinforcing or cardiovascular effects of smoked cannabis. Neuropsychopharmacology. 2016;41:1974–1982. doi: 10.1038/npp.2015.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte-Hargrove LC, Dow-Edwards DL. Withdrawal from THC during adolescence: Sex differences in locomotor activity and anxiety. Behav. Brain Res. 2012;231:48–59. doi: 10.1016/j.bbr.2012.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K, Mishima K, Hazekawa M, Sano K, Irie K, Orito K, Egawa T, Kitamura Y, Uchida N, Nishimura R, Egashira N, Iwasaki K, Fujiwara M. Cannabidiol potentiates pharmacological effects of Δ9-tetrahydrocannabinol via CB1 receptor-dependent mechanism. Brain Res. 2008;1188:157–164. doi: 10.1016/j.brainres.2007.09.090. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Herkenham M. Localization of cannabinoid CB1 receptor mRNA in neuronal subpopulations of rat striatum: A double-label in situ hybridization study. Synapse. 2000;37:71–80. doi: 10.1002/(SICI)1098-2396(200007)37:1<71::AID-SYN8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Holland ML, Panetta JA, Hoskins JM, Bebawy M, Roufogalis BD, Allen JD, Arnold JC. The effects of cannabinoids on p-glycoprotein transport and expression in multidrug resistant cells. Biochem. Pharmacol. 2006;71:1146–1154. doi: 10.1016/j.bcp.2005.12.033. [DOI] [PubMed] [Google Scholar]
- Holland ML, Lau DTT, Allen JD, Arnold JC. The multidrug transporter ABCG2 (BCRP) is inhibited by plant-derived cannabinoids. Br. J. Pharmacol. 2007;152:815–824. doi: 10.1038/sj.bjp.0707467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guide for the Care and Use of Laboratory Animals. 8. Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council; Washington, DC: 2011. [Google Scholar]
- Jaeger W, Benet LZ, Bornheim LM. Inhibition of cyclosporine and tetrahydrocannabinol metabolism by cannabidiol in mouse and human microsomes. Xenobiotica. 1996;26:275–284. doi: 10.3109/00498259609046707. [DOI] [PubMed] [Google Scholar]
- Johnson JR, Burnell-Nugent M, Lossignol D, Ganae-Motan ED, Potts R, Fallon MT. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC: CBD extract and THC extract in patients with intractable cancer-related pain. J. Pain Symptom Manage. 2010;39:167–179. doi: 10.1016/j.jpainsymman.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Karniol IG, Carlini EA. Pharmacological interaction between cannabidiol and delta 9-tetrahydrocannabinol. Psychopharmacologia. 1973;33:53–70. doi: 10.1007/BF00428793. [DOI] [PubMed] [Google Scholar]
- Klein C, Karanges E, Spiro A, Wong A, Spencer J, Huynh T, Gunasekaran N, Karl T, Long LE, Huang XF, Lui K, Arnold JC, McGregor IS. Cannabidiol potentiates Δ9-tetrahydrocannabinol (THC) behavioural effects and alters THC pharmacokinetics during acute and chronic treatment in adolescent rats. Psychopharmacology. 2011;218:443–457. doi: 10.1007/s00213-011-2342-0. [DOI] [PubMed] [Google Scholar]
- Laprairie RB, Bagher AM, Kelly MEM, Denovan-Wright EM. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol. 2015;172:4790–4805. doi: 10.1111/bph.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch ME, Ware MA. Cannabinoids for the treatment of chronic non-cancer pain: An updated systematic review of randomized controlled trials. J. Neuroimmune Pharmacol. 2015;10:293–301. doi: 10.1007/s11481-015-9600-6. [DOI] [PubMed] [Google Scholar]
- Mao J, Price DD, Lu J, Keniston L, Mayer DJ. Two distinctive antinociceptive systems in rats with pathological pain. Neurosci. Lett. 2000;280:13–16. doi: 10.1016/s0304-3940(99)00998-2. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Rossi S, Bari M, De Chiara V, Fezza F, Musella A, Gasperi V, Prosperetti C, Bernardi G, Finazzi-Agro A, Cravatt BF, Centozone D. Anandamide inhibits metabolism and physiological actions of 2-arachidonoylglycerol in the striatum. Nat. Neurosci. 2008;11:152–159. doi: 10.1038/nn2042. [DOI] [PubMed] [Google Scholar]
- Marcu JP, Christian RT, Lau D, Zielinski AJ, Horowitz MP, Lee J, Pakdel A, Allison J, Limbad C, Moore DH, Yount GL, Desprez PY, McAllister SD. Cannabidiol enhances the inhibitory effects of delta9-tetrahydrocannabinol on human glioblastoma cell proliferation and survival. Mol. Cancer Ther. 2010;9:180–189. doi: 10.1158/1535-7163.MCT-09-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musella A, De Chiara V, Rossi S, Prosperetti C, Bernardi G, Maccarrone M, Centonze D. TRPV1 channels facilitate glutamate transmission in the striatum. Mol. Cell Neurosci. 2009;40:89–97. doi: 10.1016/j.mcn.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Nadulski T, Pragst F, Weinberg G, Roser P, Schnelle M, Fronk EM, Stadelmann AM. Randomized, double-blind, placebo-controlled study about the effects of cannabidiol (CBD) on the pharmacokinetics of Δ9-tetrahydrocannabinol (THC) after oral application of THC verses standardized cannabis extract. Ther. Drug Monit. 2005;27:799–810. doi: 10.1097/01.ftd.0000177223.19294.5c. [DOI] [PubMed] [Google Scholar]
- Narimatsu S, Watanabe K, Yamamoto I, Yoshimura H. Sex difference in the oxidative metabolism of Δ9-tetrahydrocannabinol in the rat. Biochem. Pharmacol. 1991;41:1187–1194. doi: 10.1016/0006-2952(91)90657-q. [DOI] [PubMed] [Google Scholar]
- Nicholson AN, Turner C, Stone BM, Robson PJ. Effect of Δ-9-tetrahydrocannabinol and cannabidiol on nocturnal sleep and early-morning behavior in young adults. J. Clin. Psychopharmacol. 2004;24:305–313. doi: 10.1097/01.jcp.0000125688.05091.8f. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes M, Timmons MC, Davis KH, Wall EM. A comparison of the pharmacological activity in man of intravenously administered delta9-tetrahydrocannabinol, cannabinol, and cannabidiol. Experientia. 1973;29:1368–1369. doi: 10.1007/BF01922823. [DOI] [PubMed] [Google Scholar]
- Reid M, Bornheim LM. Cannabinoid-induced alterations in brain disposition of drugs of abuse. Biochem. Pharmacol. 2001;61:1357–1367. doi: 10.1016/s0006-2952(01)00616-5. [DOI] [PubMed] [Google Scholar]
- Rog DJ, Nurmikko TJ, Young CA. Oromucosal Δ9-tetrahydrocannabinol/cannabidiol for neuropathic pain associated with multiple sclerosis: an uncontrolled, open-label, 2-year extension trial. Clin. Ther. 2007;29:2068–2079. doi: 10.1016/j.clinthera.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Romero EM, Fernández B, Sagredo O, Gomez N, Urigüen L, Guaza C, De Miguel R, Ramos JA, Viveros MP. Antinociceptive, behavioural and neuroendocrine effects of CP 55,940 in young rats. Brain. Res Dev. Brain Res. 2002;136:85–92. doi: 10.1016/s0165-3806(02)00306-1. [DOI] [PubMed] [Google Scholar]
- Sanders J, Jackson DM, Starmer GA. Interactions among the cannabinoids in the antagonism of the abdominal constriction response in the mouse. Psychopharmacology. 1979;61:281–285. doi: 10.1007/BF00432273. [DOI] [PubMed] [Google Scholar]
- Schley M, Legler A, Skopp G, Schmelz M, Konrad C, Rukwied R. Delta-9-THC based monotherapy in fibromyalgia patients on experimentally induced pain, axon reflex flare, and pain relief. Curr. Med. Res. Opin. 2006;22:1269–1276. doi: 10.1185/030079906x112651. [DOI] [PubMed] [Google Scholar]
- Sciolino NR, Bortolato M, Eisenstein SA, Fu J, Oveisi F, Hohmann AG, Piomelli D. Social isolation and chronic handling alter endocannabinoid signaling and behavioral reactivity to context in adult rats. Neurosci. 2010;168:371–386. doi: 10.1016/j.neuroscience.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FL, Fujimori K, Lowe J, Welch SP. Characterization of Δ9-tetrahydrocannabinol and anandamide antinociception in nonarthritic and arthritic rats. Pharmacol. Biochem. Behav. 1998;60:183–191. doi: 10.1016/s0091-3057(97)00583-2. [DOI] [PubMed] [Google Scholar]
- Sofia RD, Vassar HB, Knobloch LC. Comparative analgesic activity of various naturally occurring cannabinoids in mice and rats. Psychopharmacologia. 1975;40:285–295. doi: 10.1007/BF00421466. [DOI] [PubMed] [Google Scholar]
- Spiro AS, Wong A, Boucher AA, Arnold JC. Enhanced brain disposition and effects of Δ9-tetrahydrocannabinol in P-glycoprotein and breast cancer resistance protein knockout mice. PloS One. 2012;7:e35937. doi: 10.1371/journal.pone.0035937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd SM, Arnold JC. Neural correlates of interactions between cannabidiol and Δ9-tetrahydrocannabinol in mice: implications for medical cannabis. Br. J. Pharmacol. 2016;173:53–65. doi: 10.1111/bph.13333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng AH, Craft RM. Sex differences in antinociceptive and motoric effects of cannabinoids. Eur. J. Pharmacol. 2001;430:41–47. doi: 10.1016/s0014-2999(01)01267-5. [DOI] [PubMed] [Google Scholar]
- Tseng AH, Harding JW, Craft RM. Pharmacokinetic factors in sex differences in Δ9-tetrahydrocannabinol-induced behavioral effects in rats. Behav. Brain Res. 2004;154:77–83. doi: 10.1016/j.bbr.2004.01.029. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Bridgen DT, Tao Q, Thomas BF, Martin BR, Lichtman AH. Δ9-tetrahydrocannbinol accounts for the antinociceptive, hypothermic, and cataleptic effects of marijuana in mice. J. Pharmacol. Exp. Ther. 2005;314:329–337. doi: 10.1124/jpet.104.080739. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Wiley WL, Yang R, Bridgen DT, Long K, Lichtman AH, Martin BR. Interactions between THC and cannabidiol in mouse models of cannabinoid activity. Psychopharmacology. 2006;186:226–234. doi: 10.1007/s00213-006-0356-9. [DOI] [PubMed] [Google Scholar]
- Wade DT, Makela P, Robson P, House H, Bateman C. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Mult. Scler. 2004;10:434–441. doi: 10.1191/1352458504ms1082oa. [DOI] [PubMed] [Google Scholar]
- Wall ME, Sadler BM, Brine D, Taylor H, Perez-Reyes M. Metabolism, disposition, and kinetics of delta-9-tetrahydrocannabinol in men and women. Clin. Pharmacol. Ther. 1983;34:352–363. doi: 10.1038/clpt.1983.179. [DOI] [PubMed] [Google Scholar]
- Ward SJ, McAllister SD, Kawamura R, Murase R, Neelakantan H, Walker EA. Cannabidiol inhibits paclitaxel-induced neuropathic pain through 5-HT1A receptors without diminishing nervous system function or chemotherapy efficacy. Br. J. Pharmacol. 2014;171:636–645. doi: 10.1111/bph.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Yamaori S, Funahashi T, Kimura T, Yamamoto I. Cytochrome P450 enzymes involved in the metabolism of tetrahydrocannabinols and cannabinol by human hepatic microsomes. Life Sci. 2007;80:1415–1419. doi: 10.1016/j.lfs.2006.12.032. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Burston JJ. Sex differences in Δ9-tetrahydrocannabinol metabolism and in vivo pharmacology following acute and repeated dosing in adolescent rats. Neurosci. Lett. 2014;576:51–55. doi: 10.1016/j.neulet.2014.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, O’Connell MM, Tokarz ME, Wright MJ. Pharmacological effects of acute and repeated administration of Δ9-tetrahydrocannabinol in adolescent and adult rats. J. Pharmacol. Exp. Ther. 2007;320:1097–1105. doi: 10.1124/jpet.106.108126. [DOI] [PubMed] [Google Scholar]
- Zhu HJ, Wang JS, Markowitz JS, Donovan JL, Gibson BB, Gefroh HA, DeVane CL. Characterization of p-glycoprotein inhibition by major cannabinoids from marijuana. J. Pharmacol. Exp. Ther. 2006;317:850–857. doi: 10.1124/jpet.105.098541. [DOI] [PubMed] [Google Scholar]
- Zuardi AW, Hallak JEC, Crippa JAS. Interaction between cannabidiol (CBD) and Δ9-tetrahydrocannabinol (THC): influence of administration interval and dose ratio between the cannabinoids. Psychopharmacology. 2012;219:247–249. doi: 10.1007/s00213-011-2495-x. [DOI] [PubMed] [Google Scholar]
- Zuardi AW, Teixeira NA, Karniol IC. Pharmacological interaction of the effects of delta 9-trans-tetrahydrocannabinol and cannabidiol on serum corticosterone levels in rats. Arch. Int. Pharmacodyn. Ther. 1984;269:12–19. [PubMed] [Google Scholar]