Introduction
The past decade has seen tremendous progress in malaria control worldwide, as 57 out of 106 countries have shown a sharp reduction of about 75% in malaria incidence [1]. Despite this progress, many febrile patients are still treated with antimalarial drugs without a confirmed malaria diagnosis, because malaria diagnosis is mainly based on microscopic examination of blood smears [2, 3]. Although rapid diagnostic tests (RDTs) are easy and reliable tools for diagnosis of Plasmodium falciparum and P. vivax, RDTs are unable to differentiate mixed infection with uncommon parasite species, i.e., P. ovale and P. malariae [4, 5]. In this report, we are presenting a rare case having all 4 species of Plasmodium in peripheral blood of a young boy from a remote community health centre (CHC), Darbha, in Bastar district of Chhattisgarh state, India (Fig 1). Malaria is a major health problem in Bastar district [6]. This area is also having serious problems of insurgency, which are affecting the health services of the area adversely [7, 8]. This is the first report from India of 4 Plasmodium species in 1 case. Such rare cases of malaria are a diagnostic and clinical challenge.
Fig 1. Map of India (A) showing Madhya Pradesh National Institute for Research in Tribal Health (NIRTH) and Chhattisgarh (B) and Darbha community health centre, Bastar district (C).
Diagnosis
A malaria clinic of National Institute for Research in Tribal Health (NIRTH) of the Indian Council of Medical Research (ICMR) was established at Darbha CHC of Bastar district in 2015 to provide prompt diagnosis and treatment of malaria in difficult and conflict-affected areas, as the villages in Darbha CHC are inaccessible and this region has been under continuous attack by the Maoists. There is no public transport available in the villages because of thick forest. People visit the hospital when they come to the market to purchase their daily needs.
Symptomatic patients were screened for malaria by bivalent RDT, SD Bioline Malaria Antigen P.f./P.v. (Bio Standard Diagnostics Pvt. Ltd., India), and microscopy. A finger-prick blood sample was collected from patients after taking written informed consent. Patients having doubtful parasite species were identified by molecular methods. Genomic DNA was isolated and polymerase chain reaction (PCR) was carried out for Plasmodium species identification using standard protocol [9]. Universal primers were designed for all 4 species of Plasmodium, with restriction sites for enzymes XhoI and BamHI. The full length of 18S rRNA gene was amplified with Phusion High Fidelity DNA Polymerase (New England Biolabs, USA) and cloned into pBluescript SK (+) vector through restriction and ligation using DH5α competent cells. Amplification of the partial 18S rRNA gene was also performed using vector-specific M13 forward primer and Plasmodium species-specific reverse primer from ligated product. Sequencing of 18S rRNA from clones and ligated product was done by Di-deoxy chain termination method using 3130xl genetic analyser (Applied biosystems, USA). Genes specific to each species, P. falciparum dihydropteroate synthase gene (Pf-dhps), P. vivax dihydropteroate synthase gene (Pv-dhps), P. malariae merozoite surface protein 1 gene (Pm-msp1), and P. ovale reticulocyte binding protein gene (Po-rbp) were also amplified and sequenced [10, 11].
Ethical approval
This study protocol for collection of blood samples from patients with malaria infection was approved by the Institutional Ethics Committee of NIRTH, Jabalpur (Madhya Pradesh), India.
Case presentation
A 12-year-old boy from a very remote village attended the Darbha CHC hospital with the complaint of repeated history of fever with no other complications.
At the time of admission, he gave history of 4 days’ fever and his body temperature was recorded as 100.8°F. His pulse rate was 101/min and his respiratory rate was 28/m. The laboratory findings of blood test at the time of admission were Hb≤10 gm/dL, blood glucose = 88 mg/dL, total leukocyte count (TLC) = 4,000/mm3, differential leukocyte count (DLC) = neutrophils (52%), lymphocyte (45%), eosinophils (2%), monocyte (1%), and serum creatinine = 0.5 mg/dL. The bivalent RDT showed the presence of P. falciparum only. Subsequently, peripheral blood smear was found positive for P. falciparum along with some doubtful structures, which a microscopist was unable to identify. He was given oral artesunate plus sulphadoxine/pyrimethamine (AS+SP). As fever was not coming down and Hb had lowered to <8 gm/dL, the patient was referred to district hospital where he was treated with intravenous antimalarial (quinine 20 mg/kg body weight on admission followed by maintenance dose of 10 mg/kg 8 hourly) along with other supportive treatment, as per the duty physician. However, he did not take the complete treatment and left hospital against medical advice. Follow-up of the patient was not possible due to inaccessibility of the area.
Case discussion
In this study, 23% mixed infections were found with 2 or more species out of 160 cases of doubtful identification (Fig 2). Molecular analysis revealed that mixed infections of P. vivax and P. falciparum were highest (19%), followed by P. falciparum, P. vivax, and P. malariae (2.5%); P. falciparum and P. malariae (1.3%); and only 1 case with all 4 species (Fig 3a & 3b). All these mixed infections were mild and did not show any complications. The analysed sequences of 4 species were submitted to the GenBank database (accession numbers KU510226-KU510234 and KY202757-KY202761). Of the 4 species, P. falciparum causes the most severe symptoms, i.e., severe anaemia, cerebral malaria, multiorgan failure, and death [6, 12]. P. vivax and P. ovale, although responsible for mild infection, may persist within the liver as hypnozoites, causing relapses even after treatment with blood schizonticides [13]. P. malariae is also mild, may persist in the human population at very low density, and may cause renal failure [14]. The available results from various studies are contradictory. The studies carried out in Ivory Coast [15], Sri Lanka, [16], Thailand [17], and Vanuatu [18] revealed that in mixed-species infection, the severity may be modulated. On the contrary, others reported that mixed infection of P. falciparum and P. vivax affected the clinical outcome in patients [19–21]. It is well known that the frequencies of less common species such as P. malariae and P. ovale are largely underestimated by microscopy [4, 22]. The low sensitivity of microscopy has 2 major consequences in malaria-control efforts. First, low-density parasitemia may serve as a reservoir for infections; second, in mixed infections, the tendency of 1 parasite to dominate the other lowers the efficiency of microscopic detection of 2 species in the same sample [23]. In this study, initial inappropriate treatment was given due to incorrect diagnosis of parasite species, as it is known that SP efficacy against vivax malaria is imperfect. Incorrect malaria diagnosis is a severe public health concern, as misidentification of malaria parasites could lengthen the parasite-clearance time and lead to recrudescence [19] and drug resistance [24]. This study indicates the need of adequate training of health staff involved in malaria diagnosis and the inherent limitations of microscopic diagnosis, which is prerequisite for malaria elimination.
Fig 2. Gel image showing diagnosis of 4 Plasmodium species.
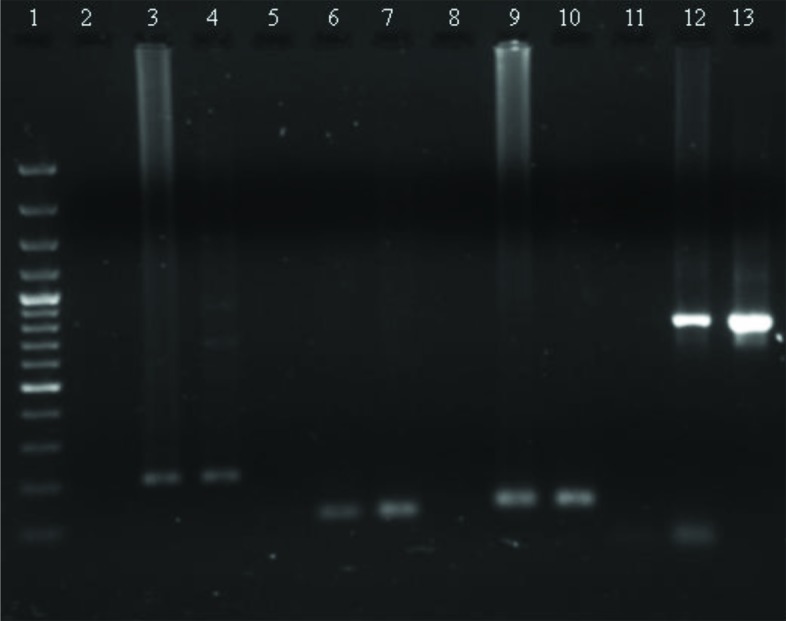
1: 100 base pair (bp) ladder; P. falciparum (2: NC, 3: PC, 4: sample); P. vivax (5: NC, 6: PC, 7: sample); P. malariae (8: NC, 9: PC, 10: sample); P. ovale (11: NC, 12: PC, 13: sample). Abbreviations: NC, negative control; PC, positive control.
Fig 3.
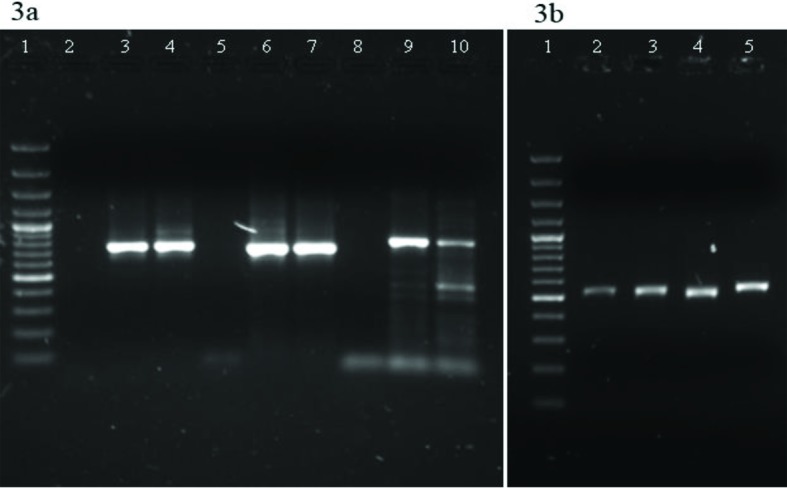
(a) Gel image showing amplification of Pf-dhps, Pv-dhps, and Po-rbp. 1: 100 base pair (bp) ladder; Pf-dhps (2: NC, 3: PC, 4: sample); Pv-dhps (5: NC, 6: PC, 7: sample); Po-rbp (8: NC, 9: PC, 10: sample) (b) Gel image showing amplification of Pm-msp1 gene from genomic DNA sample. 1: 100 bp ladder; 2–5: samples. Abbreviations: NC, negative control; PC, positive control.
Key learning points
A case of mixed infection of 4 Plasmodium species was found, which is very rare, and this is the first report of such a case in India.
Microscopy is the gold standard for malaria diagnosis, but in case of mixed infection, 1 or more species may be missed, especially in case of low parasitemia.
The study showed high proportion of mixed infection by molecular methods.
High proportion of mixed infections signifies the need of adequate training of health staff involved in malaria diagnosis, which is prerequisite for malaria elimination.
Acknowledgments
We are grateful to the study participant and guardians for their cooperation during enrollment. We are also thankful to our field staff for appreciable hard work in a remote area.
Funding Statement
This study was funded by Indian Council of Medical Research, New Delhi, India. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization. World Malaria Report 2015. http://apps.who.int/iris/bitstream/10665/200018/1/9789241565158_eng.pdf. Accessed 10 May 2016.
- 2.Bell DR, Wilson DW, Martin LB. False-positive results of a Plasmodium falciparum histidine-rich protein 2-detecting malaria rapid diagnostic test due to high sensitivity in a community with fluctuating low parasite density. Am J Trop Med Hyg. 2005; 73: 199–203. [PubMed] [Google Scholar]
- 3.White NJ, Pukrittayakamee S, Hien TT, Faiz MA, Mokuolu OA, Dondorp AM. Malaria. Lancet. 2014; 383: 723–735. doi: 10.1016/S0140-6736(13)60024-0 [DOI] [PubMed] [Google Scholar]
- 4.Bharti PK, Chand SK, Singh MP, Mishra S, Shukla MM, Singh R, et al. Emergence of a new focus of Plasmodium malariae in forest villages of district Balaghat, Central India: implications for the diagnosis of malaria and its control. Trop Med Int Health. 2013; 18: 12–17. doi: 10.1111/tmi.12005 [DOI] [PubMed] [Google Scholar]
- 5.Krishna S, Bharti PK, Chandel HS, Ahmad A, Kumar R, Singh PP, et al. Detection of Mixed Infections with Plasmodium spp. by PCR, India, 2014. Emerg Infect Dis. 2015; 21: 1853–1857. doi: 10.3201/eid2110.150678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain V, Basak S, Bhandari S, Bharti PK, Thomas T, Singh MP, et al. Burden of Complicated Malaria in a Densely Forested Bastar Region of Chhattisgarh State (Central India). PLoS ONE. 2014; 9: e115266 doi: 10.1371/journal.pone.0115266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan S. Over 200 jawans battling malaria in Chhattisgarh’s Maoist zone. Mail Today. 13 December 2014. http://indiatoday.intoday.in/story/naxals-maoists-malaria-jawans-bastar-chhattisgarh-cpim/1/406652.html. Accessed 7 July 2016.
- 8.Balagopal K. Chhattisgarh: Physiognomy of Violence. Economic & Political Weekly 2006; 3: 2183–2186. [Google Scholar]
- 9.Snounou G, Viriyakosol S, Jarra W, Thaithong S, Brown KN. Identification of the four human malaria parasite species in field samples by the polymerase chain reaction and detection of a high prevalence of mixed infections. Mol Biochem Parasitol. 1993; 58: 283–292. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed A, Bararia D, Vinayak S, Yameen M, Biswas S, Dev V, et al. Plasmodium falciparum isolates in India exhibit a progressive increase in mutations associated with sulfadoxinepyrimethamine resistance. Antimicrob Agents Chemother. 2004; 48: 879–889. doi: 10.1128/AAC.48.3.879-889.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imwong M, Pukrittayakamee S, Cheng Q, Moore C, Looareesuwan S, Snounou G, et al. Limited polymorphism in the dihydropteroate synthetase gene (dhps) of Plasmodium vivax isolates from Thailand. Antimicrob Agents Chemother. 2005; 49: 4393–4395. doi: 10.1128/AAC.49.10.4393-4395.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain V, Nagpal AC, Joel PK, Shukla M, Singh MP, Gupta RB, et al. Burden of cerebral malaria in central India (2004-2007). Am J Trop Med Hyg. 2008; 79: 636–642. [PMC free article] [PubMed] [Google Scholar]
- 13.Steenkeste N, Rogers WO, Okell L, Jeanne I, Incardona S, Duval L, et al. Sub-microscopic malaria cases and mixed malaria infection in a remote area of high malaria endemicity in Rattanakiri province, Cambodia: implication for malaria elimination. Malar J. 2010; 9: 108 doi: 10.1186/1475-2875-9-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neri S, Pulvirenti D, Patamia I, Zoccolo A, Castellino P. Acute renal failure in Plasmodium malariae infection. Neth J Med. 2008; 66: 166–168. [PubMed] [Google Scholar]
- 15.Black J, Hommel M, Snounou G, Pinder M. Mixed infections with Plasmodium falciparum and P. malariae and fever in malaria. Lancet. 1994; 343: 1095. [DOI] [PubMed] [Google Scholar]
- 16.Gunawardena DM, Carter R, Mendis KN. Patterns of acquired anti-malarial immunity in Sri Lanka. Mem Inst Oswaldo Cruz. 1994; 89: 61–63. [DOI] [PubMed] [Google Scholar]
- 17.Luxemburger C, Ricci F, Nosten F, Raimond D, Bathet S, White NJ. The epidemiology of severe malaria in an area of low transmission in Thailand. Trans R Soc Trop Med Hyg. 1997; 91: 256–262. [DOI] [PubMed] [Google Scholar]
- 18.Molineaux L, Storey J, Cohen JE, Thomas A. A longitudinal study of human malaria in the West African Savanna in the absence of control measures: relationships between different Plasmodium species, in particular P. falciparum and P. malariae. Am J Trop Med Hyg. 1980; 29: 725–737. [DOI] [PubMed] [Google Scholar]
- 19.Mayxay M, Pukrittayakamee S, Newton PN, White NJ. Mixed-species malaria infections in humans. Trends Parasitol. 2004; 20: 233–240. doi: 10.1016/j.pt.2004.03.006 [DOI] [PubMed] [Google Scholar]
- 20.Maitland K, Williams TN, Newbold CI. Plasmodium vivax and P. falciparum: biological interactions and the possibility of cross-species immunity. Parasitol Today. 1997; 13: 227–231. [DOI] [PubMed] [Google Scholar]
- 21.Smith T, Genton B, Baea K, Gibson N, Narara A, Alpers MP. Prospective risk of morbidity in relation to malaria infection in an area of high endemicity of multiple species of Plasmodium. Am J Trop Med Hyg. 2001; 64, 262–267. [DOI] [PubMed] [Google Scholar]
- 22.Chaturvedi N, Bhandari S, Bharti PK, Basak SK, Singh MP, Singh N. Sympatric distribution of Plasmodium ovale curtisi and P. ovale wallikeri in India: implication for the diagnosis of malaria and its control. Trans R Soc Trop Med Hyg. 2015; 109: 352–354. doi: 10.1093/trstmh/trv015 [DOI] [PubMed] [Google Scholar]
- 23.Sethabutr O, Brown A, Panyim S, Kain KC, Webster HK, Echeverria P. Detection of Plasmodium falciparum by polymerase chain reaction in a field study. J Infect dis. 1991; 166: 145–148. [DOI] [PubMed] [Google Scholar]
- 24.De Roode JC, Culleton R, Bell AS, Read AF. Competitive release of drug resistance following drug treatment of mixed Plasmodium chabaudi infections. Malar J. 2004; 3:33 doi: 10.1186/1475-2875-3-33 [DOI] [PMC free article] [PubMed] [Google Scholar]