Abstract
Renal hyperfiltration is closely linked to cardiometabolic disorders, and it may increase the mortality risk of the general population. Despite the well-established association between cardiometabolic diseases and sarcopenia, the relationship between renal hyperfiltration and sarcopenia has not yet been assessed.
This population-based, cross-sectional study used a nationally representative sample of 13,800 adults from the 2008 to 2011 Korea National Health and Nutrition Examination Survey. Renal hyperfiltration was defined as the age- and sex-specific glomerular filtration rate above the 90th percentile in subjects with normal kidney function (>60 mL/min/1.73 m2). Appendicular skeletal muscle (ASM), measured by dual-energy x-ray absorptiometry, was used to assess pre-sarcopenia, which the international consensus defines as both ASM per se and ASM that was adjusted for the body mass index and the height.
A total of 1402 (10.2%) participants were classified as having renal hyperfiltration. The prevalence of pre-sarcopenia ranged from 11.6% to 33.0%, by definition. Individuals with pre-sarcopenia had higher risks of renal hyperfiltration compared to those without pre-sarcopenia (10.9% vs 17.4%, P < .001; odds ratio [OR] = 1.71, 95% confidential interval [CI] = 1.48–1.99, P < .001). Multiple logistic regression analyses also demonstrated this independent association between pre-sarcopenia and renal hyperfiltration, following adjustment for confounding factors such as insulin resistance and obesity (OR = 1.84, 95% CI = 1.57–2.15, P < .001).
In the general population of healthy individuals, pre-sarcopenia might be associated with renal hyperfiltration independent of obesity or insulin resistance.
Keywords: epidemiology, insulin resistance, obesity, renal hyperfiltration, sarcopenia
1. Introduction
The decreased glomerular filtration rate (GFR) is a characteristic of chronic kidney disease (CKD), and a well-known risk factor for cardiovascular mortality and metabolic disease.[1,2] In contrast, renal hyperfiltration is an abnormally high GFR.[3] Although renal hyperfiltration occurs in a healthy population, it can indicate the preclinical stage of CKD[4] and is associated with metabolic diseases such as diabetes, hypertension, and obesity.[5–8] A pooled, international meta-analysis reported a U-shaped association of GFR with mortality, which indicates that hyperfiltration is linked to the higher mortality rate than CKD.[9]
Loss of muscle mass, or sarcopenia, is closely related to mortality regardless of age, physical performance, or health status,[10,11] and the relationship between sarcopenia and metabolic impairment has been reported by several studies.[11–14] Although the etiology of sarcopenia has not been clearly established, factors that contribute to sarcopenia, such as chronic inflammation and insulin resistance, are closely linked to renal hyperfiltration.[3,12] Furthermore, sarcopenia increases the risk of diabetes,[14] which is a determinant factor for renal hyperfiltration.[3,13] The high mortality rate of sarcopenia might be associated with renal hyperfiltration, as sarcopenia is a poor prognostic marker that is associated with increased mortality rate, independent of age,[10,11] and the U-shaped mortality curve related to kidney function.[9] In regard to mortality and cardiometabolic components, there is a possibility for sarcopenia and renal hyperfiltration to share common pathophysiology. However, no study has yet investigated the relationship between sarcopenia and renal hyperfiltration.
Based on this evidence, we hypothesized that sarcopenia and renal hyperfiltration share common pathophysiological mechanisms. The aim of the present study was to investigate the independent association between sarcopenia and renal hyperfiltration in the general population, after adjusting for the potential effects of additional risk factors such as obesity, insulin resistance, and blood pressure. To the best of our knowledge, this is the first study to explore the relationship between renal hyperfiltration and sarcopenia.
2. Materials and methods
2.1. Data source and study population
This cross-sectional study extracted data from the 2008 to 2011 Korea National Health and Nutrition Examination Survey (KNHANES). As previously described in detail,[15] each KNHANES is composed of independent data sets from the general population of Korea, similar to the National Health and Nutrition Examination Survey in the United States. Of the 37,753 participants in the 2008–2011 KNHANES, we initially selected individuals aged ≥20 years (12,160 men and 15,911 women). Exclusion criteria were missing data for appendicular skeletal muscle (ASM) mass, determined by dual-energy x-ray absorptiometry (DXA, QDR 4800A; Hologic Inc., Bedford, MA), height, body weight, and serum creatinine. We also excluded individuals with CKD (defined as GFR < 60 mL/min/1.73 m2)[16] or diabetes, both of which are considered major contributors to renal hyperfiltration.[3] A total of 13,800 subjects were included in the final analysis (Fig. 1). All participants signed written informed consent. Survey protocol was approved by the institutional review board of the Korean Centers for Disease Control and Prevention (2008-04EXP-01-C, 2009-07CON-03-2C, 2010-02CON-21-C, and 2011-02CON-06C).
Figure 1.
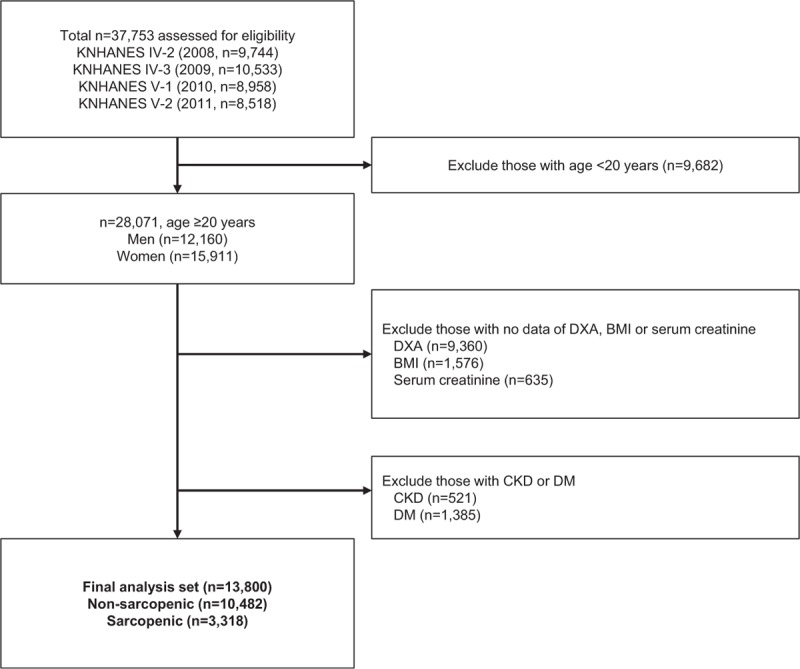
Flow diagram of inclusion and exclusion of subjects from the 2008 to 2011 Korean National Health and Nutrition Examination Surveys (KNHANES IV and V). BMI = body mass index, CKD = chronic kidney disease, DM = diabetes mellitus, DXA = dual-energy x-ray absorptiometry.
2.2. Definitions of pre-sarcopenia and renal hyperfiltration
DXA has been used to assess body muscle distribution as well as bone density with minimal radiation exposure.[17] In addition to the assessment of subjects’ muscle distributions throughout the whole body, ASM was calculated as the sum of lean soft tissue masses in the arms and legs, excluding all other body parts. Since the KNHANES data used in the current study evaluated the muscle mass, and not the muscle quality or strength, we used the term “pre-sarcopenia” according to the European consensus guideline.[18] We applied multiple pre-sarcopenia definitions that are recommended by international consensus, as follows: ASM/height[2] (<7.0 kg/m2 for men, <5.4 kg/m2 for women)[19]; ASM/body mass index (BMI) (sex-specific lowest 25th percentile, <0.909 for men, <0.603 for women); and ASM (<19.75 kg for men, <15.02 kg for women).[20] According to the cut points set by National Institutes of Health (FNIH), which are defined as ASM/BMI of <0.789 for men and <0.512 for women, the prevalence of pre-sarcopenia in the study population was 4.1%, which was not enough for data analysis. Therefore, we determined other values that are the FNIH suggested as alternative definitions of ASM per se,[20] as well as the sex-specific, lowest 25th percentile for ASM/BMI. Estimated GFR was calculated using the Chronic Kidney Disease Epidemiology collaboration (CKD-EPI) equation,[21] and renal hyperfiltration was defined as previously described[22]: GFR > 90th percentile adjusted for sex and age.
2.3. Measurements of biochemical analysis, clinical parameters, and lifestyle factors
KNHANES data include a three-part medical history, information on dietary intake, and laboratory tests. Medical history, which included smoking status, alcohol consumption, exercise, diagnoses, and treatments, was evaluated based on direct interviews and self-report questionnaires. Dietary intake information was collected by well-trained dietary interviewers, using a 24-hour recall method. Blood pressure was manually measured by well-trained nurses using mercury sphygmomanometers (Baumanometer; W.A. Baum, Copiague, NY). After an overnight (at least 8-hour) fast, blood and spot urine samples were collected, refrigerated, and transported to the central laboratory (NeoDin Medical Institute, Seoul, South Korea) within 24 hours. Serum lipid profiles, as well as levels of glucose, insulin, 25(OH)D concentration, creatinine, and other biochemical variables (white blood cell, liver enzymes, etc.) from blood samples were measured. Proteinuria was characterized as more than 1 positive dip-stick test.
Regular exercise was referred to engaging in moderate physical activity (slow-pace swimming, doubles tennis, volleyball, badminton, table tennis, moving light objects, etc.) involved 20-minute sessions (or longer each time) 3 days a week. Participants were considered to be heavy drinkers if their alcohol consumption exceeded 140 g/week for men and 70 g/week for women.[23] Hypertension was defined as systolic/diastolic blood pressure ≥140/90 mm Hg, or taking anti-hypertensive medications. Impaired fasting glucose (IFG) was considered if subject's fasting blood glucose was 100 mg/dL or more. Metabolic syndrome was defined according to the National Cholesterol Education Program Adult Treatment Panel III criteria adopted for the Korean population.[24] Central obesity was characterized using the following cut-offs adopted for the Korean population: waist circumference ≥90 and ≥85 cm for men and women, respectively [24]. The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as previously described,[25] and insulin resistance was defined as HOMA-IR > 2.5. Because vitamin D deficiency is known to be associated with sarcopenia, in regard to both muscle mass and strength,[26,27] we defined vitamin D deficiency as 25(OH)D < 20 ng/dL, based on the clinical practice guideline of the Endocrine Society.[28]
2.4. Statistical analyses
We analyzed participant characteristics according to each individual's renal hyperfiltration status, using Student's t-tests for continuous variables and chi-square tests for categorical variables. Data are presented as mean ± standard deviation, number, or percent. Because fasting insulin, total cholesterol, triglyceride, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol values were not normally distributed, analyses were performed using log- and back-transformed data. Multiple logistic regression analysis was used to compute odds ratios (ORs) to estimate the association between pre-sarcopenia and renal hyperfiltration. To evaluate the relationship between pre-sarcopenia and renal hyperfiltration, anthropometric characteristics (age, sex, obesity, blood pressure), lifestyle patterns (exercise, alcohol consumption, cigarette smoking), and biochemical values (fasting plasma glucose, HOMAIR, lipid panels) were determined as covariates. Statistical analyses were performed using IBM SPSS version 23.0 for Windows (IBM Corp., Armonk, NY); P <.05 was considered significant.
3. Results
The mean age of current study population was 47.2 years, and less than half (42.5%) of the subjects were men. Although the mean BMI was 23.4 kg/m2, 32.6% of participants were classified as having central obesity. The prevalence of pre-sarcopenia differed for varying definitions: 11.8% (ASM/BMI), 15.2% (ASM/height[2]), and 12.7% (ASM). In addition, the ratio of individuals with pre-sarcopenia was higher in the older age group (>60 years) than the young (49.7% vs 17.0%, 14.2% vs 10.8%, 27.3% vs 50.8%; all P < .001, ASM/BMI, ASM/height[2], ASM definitions, respectively). The prevalence of renal hyperfiltration was 10.2% in the overall study population. The mean age- and sex-specific GFR and 90th percentile cutoff points are shown in Fig. 2. The cutoff values for renal hyperfiltration ranged from 90.5 to 122.5 mL/min/1.73 m2 for men and from 92.0 to 128.9 mL/min/1.73 m2 for women. The prevalence of pre-sarcopenia gradually increased with age (P for trend < .001, Fig. 3A), whereas the prevalence of renal hyperfiltration was similar among age groups (P for trend = .302, Fig. 3B).
Figure 2.
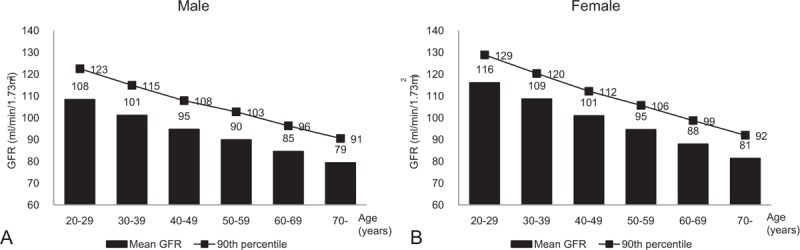
Distribution of estimated glomerular filtration rate by sex and age. The 90th percentiles (line graph) and mean values (bar graph) are shown for each 10-year age group in (A) men and (B) women.
Figure 3.
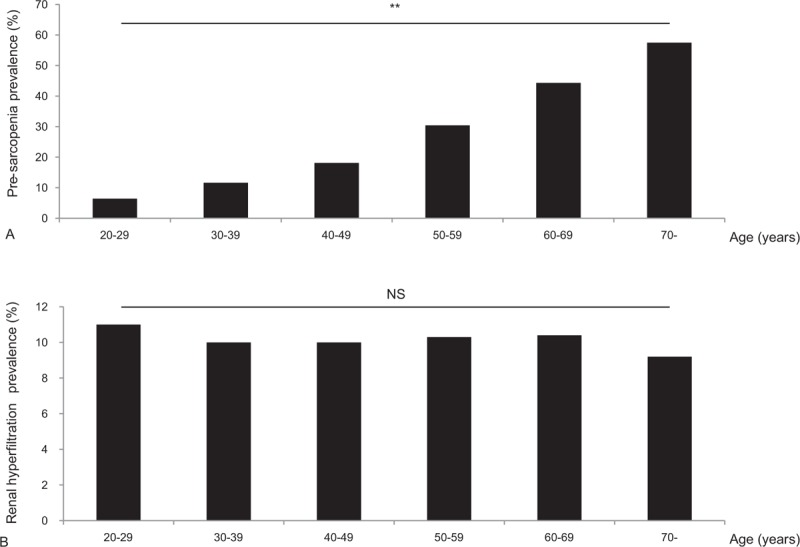
Prevalence of pre-sarcopenia and renal hyperfiltration according to age. Prevalence of (A) pre-sarcopenia and (B) renal hyperfiltration. Sarcopenia was defined by sex-specific skeletal muscle mass adjusted by body mass index. NS = nonsignificant, ∗∗P for trend <.001.
3.1. Characteristics of subjects with renal hyperfiltration
We compared the baseline characteristics of 1402 individuals with renal hyperfiltration and 12,398 participants without renal hyperfiltration (Table 1). Participants with renal hyperfiltration were relatively younger, with lower BMI, waist circumference and ASM. Although the fasting plasma glucose level slightly increased in participants without renal hyperfiltration, fasting insulin, HOMA-IR, and the prevalence of IFG were similar to those of renal hyperfiltration population (all P>.05). Renal hyperfiltration individuals showed higher serum triglycerides and low 25(OH)D concentration, with slight decreases in the prevalence of hypertension and metabolic syndrome. However, we found more current smokers and heavy alcohol drinkers in renal hyperfiltration population. In addition, we observed no difference in the level of dietary intake between with and without renal hyperfiltration groups. The proportions of individuals with renal hyperfiltration significantly increased in the pre-sarcopenic group, regardless of pre-sarcopenia definitions (Fig. 4). The prevalence of proteinuria was higher in the hyperfiltration group; however, there was no statistical difference in proteinuria prevalence between groups (P = .612) due to the low proteinuria prevalence (0.6%).
Table 1.
Characteristics of study participants according to renal hyperfiltration status.
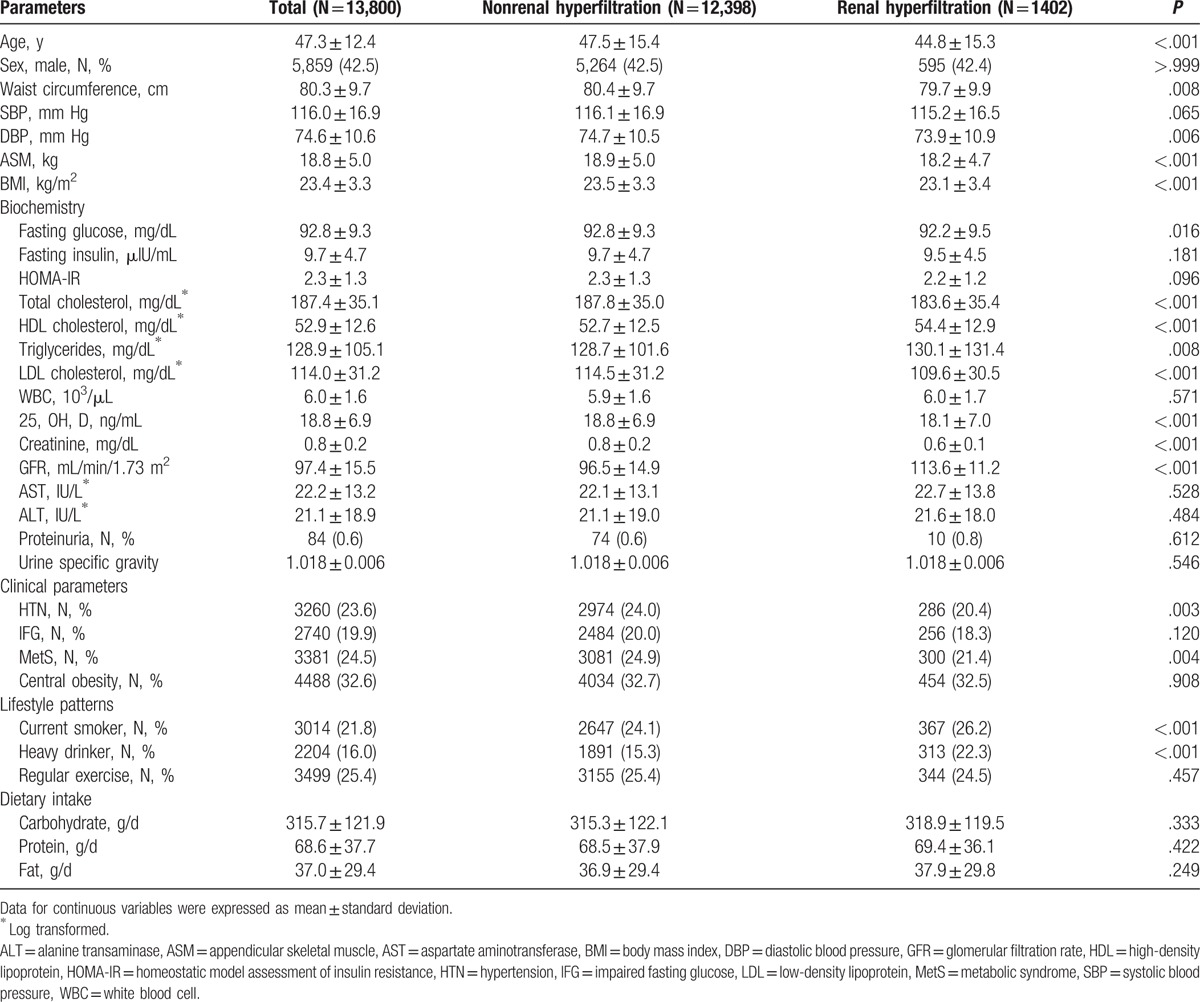
Figure 4.
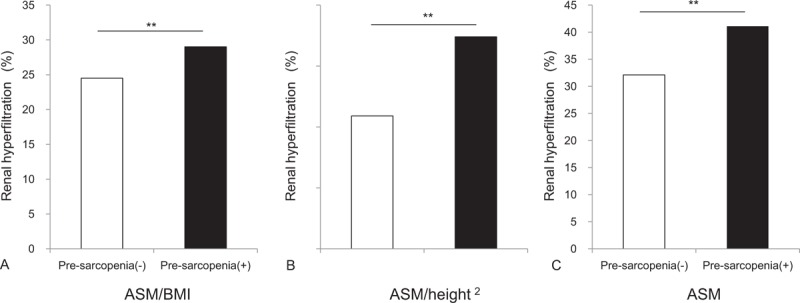
Proportion of individuals with sarcopenia and renal hyperfiltration according to pre-sarcopenia definitions. Pre-sarcopenia was defined by (A) ASM/BMI, (B) ASM/height2, and (C) ASM. ASM = appendicular skeletal muscle, BMI = body mass index, ∗∗P < .001.
3.2. Multiple logistic regression analysis to evaluate the relationship between pre-sarcopenia and renal hyperfiltration
Several multivariable logistic regression models were applied to adjust for conventional confounding covariates that affect renal hyperfiltration (Table 2). In the basic adjusted model with age and sex (model 1), pre-sarcopenia significantly raised the risk of renal hyperfiltration regardless of pre-sarcopenia definitions (OR ranges 1.57–1.79, all P < .001). This association remained even after adjusting factors that could affect renal hyperfiltration, including lipids (triglyceride, total cholesterol), plasma glucose, insulin resistance (HOMA-IR), vitamin D deficiency, blood pressure, central obesity, and lifestyle patterns (exercise, alcohol drink, and cigarette smoke). As shown in Model 3, pre-sarcopenia continued to increase the risk of renal hyperfiltration (OR ranges 1.62–1.99, all P < .001).
Table 2.
Odds ratio and 95% confidential interval of renal hyperfiltration according to pre-sarcopenia definitions.
4. Discussion and conclusions
The results of this nationally representative, population-based study showed that, in comparison to individuals with preserved muscle mass, subjects with pre-sarcopenia had an increased risk of renal hyperfiltration independent of glucose impairment, insulin resistance, and central obesity. Our multivariable logistic regression analyses clearly showed a significant association between pre-sarcopenia and renal hyperfiltration, regardless of pre-sarcopenia definitions.
Renal hyperfiltration is a complication of diabetes and a predictor of complication progression including diabetic nephropathy.[29,30] Furthermore, a recent study concluded that renal hyperfiltration is associated with a significantly increased risk of metabolic impairment in the absence of diabetes and, individuals with renal hyperfiltration had approximately 60% increased risk of carotid atherosclerosis and left ventricular hypertrophy, although none of the study population had diabetes or prior cardiovascular disease.[31] In addition, other epidemiologic studies have reported that renal hyperfiltration may increase the mortality rates of general populations by 27 to 37%.[9,32]
The mechanisms underlying renal hyperfiltration and its adverse outcomes are not clearly understood, but proposed factors contributing to this condition include inflammation,[33] insulin resistance,[34] and overactivation of the sympathetic nervous system or renin-angiotensin-aldosterone system (RAAS).[31] Oxidative stress and inflammation could initiate glomerular structural change, increase glomerular permeability, and ultimately cause renal hyperfiltration.[33] Inflammation also plays a key role in the pathogenesis of insulin resistance,[35] causing renal hyperfiltration by altering renal endothelial function and inducing afferent arteriole relaxation.[36] The angiotensin-converting enzyme in vascular endothelial cells inactivates bradykinin, which stimulates nitric oxide release and counteracts angiotensin II-mediated effects.[37] Angiotensin II activation increases TGF-β upregulation, thereby accelerates glomerular and tubulointerstitial injury.[38] Conversely, RAAS blockade or sympathetic nerve denervation could decrease renal hyperfiltration.[39,40] In addition, vitamin D deficiency is associated with RAAS and NF-κB pathway, regulating renal hyperfiltration.[41] These factors are also involved in muscle wasting. Chronic inflammation could influence protein metabolism and contribute to progression of muscle wasting.[33] As insulin has an anabolic effect that stimulates muscle hypertrophy, insulin resistance, which is an impairment in insulin signaling, could induce muscle synthesis.[42,43] In addition, overactivation of RAAS with TGF-β activation attenuates muscle structure,[44] whereas vitamin D deficiency is positively correlated with muscle wasting .[45]
The most important limitation of the current study was its cross-sectional design, which precludes conclusions about causality. However, we analyzed the risks of pre-sarcopenia and renal hyperfiltration and fully adjusted for confounding factors. Our results indicate that pre-sarcopenia may increase the risk of renal hyperfiltration, suggesting that there are shared underlying mechanisms. Second, in GFR assessment, the CKD-EPI equation based on serum creatinine was used to determine renal hyperfiltration in the current study. Ideally, inulin has been considered to be the marker for determining GFR. However, it is difficult to use inulin in primary care; thus, indirect markers using equations are commonly used. In addition, considering the close relationship between sarcopenia and albuminuria,[46] and albuminuria to renal hyperfiltration,[3] the association between renal hyperfiltration and sarcopenia is plausible. Although the CKD-EPI equation is used to estimate GFR in many epidemiology studies, it cannot completely substitute the gold standard method based on inulin clearance or cystatin C. Third, pre-sarcopenia was assessed only by dual-energy x-ray absorptiometry, which reflects muscle mass but not skeletal muscle function. We only determined the muscle mass, and neither the muscle strength nor physical performance, due to lack of information.
To the best of our knowledge, this is the first study to report an independent association between renal hyperfiltration and pre-sarcopenia in the general population. In addition, this is a large population-based study based on national data, which strengthens the statistical reliability of the results. KNHANES represents the non-institutionalized general population of an Asian country; therefore, selection bias is minimized. Furthermore, we showed strong evidence for a close relationship between pre-sarcopenia and renal hyperfiltration by adjusting for confounders, and this association remained regardless of pre-sarcopenia definitions.
In conclusion, the present study shows that pre-sarcopenia is associated with renal hyperfiltration in healthy general populations. This association was independent of insulin resistance and central obesity. Prospective longitudinal studies are warranted to assess causality and identify shared mechanisms.
Acknowledgments
The authors thank to all participants in this study.
Footnotes
Abbreviations: ASM = appendicular skeletal muscle, BMI = body mass index, CKD = chronic kidney disease, CKD-EPI = Chronic Kidney Disease Epidemiology collaboration, DXA = dual-energy x-ray absorptiometry, GFR = glomerular filtration rate, HOMA-IR = homeostasis model assessment of insulin resistance, IFG = impaired fasting glucose, KNHANES = Korea National Health and Nutrition Examination Survey, OR = odds ratio, RAAS = renin-angiotensin-aldosterone system.
Authorship: Study concept and design—EH, Y-hL, and B-SC.
Analysis and interpretation of data—EH, Y-hL, and B-SC.
Drafting of the manuscript: EH and Y-hL.
Critical revision of the manuscript for important intellectual content—ESK and B-WL.
Statistical analysis—EH and Y-hL.
Administrative, technical, or material support—ESK, B-WL, and B-SC.
EH and Y-hL equally contributed to this study.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Nitsch D, Grams M, Sang Y, et al. Associations of estimated glomerular filtration rate and albuminuria with mortality and renal failure by sex: a meta-analysis. BMJ 2013;346:f324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chen J, Muntner P, Hamm LL, et al. The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Int Med 2004;140:167–74. [DOI] [PubMed] [Google Scholar]
- [3].Helal I, Fick-Brosnahan GM, Reed-Gitomer B, et al. Glomerular hyperfiltration: definitions, mechanisms and clinical implications. Nat Rev Nephrol 2012;8:293–300. [DOI] [PubMed] [Google Scholar]
- [4].Brenner BM, Lawler EV, Mackenzie HS. The hyperfiltration theory: a paradigm shift in nephrology. Kidney Int 1996;49:1774–7. [DOI] [PubMed] [Google Scholar]
- [5].Nelson RG, Bennett PH, Beck GJ, et al. Development and progression of renal disease in Pima Indians with non-insulin-dependent diabetes mellitus. Diabetic Renal Disease Study Group. N Engl J Med 1996;335:1636–42. [DOI] [PubMed] [Google Scholar]
- [6].Vora JP, Dolben J, Dean JD, et al. Renal hemodynamics in newly presenting non-insulin dependent diabetes mellitus. Kidney Int 1992;41:829–35. [DOI] [PubMed] [Google Scholar]
- [7].Schmieder RE, Messerli FH, Garavaglia G, et al. Glomerular hyperfiltration indicates early target organ damage in essential hypertension. JAMA 1990;264:2775–80. [PubMed] [Google Scholar]
- [8].Chagnac A, Weinstein T, Herman M, et al. The effects of weight loss on renal function in patients with severe obesity. J Am Soc Nephrol 2003;14:1480–6. [DOI] [PubMed] [Google Scholar]
- [9].Matsushita K, van der Velde M, et al. Chronic Kidney Disease Prognosis Consortium. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010;375:2073–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Szulc P, Munoz F, Marchand F, et al. Rapid loss of appendicular skeletal muscle mass is associated with higher all-cause mortality in older men: the prospective MINOS study. Am J Clin Nutr 2010;91:1227–36. [DOI] [PubMed] [Google Scholar]
- [11].Landi F, Cruz-Jentoft AJ, Liperoti R, et al. Sarcopenia and mortality risk in frail older persons aged 80 years and older: results from ilSIRENTE study. Age Ageing 2013;42:203–9. [DOI] [PubMed] [Google Scholar]
- [12].Lee YH, Jung KS, Kim SU, et al. Sarcopaenia is associated with NAFLD independently of obesity and insulin resistance: Nationwide surveys (KNHANES 2008–2011). J Hepatol 2015;63:486–93. [DOI] [PubMed] [Google Scholar]
- [13].Kim TN, Park MS, Yang SJ, et al. Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes: the Korean Sarcopenic Obesity Study (KSOS). Diabetes Care 2010;33:1497–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Srikanthan P, Hevener AL, Karlamangla AS. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: findings from the National Health and Nutrition Examination Survey III. PloS One 2010;5:e10805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lee YH, Kim JE, Roh YH, et al. The combination of vitamin D deficiency and mild to moderate chronic kidney disease is associated with low bone mineral density and deteriorated femoral microarchitecture: results from the KNHANES 2008–2011. J Clin Endocrinol Metab 2014;99:3879–88. [DOI] [PubMed] [Google Scholar]
- [16].National Kidney F. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39(2 suppl 1):S1–266. [PubMed] [Google Scholar]
- [17].Chen Z, Wang Z, Lohman T, et al. Dual-energy X-ray absorptiometry is a valid tool for assessing skeletal muscle mass in older women. J Nutr 2007;137:2775–80. [DOI] [PubMed] [Google Scholar]
- [18].Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chen LK, Liu LK, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Directors Assoc 2014;15:95–101. [DOI] [PubMed] [Google Scholar]
- [20].Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. The journals of gerontology. J Gerontol A Biol Sci Med Sci 2014;69:547–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Int Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Melsom T, Mathisen UD, Ingebretsen OC, et al. Impaired fasting glucose is associated with renal hyperfiltration in the general population. Diabetes Care 2011;34:1546–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Han E, Kim G, Hong N, et al. Association between dietary acid load and the risk of cardiovascular disease: nationwide surveys (KNHANES 2008–2011). Cardiovasc Diabetol 2016;15:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Park HS, Park CY, Oh SW, et al. Prevalence of obesity and metabolic syndrome in Korean adults. Obesity Rev 2008;9:104–7. [DOI] [PubMed] [Google Scholar]
- [25].Yokoyama H, Emoto M, Fujiwara S, et al. Quantitative insulin sensitivity check index and the reciprocal index of homeostasis model assessment in normal range weight and moderately obese type 2 diabetic patients. Diabetes Care 2003;26:2426–32. [DOI] [PubMed] [Google Scholar]
- [26].Visser M, Deeg DJ, Lips P, et al. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab 2003;88:5766–72. [DOI] [PubMed] [Google Scholar]
- [27].Scott D, Blizzard L, Fell J, et al. A prospective study of the associations between 25-hydroxy-vitamin D, sarcopenia progression and physical activity in older adults. Clin Endocrinol 2010;73:581–7. [DOI] [PubMed] [Google Scholar]
- [28].Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–30. [DOI] [PubMed] [Google Scholar]
- [29].Dahlquist G, Stattin EL, Rudberg S. Urinary albumin excretion rate and glomerular filtration rate in the prediction of diabetic nephropathy; a long-term follow-up study of childhood onset type-1 diabetic patients. Nephrol Dialysis Transplant 2001;16:1382–6. [DOI] [PubMed] [Google Scholar]
- [30].Ruggenenti P, Porrini EL, Gaspari F, et al. Glomerular hyperfiltration and renal disease progression in type 2 diabetes. Diabetes Care 2012;35:2061–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Eriksen BO, Lochen ML, Arntzen KA, et al. Subclinical cardiovascular disease is associated with a high glomerular filtration rate in the nondiabetic general population. Kidney Int 2014;86:146–53. [DOI] [PubMed] [Google Scholar]
- [32].Park M, Yoon E, Lim YH, et al. Renal hyperfiltration as a novel marker of all-cause mortality. J Am Soc Nephrol 2015;26:1426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Timmerman KL, Volpi E. Endothelial function and the regulation of muscle protein anabolism in older adults. Nutr Metab Cardiovasc Dis 2013;23(suppl 1):S44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Dengel DR, Goldberg AP, Mayuga RS, et al. Insulin resistance, elevated glomerular filtration fraction, and renal injury. Hypertension 1996;28:127–32. [DOI] [PubMed] [Google Scholar]
- [35].Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 2006;116:1793–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Knight SF, Imig JD. Obesity, insulin resistance, and renal function. Microcirculation 2007;14:349–62. [DOI] [PubMed] [Google Scholar]
- [37].Sumukadas D, Struthers AD, McMurdo ME. Sarcopenia—a potential target for angiotensin-converting enzyme inhibition? Gerontology 2006;52:237–42. [DOI] [PubMed] [Google Scholar]
- [38].Sharma K, Ziyadeh FN. Renal hypertrophy is associated with upregulation of TGF-beta 1 gene expression in diabetic BB rat and NOD mouse. Am J Physiol 1994;267:F1094–101. [DOI] [PubMed] [Google Scholar]
- [39].Luippold G, Beilharz M, Muhlbauer B. Chronic renal denervation prevents glomerular hyperfiltration in diabetic rats. Nephrol Dialysis Transplant 2004;19:342–7. [DOI] [PubMed] [Google Scholar]
- [40].Sochett EB, Cherney DZ, Curtis JR, et al. Impact of renin angiotensin system modulation on the hyperfiltration state in type 1 diabetes. J Am Soc Nephrol 2006;17:1703–9. [DOI] [PubMed] [Google Scholar]
- [41].Li YC. Renoprotective effects of vitamin D analogs. Kidney Int 2010;78:134–9. [DOI] [PubMed] [Google Scholar]
- [42].Kalyani RR, Corriere M, Ferrucci L. Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol 2014;2:819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Abbatecola AM, Paolisso G, Fattoretti P, et al. Discovering pathways of sarcopenia in older adults: a role for insulin resistance on mitochondria dysfunction. J Nutr Health Aging 2011;15:890–5. [DOI] [PubMed] [Google Scholar]
- [44].Sumukadas D, Witham MD, Struthers AD, et al. Ace inhibitors as a therapy for sarcopenia—evidence and possible mechanisms. J Nutr Health Aging 2008;12:480–5. [DOI] [PubMed] [Google Scholar]
- [45].Kim MK, Baek KH, Song KH, et al. Vitamin D deficiency is associated with sarcopenia in older Koreans, regardless of obesity: the Fourth Korea National Health and Nutrition Examination Surveys (KNHANES IV) 2009. J Clin Endocrinol Metab 2011;96:3250–6. [DOI] [PubMed] [Google Scholar]
- [46].Han E, Lee YH, Kim G, et al. Sarcopenia is associated with albuminuria independently of hypertension and diabetes: KNHANES 2008-2011. Metabolism 2016;65:1531–40. [DOI] [PubMed] [Google Scholar]


