Abstract
High blood pressure (BP) is a major risk factor associated with stroke in China. This is a subanalysis of patients from the China Status II study, aimed to evaluate the effectiveness and safety of valsartan/amlodipine (Val/Aml) single-pill combination (SPC) in hypertensive patients with different stroke subtypes (hemorrhagic, ischemic, or mixed).
China Status II was a multicenter, postmarketing, prospective observational study in hypertensive patients uncontrolled on monotherapy. The study was an 8-week open-label treatment period with 2 4-week follow-ups. Change in BP from baseline to weeks 4 and 8, BP control rate, and response rate at weeks 4 and 8, and safety of 8-week treatment with Val/Aml (80/5 mg) were assessed.
A total of 565 hypertensive patients with different types of stroke were analyzed in this China Status II substudy. Significant mean sitting systolic/diastolic BP (MSSBP/MSDBP) reductions from baseline to week 8 were observed across all stroke subtypes (P < .0001). At week 8, percentages of patients achieving MSSBP response (≥20 mm Hg reduction from baseline) were 76.3%, 74.4%, and 85.7%, MSDBP response (≥10 mm Hg reduction from baseline) were 67.8%, 65.9%, and 64.3%, and BP control (<140/90 mm Hg) were 74.6%, 80.5%, and 92.9%, in the hemorrhagic, ischemic, and mixed stroke subgroups, respectively. Adverse events (AEs) and serious AEs were reported in 5 patients (1%) and 1 patient (0.2%), respectively, in the ischemic stroke subgroup, while no AEs were reported in hemorrhagic and mixed stroke subgroups.
Val/Aml SPC was effective in hypertensive patients with different stroke subtypes and was well tolerated.
Keywords: blood pressure, China, hypertension, single-pill combination, stroke, valsartan/amlodipine
1. Introduction
Stroke is one of the leading causes of mortality and disease burden (as measured in disability-adjusted life years [DALYs]).[1] In 2005, the global prevalence of stroke survivors (whether or not disabled as a consequence of stroke) was estimated to be 62 million, and is projected to rise to 77 million by 2030.[2] In 2010, 11.1% of all deaths worldwide were due to stroke, equally divided between hemorrhagic, ischemic, and other nonischemic stroke types,[3] and approximately 4% of global DALYs were due to stroke.[4] The majority of global stroke burden is in low- and middle-income countries, with 57% and 84% deaths and 64% and 85% DALYs lost in low- and middle-income countries, respectively.[5]
Stroke is the second most common cause of death among both urban and rural residents of China.[6] The overall incidence of stroke in China is projected to increase by 50% from 2010 to 2030. Based on an epidemiology survey of cerebrovascular disease conducted in 7 cities and 21 rural provinces in China, morbidity, mortality, and point prevalence was 219, 116, and 719 per 10 million in cities, and 185, 142, and 394 per 10 million in rural areas, respectively. Prevalence of patients with new-onset of stroke was estimated to be approximately 2 million, while nearly 1.5 million die of cerebrovascular disease.[7] Compared to developed countries, in China and many developing countries, stroke is the predominant form of cardiovascular disease and the incidence of both ischemic and of hemorrhagic stroke exceeds the incidence of ischemic heart disease.[8]
Blood pressure (BP) is the most consistent and powerful predictor of stroke. This is further supported by the fact that population mortality trends for stroke parallel those for hypertension.[9] In China, hypertension is the most important risk factor. A meta-analysis of 12 epidemiological studies with 2379 stroke cases confirmed that the overall relative risk of stroke associated with hypertension was 5.43.[10] In the INTERSTROKE study, self-reported history of hypertension was a significant risk factor for stroke, accounting for close to 50% of population at risk.[11] Guidelines recommend that for patients with hypertension and stroke, BP should be <140/90 mm Hg.[12–14]
Angiotensin receptor blockers (ARBs) can effectively control BP and reduce the incidence of stroke and hypertension associated with diabetes and atrial fibrillation; therefore, an ARB is recommended as a first-line treatment for stroke prevention in patients with hypertension.[15] Long-term use of calcium channel blockers (CCBs) was effective in reducing BP steadily, thereby helping to prevent atherosclerosis. CCBs can be the first choice of treatment for high BP and cerebrovascular disease.[14]
Previously conducted randomized controlled trials have shown that the ARB/CCB combination, valsartan/amlodipine (Val/Aml) (80/5 mg) single-pill combination (SPC) was superior to Val or Aml monotherapy in lowering BP and achieving BP control in Chinese patients with mild to moderate hypertension who were inadequately controlled by either monotherapy.[16] Aml and Val, 2 widely used antihypertensive drugs, have also been established to improve stroke prognosis in the VALUE study.[17,18]
China Status II, an observational study, has shown the effectiveness and safety of Val/Aml (80/5 mg) SPC in Chinese hypertensive patients uncontrolled by monotherapy.[19] The present study is a subanalysis of China Status II, evaluating the effectiveness and safety of 8-week treatment with Val/Aml SPC in hypertensive patients with stroke.
2. Materials and methods
2.1. Study design
This study was a post hoc subgroup analysis of the China Status II study based on stroke subtypes. China Status II was a multicenter, postmarketing, prospective observational study conducted in patients with essential hypertension whose BP was not adequately controlled by monotherapy. The study design and overall results have been described in detail elsewhere.[19] Briefly, the study consisted of an 8-week, open-label treatment period with 2 4-week follow-ups. An additional antihypertensive agent was added to the treatment regimens of those patients whose BP was not controlled at follow-up after 4 weeks. The study was conducted in accordance with the International Conference on Harmonization Good Clinical Practice, applicable local regulations, and routine clinical outpatient practice in China. All procedures followed conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
2.2. Study population
Briefly, the study population included adult Chinese patients (both male and female patients aged ≥18 years) with primary hypertension and stroke whose BP was not adequately controlled by monotherapy. Patients were categorized into 3 subgroups based on stroke subtype, namely, hemorrhagic, ischemic, or mixed stroke. All patients were administered Val/Aml (80/5 mg) SPC. Patients on antihypertensive therapy [angiotensin-converting enzyme inhibitors (ACEIs), angiotensin-II receptor blockers (ARBs), CCBs, diuretics, or β-blockers] and those with diabetes or renal insufficiency were included. Patients were prescribed Val/Aml SPC based on the clinical judgment of the investigators according to the patient's condition and taking into consideration the package insert. Signed informed consent was obtained from all patients before study enrollment. Patients were excluded if they had any conditions that precluded administration of the drug based on the investigator's discretion. Women were also excluded if they were pregnant, lactating, or of child-bearing potential and not using adequate contraception measures. Details of inclusion/exclusion criteria, treatment assignment, and outcome measures have been previously described.[19]
2.3. Effectiveness assessments
Changes in MSSBP and MSDBP from baseline to week 4 and week 8 (study endpoint) were assessed. Also, BP control (defined as the proportion of patients achieving MSSBP/MSDBP, <140/90 mm Hg), SBP, and DBP response rates were assessed.
2.4. Safety assessments
Safety assessments included recording and measuring all AEs and vital signs in the population. The incidence of AEs was recorded at weeks 4 and 8 of the study period. Each AE was defined by its duration, severity, and relationship to the study drug.
2.5. Statistical analysis
The full analysis set and safety set for the subset of patients with stroke included patients with at least one postbaseline efficacy and safety evaluation, respectively. The full analysis set was used for all efficacy analyses.
All statistical analyses were performed using SAS Software version 9.2 (SAS Institute, Inc., Cary, NC) at 2-sided significance level (P) of <.05. Demographic and baseline variables were summarized using descriptive statistics, including the mean, standard deviation, median, minimum, and maximum values for numeric variables, and the count number and percentage for categorical variables. Paired t test, 2-way analysis of variance, the Chi-square test, and logistic regression were used to analyze effectiveness end points, including age, height, weight, waistline, and average history of hypertension. The efficacy, tolerability, and medication compliance of Val/Aml SPC evaluated by the investigator and the patient were classified as “very good,” “good,” “general,” and “not good.”
3. Results
A total of 565 hypertensive patients with different types of stroke were analyzed in this China Status II substudy. Detailed demographic and baseline characteristics of these patients are presented in Table 1.
Table 1.
Demographic and baseline characteristics of hypertensive patients with different stroke subtypes.
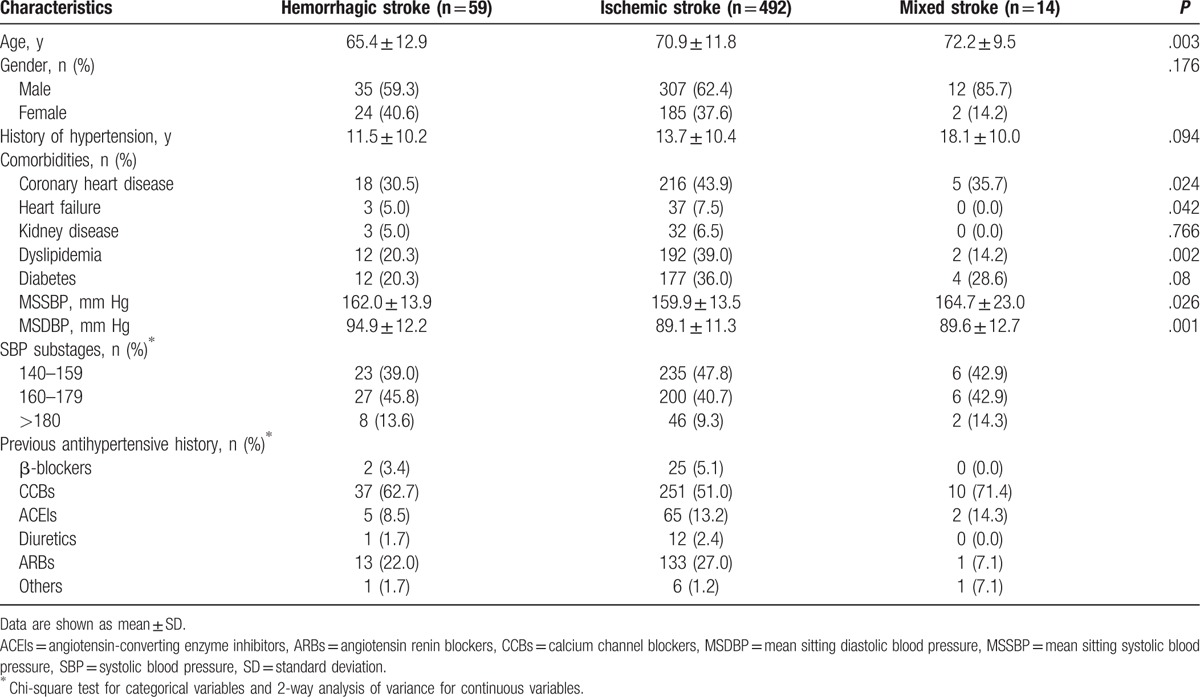
Of 565 patients, 59 (10.4%), 492 (87.1%), and 14 (2.5%) patients belonged to the hemorrhagic, ischemic, and mixed stroke subgroups, respectively. The average age of patients in the hemorrhagic, ischemic, and mixed stroke subgroups was 65.4 ± 13.0, 70.9 ± 11.9, and 72.2 ± 9.6 years, respectively and the proportion of men was 59.3%, 62.4%, and 85.7%, respectively. The average history of hypertension in the hemorrhagic, ischemic, and mixed stroke subgroups was 11.5 ± 10.2, 13.7 ± 10.4, and 18.1 ± 10.1 years, respectively. The mean baseline SBP was 162.0 ± 13.9, 159.9 ± 13.5, and 164.7 ± 23.0 mm Hg in the hemorrhagic, ischemic, and mixed stroke subgroups, respectively.
Most common comorbidities in the 3 stroke subgroups were coronary heart disease (CHD), diabetes, and dyslipidemia. Overall, 42.0%, 37.1%, and 34.5% of patients with hypertension and stroke had comorbid coronary heart disease, dyslipidemia, and diabetes, respectively. Among patients with hemorrhagic stroke, 30.5% and 20.3% of patients each had CHD and diabetes/dyslipidemia, respectively. Prevalence of comorbidities in each stroke subgroup is presented in Table 1.
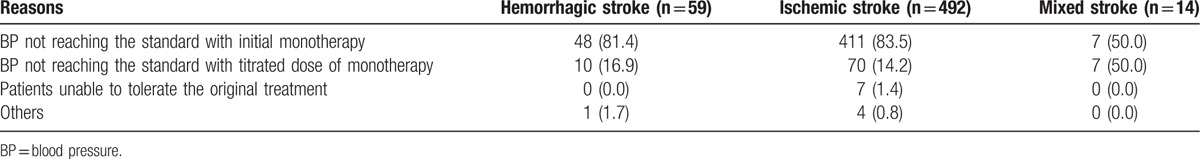
ARBs (52.6%) and CCBs (25.4%) were the most widely used antihypertensive drugs across the 3 stroke subgroups. The primary reason for patients switching to Val/Aml was “BP not reaching the standard with initial monotherapy,” indicated by 81.1% of patients, while 16.7% of patients switched to Val/Aml due to “BP not reaching the standard with titrated dose of monotherapy” (Table 2). Other antihypertensive drugs were added at week 4 in 2 patients (3.4%), 43 patients (8.7%), and 1 patient (7.1%) in the hemorrhagic, ischemic, and mixed stroke subgroups, respectively.
Table 2.
Reasons for switching to valsartan/amlodipine by hypertensive patients with different stroke subtypes.
3.1. Effectiveness
Across all stroke subgroups, Val/Aml SPC resulted in significant (P < .0001) overall MSSBP/MSDBP reductions of 22.5/9.5 and 28.5/12.9 mm Hg from baseline to week 4 and week 8, respectively. In hemorrhagic, ischemic, and mixed stroke subgroups, Val/Aml SPC resulted in significant MSSBP/MSDBP reductions of 29.0/14.8, 27.9/12.6, and 34.7/10.2 mm Hg, by week 8 (Fig. 1).
Figure 1.
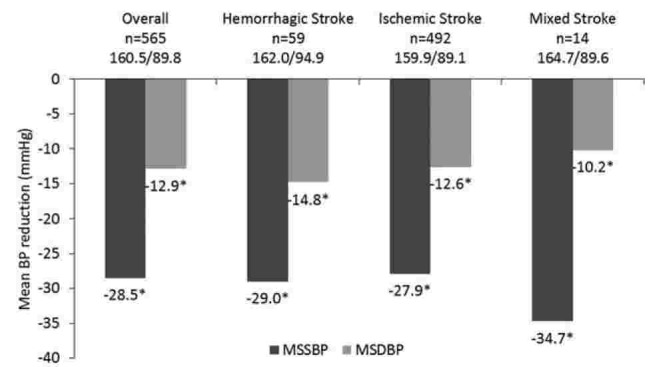
MSSBP and MSDBP reductions in hypertensive patients with different stroke subtypes at week 8. ∗P < .0001 versus baseline. BP = blood pressure, MSDBP = mean sitting diastolic blood pressure, MSSBP = mean sitting systolic blood pressure.
After 4 weeks of Val/Aml SPC treatment, BP control was achieved by 50.1% of patients while after 8 weeks, BP control was attained by 80.2% of patients in the overall population (Fig. 2). BP control rates in each stroke subgroup at week 4 and week 8 are presented in Table 3. At week 4, 47.5% to 78.6% of patients achieved BP control, while the proportion of patients attaining BP control increased at week 8, with a range of 74.6% to 92.9%, across the 3 stroke subgroups.
Figure 2.
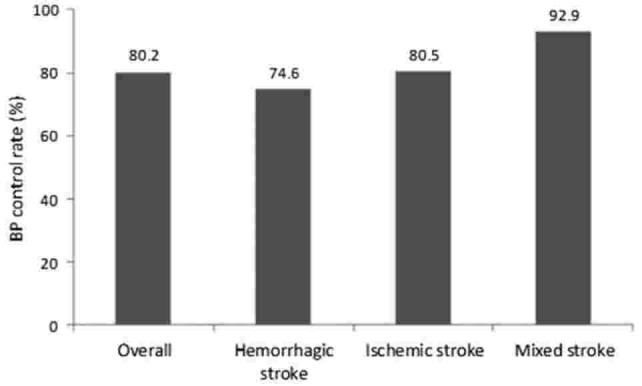
BP control rate (proportion of patients achieving < 140/90 mm Hg) in hypertensive patients with different stroke subtypes at week 8. BP = blood pressure.
Table 3.
Mean blood pressure, blood pressure control, and response rates at week 4 and week 8 in hemorrhagic, ischemic, and mixed stroke subgroups.
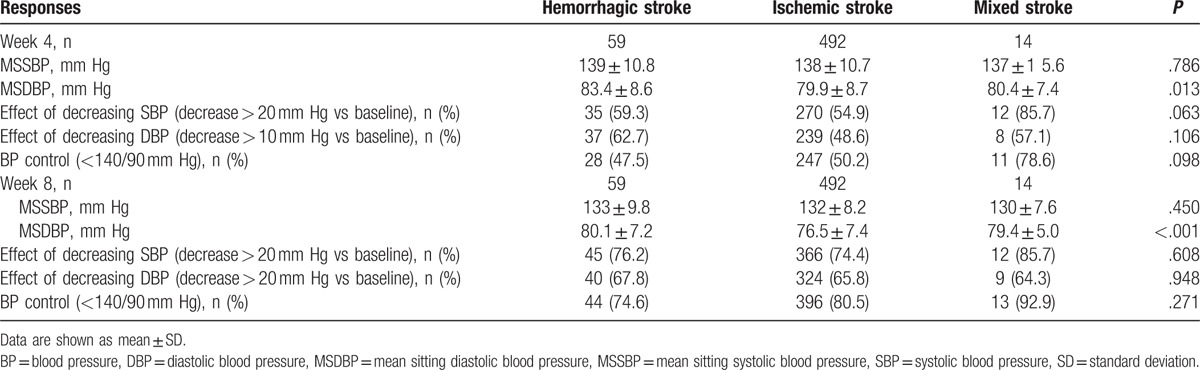
After 4 weeks of Val/Aml SPC treatment, the rate of patients not achieving BP control was less than 9%, with 3.4%, 8.7%, and 7.1% in hemorrhagic, ischemic, and mixed stroke subgroups, respectively. At week 4, SBP response (decreasing by ≥20 mm Hg vs baseline) was achieved by 54.9%, 54.9%, and 85.7% of patients, while DBP response (decreasing by ≥10 mm Hg vs baseline) was achieved by 62.7%, 48.6%, and 57.1% of patients in hemorrhagic, ischemic, and mixed stroke subgroups, respectively (Table 3). After 8 weeks of Val/Aml SPC treatment, SBP response increased to 76.2%, 74.4%, and 85.7% and DBP response increased to 67.8%, 65.8%, and 64.3% of patients in hemorrhagic, ischemic, and mixed stroke subgroups, respectively. After 4 weeks of Val/Aml SPC treatment, SBP and DBP control rates were 56.2% and 50.6%, respectively, and after 8 weeks, the rates were 74.6% and 66.3%, respectively, in the overall population.
3.2. Safety and tolerability
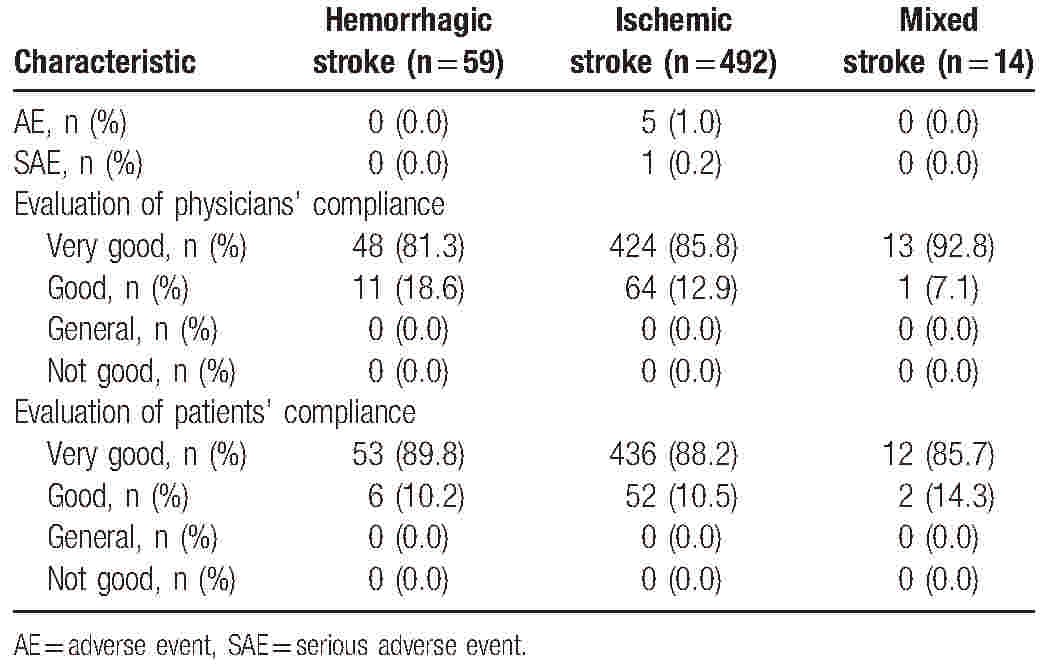
Val/Aml SPC treatment was well tolerated in the 565 patients included in the study. AEs and SAEs were reported in 5 patients (1%) and 1 patient (0.2%), respectively, in the ischemic stroke subgroup, while no AEs were reported in hemorrhagic and mixed stroke subgroups. More than 98.8% patients had higher tolerability. The majority of patients and physicians (at least 80%) across the 3 stroke subgroups rated drug compliance as “very good” (Table 4).
Table 4.
Safety and compliance in hemorrhagic, ischemic, and mixed stroke subgroups.
4. Discussion
China Status II, a multicenter, observational, real-world study, reported the effectiveness and safety of Val/Aml SPC (80/5 mg) in a very large population of Chinese patients with hypertension whose BP was inadequately controlled by monotherapy.[19] The present analysis of patients from the China Status II study stratified based on 3 stroke subtypes, confirmed the BP-lowering effectiveness and safety of Val/Aml 80/5 mg SPC in Chinese hypertensive patients with hemorrhagic, ischemic, or mixed stroke. The majority of patients achieved BP control, SBP response, and DBP response in all stroke subgroups at the study endpoint at week 8. Val/Aml SPC was safe and well-tolerated in hypertensive patients across all stroke subgroups.
Increased BP, specifically SBP > 140 mm Hg, was observed in >60% of patients during the acute phase of a hemorrhagic or an ischemic stroke.[20,21] Such increases in BP during the acute phase of a stroke have been associated with poor short-term and long-term outcomes[20,22,23] and an increased risk of early recurrence.[24] A meta-analysis of 16 randomized controlled trials comparing 95 antihypertensive drugs versus placebo in 70,664 prehypertensive patients with baseline BP ranging from 120 to 139/80 to 89 mm Hg showed that antihypertensive therapy significantly reduced the risk of stroke by 22% compared with placebo.[25] Therefore, lowering BP with antihypertensive drug in patients with hypertension during stroke would improve cardiovascular and cerebrovascular outcomes.
The European Society of Hypertension (ESH) and European Society of Cardiology (ESC) 2013 guidelines recommend antihypertensive treatment in hypertensive patients with a history of stroke or transient ischemic attack, even when initial SBP is within the range from 140 to 159 mm Hg.[13] All drug regimens (ACEI, ARB, CCB, or diuretics as monotherapies or dual/triple combination therapies) are recommended for stroke prevention, provided that BP is effectively reduced. In addition, ESC/ESH 2013 guidelines recommend initiation of combination therapy containing agents with complementary mechanisms of action (e.g., renin-angiotensin-aldosterone system blockers such as ACEIs or ARBs with CCBs and/or diuretics) in patients with markedly high baseline BP or at high cardiovascular risk. Further, SPCs are recommended as they improve compliance in patients with hypertension, usually with low adherence, thereby increasing rates of BP control and ultimately, leading to cardiovascular benefits of BP lowering.[13]
In a meta-analysis of 9 randomized clinical trials comparing antihypertensive therapies in 62,605 patients, CCBs provided more reduction in the risk of stroke compared to other classes.[26] Similar results suggesting that CCBs may have a slightly greater effectiveness on stroke prevention have also been reported by other meta-analyses and meta-regression analyses.[27,28] From experimental studies, the protective effects of CCBs on stroke might be explained by a specific role of intracellular calcium in triggering ischemic cell death. CCBs block the central neuronal calcium influx, thus reducing ischemic injury and necrosis of neurons in the ischemic brain area.[29] ARBs have also been reported to have greater cerebrovascular protective effects versus other drugs in randomized controlled trials and this was further confirmed by a meta-analysis of ∼50,000 patients, where treatment with ARBs was associated with a significant reduction of stroke risk (∼8%) compared with ACEIs.[30,31] ARBs exert their beneficial effects on BP lowering, cardiovascular remodeling, and stroke prevention by selectively blocking the angiotensin I receptors and allowing angiotensin II to stimulate the unoccupied angiotensin 2 receptors. This dual effect of ARBs may explain their superiority over ACEIs in stroke protection.[32]
Previous randomized clinical trials have reported significant BP-lowering effects of the Val/Aml combination therapy in patients with hypertension,[33–35] including Chinese patients with hypertension inadequately controlled by Val or Aml monotherapy.[16,36–39] Further, patients using valsartan-based SPCs are significantly more likely to achieve their BP goal than those treated with ARB-based free combinations in real-world clinical practice.[40] In a recent real-world study in Chinese patients with uncontrolled hypertension and a history of stroke, initial dual combination therapy during the first 6 months reduced stroke incidence to a greater extent than monotherapy. Also, initial therapy and maintenance therapy were mainly CCB-based (alone or in combination), and ARB/CCB combination was the dominant therapy after medication switching in patients taking combination therapy.[41] CCBs and ARBs were the most common antihypertensive drugs in our study population. In a meta-analysis of 8 RCTs in 20,451 hypertensive patients, ACEI/ARB plus CCB combination therapy was superior to other combinations in lowering the incidence of cardiovascular events, including stroke.[42] Based on the above evidence, ARB/CCB combination has the potential to reduce the risk of stroke.
The neuroprotective effect of ARBs and CCBs has been shown in a few studies. In the LIFE study conducted in hypertensive patients with left ventricular hypertrophy, losartan significantly reduced the rate of fatal and nonfatal stroke by 25%.[43] In the SCOPE study in elderly patients, candesartan-based treatment reduced nonfatal stroke by 30% and all stroke by 24% versus placebo.[44] In the ASCOT study, Aml reduced fatal and nonfatal stroke better than atenolol.[45] Nitrendipine-based treatment reduced the incidence of fatal and nonfatal stroke in elderly Chinese patients with isolated systolic hypertension.[46] An analysis of 6 actively controlled studies with Aml-based treatment showed that Aml provided more protection against stroke than other antihypertensive agent.[47] Our results show that Val/Aml SPC resulted in significant BP reductions from baseline and high BP control rates at week 8 across all stroke subtypes in patients with hypertension. The above evidence demonstrating the protective effects of Val- or Aml-based therapies against stroke, coupled with the greater BP-lowering efficacy of Val/Aml combination than either monotherapy in several studies, suggest that this combination might be an effective approach for stroke protection and prevention.
To name a few study limitations, there was no washout period in this study. The addition of treatments at week 4 might have influenced effectiveness of Val/Aml at week 8, and this impact has not been determined. Moreover, lack of a comparable group in the study introduces the potential for selection bias, which precludes generalization of the results to the entire population.
The major study limitation is the nonrandomized, open-label, single-arm design, which does not allow the comparison between treatment arm and control arm. However this study represents actual clinical practice. And it is still possible to evaluate the BP change in real world setting. A patient was prescribed the Val/Aml SPC based on the clinical judgment of the investigators. And then after 4 weeks the additional treatment was adopted when BP was not controlled at 4 weeks. Changes in MSSBP and MSDBP from baseline to week 4 and week 8 were observed. Val/Aml SPC resulted in significant overall MSSBP/MSDBP reductions from baseline to week 4 and week 8, respectively.
5. Conclusion
The present findings of the China Status II study post hoc analysis confirmed the effectiveness of Val/Aml (80/5 mg) SPC in achieving BP control and its tolerability in Chinese hypertensive patients with hemorrhagic, ischemic, or mixed stroke, in whom BP was inadequately controlled by antihypertensive monotherapy.
Acknowledgments
Writing support was provided by Parvathy Ramakrishnan from Novartis Healthcare Private Limited, India. The study was sponsored by Novartis.
Footnotes
Abbreviations: ACEI = angiotensin-converting enzyme inhibitor, ARB = angiotensin receptor blocker, BP = blood pressure, CCB = calcium channel blocker, DALYs = disability-adjusted life years, ESC = European Society of Cardiology, ESH = European Society of Hypertension, MSDBP = mean sitting diastolic blood pressure, MSSBP = mean sitting systolic blood pressure, SD = standard deviation, SPC = single-pill combination, Val/Aml = valsartan/amlodipine.
The authors have no conflicts of interest to disclose.
WZ and JX are employees of Neurology Department, Xinjiang General Hospital of PLA, Urumqi, China.
References
- [1].Lopez AD, Mathers CD, Ezzati M, et al. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 2006;367:1747–57. [DOI] [PubMed] [Google Scholar]
- [2].Strong K, Mathers C, Bonita R. Preventing stroke: saving lives around the world. Lancet Neurol 2007;6:182–7. [DOI] [PubMed] [Google Scholar]
- [3].Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2197–223. [DOI] [PubMed] [Google Scholar]
- [5].Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 2015;131:e29–322. [DOI] [PubMed] [Google Scholar]
- [6].Liu M, Wu B, Wang WZ, et al. Stroke in China: epidemiology, prevention, and management strategies. Lancet Neurol 2007;6:456–64. [DOI] [PubMed] [Google Scholar]
- [7].Rao M. Prevention and Control Guidelines of Cerebrovascular Diseases in China. China: Neurology Branch of Chinese Medical Association; 2005. [Google Scholar]
- [8].Kim AS, Cahill E, Cheng NT. Global stroke belt: geographic variation in stroke burden worldwide. Stroke 2015;46:3564–70. [DOI] [PubMed] [Google Scholar]
- [9].Zhang H, Thijs L, Staessen JA. Blood pressure lowering for primary and secondary prevention of stroke. Hypertension 2006;48:187–95. [DOI] [PubMed] [Google Scholar]
- [10].He J, Klag MJ, Wu Z, et al. Stroke in the People's Republic of China. II. Meta-analysis of hypertension and risk of stroke. Stroke 1995;26:2228–32. [PubMed] [Google Scholar]
- [11].O’Donnell MJ, Xavier D, Liu L, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet 2010;376:112–23. [DOI] [PubMed] [Google Scholar]
- [12].Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:2160–236. [DOI] [PubMed] [Google Scholar]
- [13].Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013;31:1281–357. [DOI] [PubMed] [Google Scholar]
- [14].Neurology cerebrovascular disease study group of the Chinese Medical Association. 2014 Guidelines for secondary prevention of stroke in patients with ischemic stroke or transient ischemic attack. Chin J Neurol 2015;48:258–73. [Google Scholar]
- [15].Angiotensin E receptor antagonist stroke prevention and control group. Chin J Intern Med 2008;47:258–61. [Google Scholar]
- [16].Ke YN, Huang J, Zhu JR, et al. [Efficacy and safety of the single pill combination of valsartan 80 mg plus amlodipine 5 mg in mild to moderate essential hypertensive patients without adequate blood pressure control by monotherapy]. Zhonghua Xin Xue Guan Bing Za Zhi 2009;37:794–9. [PubMed] [Google Scholar]
- [17].Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet 2004;363:2022–31. [DOI] [PubMed] [Google Scholar]
- [18].Weber MA, Julius S, Kjeldsen SE, et al. Blood pressure dependent and independent effects of antihypertensive treatment on clinical events in the VALUE Trial. Lancet 2004;363:2049–51. [DOI] [PubMed] [Google Scholar]
- [19].Hu D, Liu L, Li W. Efficacy and safety of valsartan/amlodipine single-pill combination in 11,422 Chinese patients with hypertension: an observational study. Adv Ther 2014;31:762–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Qureshi AI, Ezzeddine MA, Nasar A, et al. Prevalence of elevated blood pressure in 563,704 adult patients with stroke presenting to the ED in the United States. Am J Emerg Med 2007;25:32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Qureshi AI. Acute hypertensive response in patients with stroke: pathophysiology and management. Circulation 2008;118:176–87. [DOI] [PubMed] [Google Scholar]
- [22].Willmot M, Leonardi-Bee J, Bath PM. High blood pressure in acute stroke and subsequent outcome: a systematic review. Hypertension 2004;43:18–24. [DOI] [PubMed] [Google Scholar]
- [23].Leonardi-Bee J, Bath PM, Phillips SJ, et al. Blood pressure and clinical outcomes in the International Stroke Trial. Stroke 2002;33:1315–20. [DOI] [PubMed] [Google Scholar]
- [24].Bogousslavsky J, Victor SJ, Salinas EO, et al. Fiblast (trafermin) in acute stroke: results of the European-Australian phase II/III safety and efficacy trial. Cerebrovasc Dis 2002;14:239–51. [DOI] [PubMed] [Google Scholar]
- [25].Sipahi I, Swaminathan A, Natesan V, et al. Effect of antihypertensive therapy on incident stroke in cohorts with prehypertensive blood pressure levels: a meta-analysis of randomized controlled trials. Stroke 2012;43:432–40. [DOI] [PubMed] [Google Scholar]
- [26].Staessen JA, Wang JG, Thijs L. Cardiovascular protection and blood pressure reduction: a meta-analysis. Lancet 2001;358:1305–15. [DOI] [PubMed] [Google Scholar]
- [27].Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009;338:b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Turnbull F. Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet 2003;362: 1527–1535. [DOI] [PubMed] [Google Scholar]
- [29].Angeli F, Verdecchia P, Reboldi GP, et al. Calcium channel blockade to prevent stroke in hypertension: a meta-analysis of 13 studies with 103,793 subjects. Am J Hypertens 2004;17:817–22. [DOI] [PubMed] [Google Scholar]
- [30].Schrader J, Luders S, Kulschewski A, et al. Morbidity and mortality after stroke, eprosartan compared with nitrendipine for secondary prevention: principal results of a prospective randomized controlled study (MOSES). Stroke 2005;36:1218–26. [DOI] [PubMed] [Google Scholar]
- [31].Reboldi G, Angeli F, Cavallini C, et al. Comparison between angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on the risk of myocardial infarction, stroke and death: a meta-analysis. J Hypertens 2008;26:1282–9. [DOI] [PubMed] [Google Scholar]
- [32].Chrysant SG. Possible pathophysiologic mechanisms supporting the superior stroke protection of angiotensin receptor blockers compared to angiotensin-converting enzyme inhibitors: clinical and experimental evidence. J Hum Hypertens 2005;19:923–31. [DOI] [PubMed] [Google Scholar]
- [33].Destro M, Luckow A, Samson M, et al. Efficacy and safety of amlodipine/valsartan compared with amlodipine monotherapy in patients with stage 2 hypertension: a randomized, double-blind, multicenter study: the EX-EFFeCTS Study. J Am Soc Hypertens 2008;2:294–302. [DOI] [PubMed] [Google Scholar]
- [34].Smith TR, Glazer RD, Koren MJ, et al. Combination therapy with amlodipine/valsartan in essential hypertension: a 52-week, randomised, open-label, extension study. Int J Clin Pract 2010;64:1367–74. [DOI] [PubMed] [Google Scholar]
- [35].Flack JM, Calhoun DA, Satlin L, et al. Efficacy and safety of initial combination therapy with amlodipine/valsartan compared with amlodipine monotherapy in black patients with stage 2 hypertension: the EX-STAND study. J Hum Hypertens 2009;23:479–89. [DOI] [PubMed] [Google Scholar]
- [36].Ke Y, Zhu D, Hong H, et al. Efficacy and safety of a single-pill combination of amlodipine/valsartan in Asian hypertensive patients inadequately controlled with amlodipine monotherapy. Curr Med Res Opin 2010;26:1705–13. [DOI] [PubMed] [Google Scholar]
- [37].Huang J, Sun NL, Hao YM, et al. Efficacy and tolerability of a single-pill combination of amlodipine/valsartan in Asian hypertensive patients not adequately controlled with valsartan monotherapy. Clin Exp Hypertens 2011;33:179–86. [DOI] [PubMed] [Google Scholar]
- [38].Zhu D, Yang K, Sun N, et al. Amlodipine/valsartan 5/160 mg versus valsartan 160 mg in Chinese hypertensives. Int J Cardiol 2013;167:2024–30. [DOI] [PubMed] [Google Scholar]
- [39].Wang JG, Zeng WF, He YS, et al. Valsartan/amlodipine compared to nifedipine GITS in patients with hypertension inadequately controlled by monotherapy. Adv Ther 2013;30:771–83. [DOI] [PubMed] [Google Scholar]
- [40].Chang J, Yang W, Fellers T, et al. Chart review of patients on valsartan-based single-pill combinations vs. ARB-based free combinations for BP goal achievement. Curr Med Res Opin 2010;26:2203–12. [DOI] [PubMed] [Google Scholar]
- [41].Yu JM, Kong QY, Shen T, et al. Benefit of initial dual-therapy on stroke prevention in Chinese hypertensive patients: a real world cohort study. J Thorac Dis 2015;7:881–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chi C, Tai C, Bai B, et al. Angiotensin system blockade combined with calcium channel blockers is superior to other combinations in cardiovascular protection with similar blood pressure reduction: a meta-analysis in 20,451 hypertensive patients. J Clin Hypertens (Greenwich) 2016;18:801–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Dahlof B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002;359:995–1003. [DOI] [PubMed] [Google Scholar]
- [44].Lithell H, Hansson L, Skoog I, et al. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens 2003;21:875–86. [DOI] [PubMed] [Google Scholar]
- [45].Dahlof B, Sever PS, Poulter NR, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet 2005;366:895–906. [DOI] [PubMed] [Google Scholar]
- [46].Wang JG, Staessen JA, Gong L, et al. Chinese trial on isolated systolic hypertension in the elderly. Systolic Hypertension in China (Syst-China) Collaborative Group. Arch Intern Med 2000;160:211–20. [DOI] [PubMed] [Google Scholar]
- [47].Wang JG, Li Y, Franklin SS, et al. Prevention of stroke and myocardial infarction by amlodipine and Angiotensin receptor blockers: a quantitative overview. Hypertension 2007;50:181–8. [DOI] [PubMed] [Google Scholar]


