Abstract
Background:
Anti-PD-1 monoclonal antibodies, nivolumab and pembrolizumab, and anti-CTLA-4 antibody ipilimumab are being in clinic trials to treat melanoma. Here, we performed a meta-analysis to evaluate the efficacy and toxicity of them against advanced melanoma.
Methods:
Eleven reports from 6 randomized control trials on treating metastatic melanoma, which were divided into 3 subgroups, nivolumab/pembrolizumab versus chemotherapy, nivolumab versus ipilimumab, and nivolumab-plus-ipilimumab versus ipilimumab, were included and the meta-analysis was performed for each subgroup. The outcome measures were objective response rates (ORR), median progression free survival (PFS), 1-year overall survival rates (OS), and toxicity estimated by grade 3 to 4 adverse events.
Results:
For nivolumab/pembrolizumab versus chemotherapy, nivolumab versus ipilimumab, and nivolumab-plus-ipilimumab versus ipilimumab, the pooled risk ratios (RR) of the ORR were 3.43 (95% CI: 2.57–4.58), 2.51 (95% CI: 2.03–3.09), and 3.28 (95% CI: 2.58–4.17), respectively. The pooled HR of PFS were 0.42 (95% CI: 0.36–0.49), 0.58 (95% CI: 0.50–0.66), and 0.41 (95% CI: 0.30–0.52), respectively. The pooled RR of 1-year OS was 1.37 (95% CI: 1.08–1.74) and 1.54 (95% CI: 0.90–2.63) for nivolumab versus ipilimumab and nivolumab-plus-ipilimumab versus ipilimumab. These results suggested that anti-PD-1 monotherapy and nivolumab-plus-ipilimumab therapy had ORR and PFS benefit compared with the control group. Anti-PD-1 treatment increased 1-year OS for patients compared with ipililumab treatment. But there is no significantly difference on 1-year OS between the nivolumab-plus-ipilimumab treatment and the ipilimumab treatment group. The toxicity analysis showed that there is less risk of adverse events in the anti-PD-1 treatment group compared with the chemotherapy and ipilimumab group. Combining nivolumab with ipilimumab increased the risk for high-grade adverse events compared with ipilimumab alone but the adverse events were generally manageable.
Conclusions:
Anti-PD-1 monotherapy and nivolumab-plus-ipilimumab therapy improved ORR and prolonged PFS of patients with advanced melanoma and the adverse events are generally manageable. The therapy is indeed a promising approach for treatment of advanced melanoma.
Keywords: anti-CTLA-4, anti-PD-1, immunotherapy, ipilimumab, melanoma, nivolumab, pembrolizumab
1. Introduction
Metastatic cutaneous melanoma is a potentially fetal form of skin cancer. The incidence of melanoma has been rising over decades among many countries. According to WHO's data, 200,000 malignant melanomas occur global each year and more than 65,000 are dead.[1,2] Five-year survival rate of malignant melanoma at the early stage (stage 0/I) is more than 90% after surgical excision. However, advanced melanoma can invade lymph nodes and other organs.[3] As an aggressive disease, the malignant melanoma at metastatic stage (stage III or IV) has only a 16% 5-year survival rate and responds poorly to most standard chemotherapies.[4]
The immune responses, especially the cell-mediated immunity, play an important role to recognize and delete tumor cells. However, cancer cells can escape from the immune system by some regulatory mechanisms such as upregulating immune inhibitors of immune checkpoints in T lymphocytes. Immune checkpoints are normal immune signals which can stop an immune response. By using immune checkpoints to block the function of effect cells, most tumors can escape immunity. Blocking the inhibitory receptor–ligand interaction of the immune checkpoints, antitumor immunity may be restored.[5] Monoclonal antibodies are capable of disrupting the ligand–receptor association for immune checkpoints and/or its functional consequences were developed.
The most effective immune checkpoint blocker developed in recent years is antibodies against programmed cell death (PD)-1 and its ligands. PD-1 is expressed in activated T-lymphocytes and T-cells with chronic stimulations. PD-1 ligands (PD-L1) are expressed in various immune cells including activated T-lymphocytes, B-lymphocytes, macrophages, and dendritic cells and in nonlymphoid cells or tissues. Accumulated studies showed that high expression of PD-L1 in tumors was correlated with poor prognosis in various malignant tumors.[6–9] With the development of immunotherapy, blocking the interaction between the PD-1 and PD-L1 by antibodies has been reported to have impressive antitumor effects.
Nivolumab and pembrolizumab are the members of PD-1 inhibitors approved by FDA for the treatment of advanced melanoma. Nivolumab has been approved by FDA in December 2014 to treat unresectable or metastatic melanoma with no response to other drugs. Pembrolizumab, formerly named lambrolizumab or MK-3475, has been approved by FDA in September 2014 for unresectable or metastatic melanoma patients and the progressed disease of treated after ipilimumab or a BRAF inhibitor.[10,11] As a coinhibitory molecule of immune system,[12] ipilimumab blocking cytotoxic T-lymphocyte antigen-4 (CTLA-4) is approved by FDA as an orphan drug for treating advanced melanoma.[13,14] PD-1 and CTLA-4 restrain T-cell activation. Combination blockade of PD-1 and CTLA-4 was shown to be synergistic in preclinical melanoma mouse models and some clinical phase1trials of the efficacy.[10,15]
There are some clinical trials that included phase 1[16–18] and randomized phase 2 and phase 3 trials[19–29] regarding the use of anti-PD-1, nivolumab, and pembrolizumab for the treatment of advanced melanoma. There were several publications[30–35] of systematic review and meta-analysis on anti-PD-1 immunotherapy in advanced melanoma until February 2017. However, previous systematic review and meta-analysis on advanced melanoma dated back to year 2015. Several new researches on randomized controlled trials (RCTs) were published in 2016 and provided new data. In addition, some of previous systematic reviews and meta-analysis mainly included phase 1 trials and it may result in a reduction in the reliability of the result. We collected 11 publications data from 6 RCTs of anti-PD-1 in the treatment of advanced melanoma including not only nivolumab/pembrolizumab monotherapy but also the combination of nivolumab with ipilimumab. Among the 6 RCTs included, 4 were randomized phase 3 trials and 2 were randomized phase 2 trials, which make the result more reliable. We performed a meta-analysis of all available data regarding the efficacy and safety of them.
2. Methods
2.1. Literature search
The databases of PubMed, EMBASE, Cochrane Library of Controlled Trials, and Web of Science were searched by using the terms as follows: “nivolumab,” “MDX-1106,” “ONO-4538,” “BMS-936558,”“Opdivo,”“pembrolizumab,”“lambrolizumab,”“keytruda,”“MK-3475,”“anti-PD-1,”“melanoma,”“randomized controlled trial,” “controlled clinical trial,” “control trial,” “double-blind,” and “clinical trial” were combined using “or” or “and” for searching for relevant studies. Only studies in English were considered. The search included literature published from 1990 to February 2017. The computer search was supplemented with manual searches for references of the included studies and for related citations. To select studies for inclusion in the analysis, we first reviewed article titles and abstracts, and then obtained full text to verify eligibility.
2.2. Studies selection
We included all randomized controlled trials that compared nivolumab or pembrolizumab to chemotherapy or ipilimumab in adult patients with advanced cutaneous melanoma.
2.3. Outcome measures
The primary outcomes were objective response rate (ORR)—the percentage of patients achieving a complete response or a partial response defined by RECIST criteria (Response Evaluation Criteria in Solid Tumors), median progression-free survival (PFS), 1-year overall survival rate (OS), and the toxicity estimated by adverse events defined as grade. The severity of adverse events was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
2.4. Data extraction
Two reviewers (CH and JT) extracted data independently using a predefined data extraction form. Disagreements were resolved by discussion with a third reviewer (BZ) . The data extracted included the first author, study characteristics, participant characteristics, anti-PD-1 of the experimental, and the control group treatments and measured outcomes.
2.5. Quality assessment
Trials fulfilling the review inclusion criteria were assessed for methodological quality by 2 reviewers. Assessment of risk bias was performed using the criteria described in the Cochrane Reviewer's handbook.[36]
2.6. Statistical methods
We did all statistical analyses with StataSE12.0 software (StataCorp, TX) . For meta-analysis, odds ratio (OR) or risk ratios (RR) were used to compare dichotomous variables. All the results were reported with 95% confidence intervals (CI). Pooled RR or OR and 95% confidence intervals for dichotomous data were estimated using the Mantel–Haenszel method. I-square (I2) test was performed to assess the impact of study heterogeneity on the results of meta-analysis. According to the Cochrane review guidelines, if severe heterogeneity was present at I2 > 50%, the random effect model was chosen; otherwise, the fixed effect model was used. Subgroup analyses were performed according to the intervention of study design: anti-PD-1 versus chemotherapy, anti-PD-1 versus ipilimumab and nivolumab-plus-ipilimumab versus ipilimumab.
2.7. Ethics
All the analyses were based on previous published studies, thus ethical approval is not necessary for systematic review and meta-analysis.
3. Results
A total of 11 reports [19–29] from 6 randomized control trials were included in this review. The search initially provided a total of 1256 publications. After excluding duplicates, 1021 reports remained. Of these, 959 studies were discarded because a review of their titles and abstracts made it clear that they did not meet the criteria. By full-text assessing, 51 reports were further excluded. Details about selection of studies are outlined in the flow diagram in Figure 1.
Figure 1.
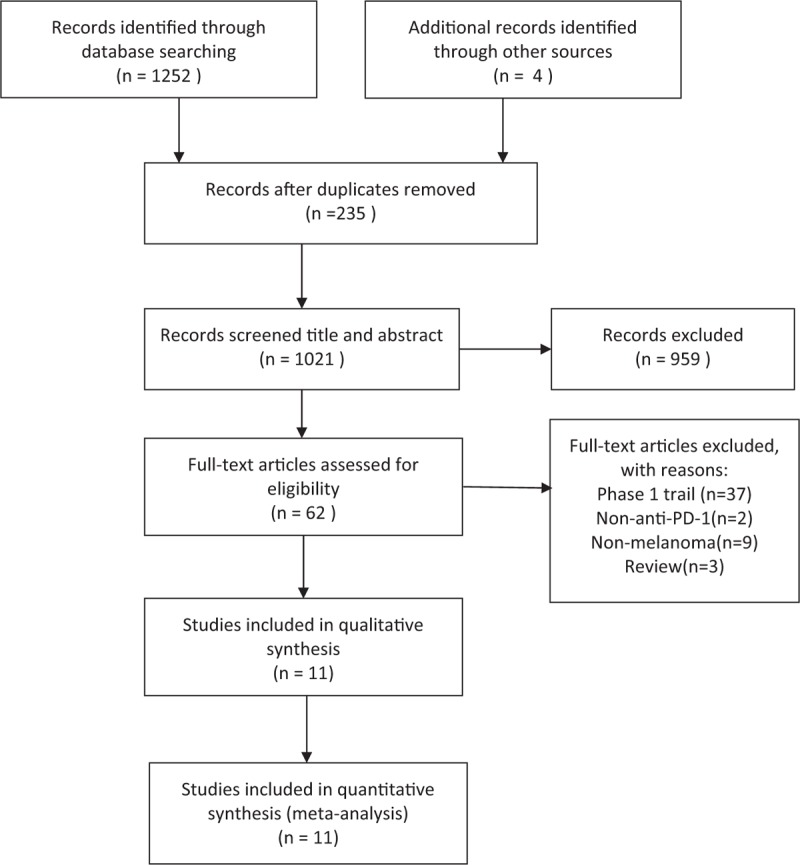
The flow diagram of the studies selection.
The quality of included studies was assessed according to the RCT quality evaluation standards of the Cochrane Reviewer handbook. Randomized sequence generation, allocation concealment, blinding of participants and personnel, incomplete outcome data, selective reporting, and other source of bias were included. If there was information for all the parameters or no information at all, then the study was assigned as low bias or high bias, respectively. If the information was partial or unclear, the risk of bias was defined as unclear (Table 1).
Table 1.
The methodological quality of included trials.
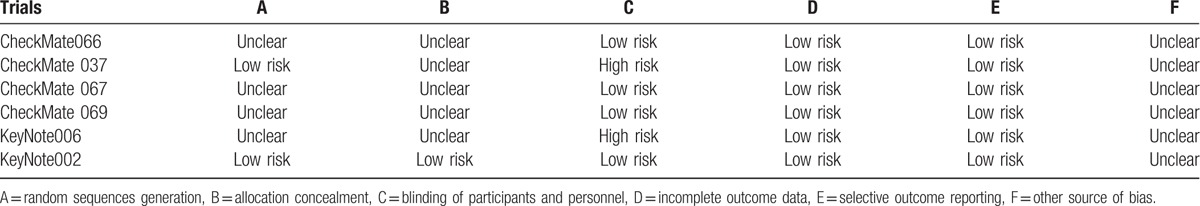
In all included 6 randomized control trials, 4 were randomized phase 3 trials and 2 were randomized phase 2 trials. The primary endpoint was PFS and OS in 4 trials and ORR in 2 trials. There were 3284 patients in this assessment. The characteristics of the included studies are presented in Table 2.
Table 2.
Characteristics of included studies in the meta-analysis.
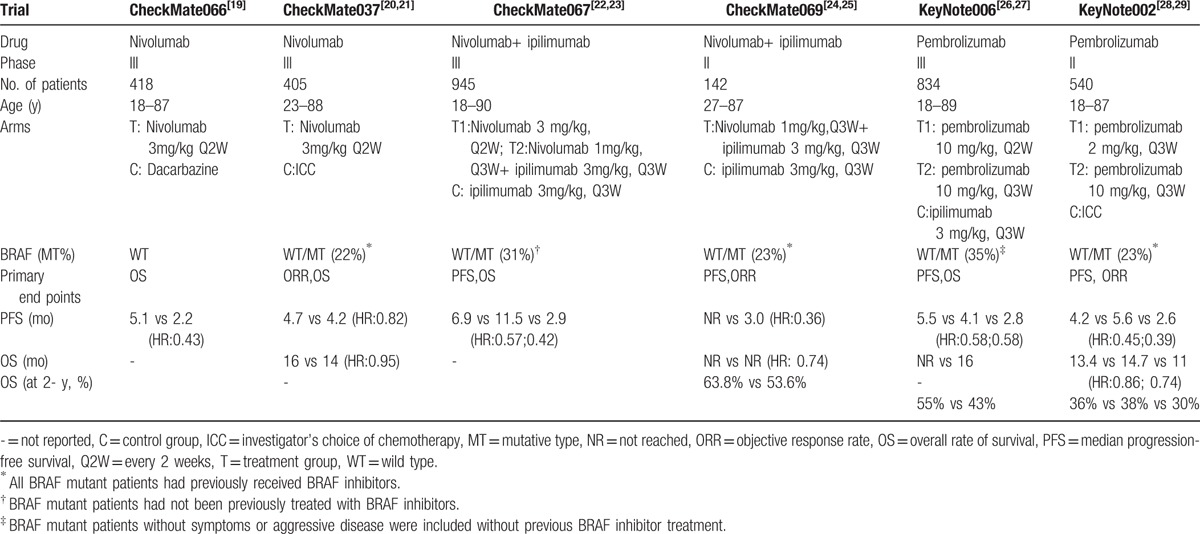
3.1. Efficacy
For efficacy, the primary outcomes were ORR, median PFS and 1-year OS. The ORR was reported by all studies included. According to the intervention of study design, subgroup analyses were performed. There is no severe heterogeneity among the studies and fixed effect model was used. The median PFS of these trials is presented in Table 2 and the hazard ratio (HR) for death or disease progression of anti-PD-1 or nivolumab-plus-ipiliumab treatment group versus control group was pooled. One-year OS was reported by some of trials.
3.1.1. Anti-PD-1 (nivolumab or pembrolizumab) versus chemotherapy
In this subgroup, the pooled RR of ORR was 3.43 (95% CI: 2.57–4.58, I2 = 11%, P = .325; Fig. 2A). The result showed that anti-PD-1 monoclonal antibody intervention is benefit for the patients compared with the chemotherapy group. In the anti-PD-1 intervention group, a significantly longer PFS was observed (Table 2). The pooled HR for the comparison between the anti-PD-1 group and the chemotherapy group was 0.42 (95% CI: 0.36–0.49, I2 = 0, P = .384; Fig. 3A). The result indicated that anti-PD-1 intervention can significantly prolong the median PFS for advanced melanoma patients. Only 1 trail reported 1-year OS in this subgroup, so there was no data pooled about OS.
Figure 2.
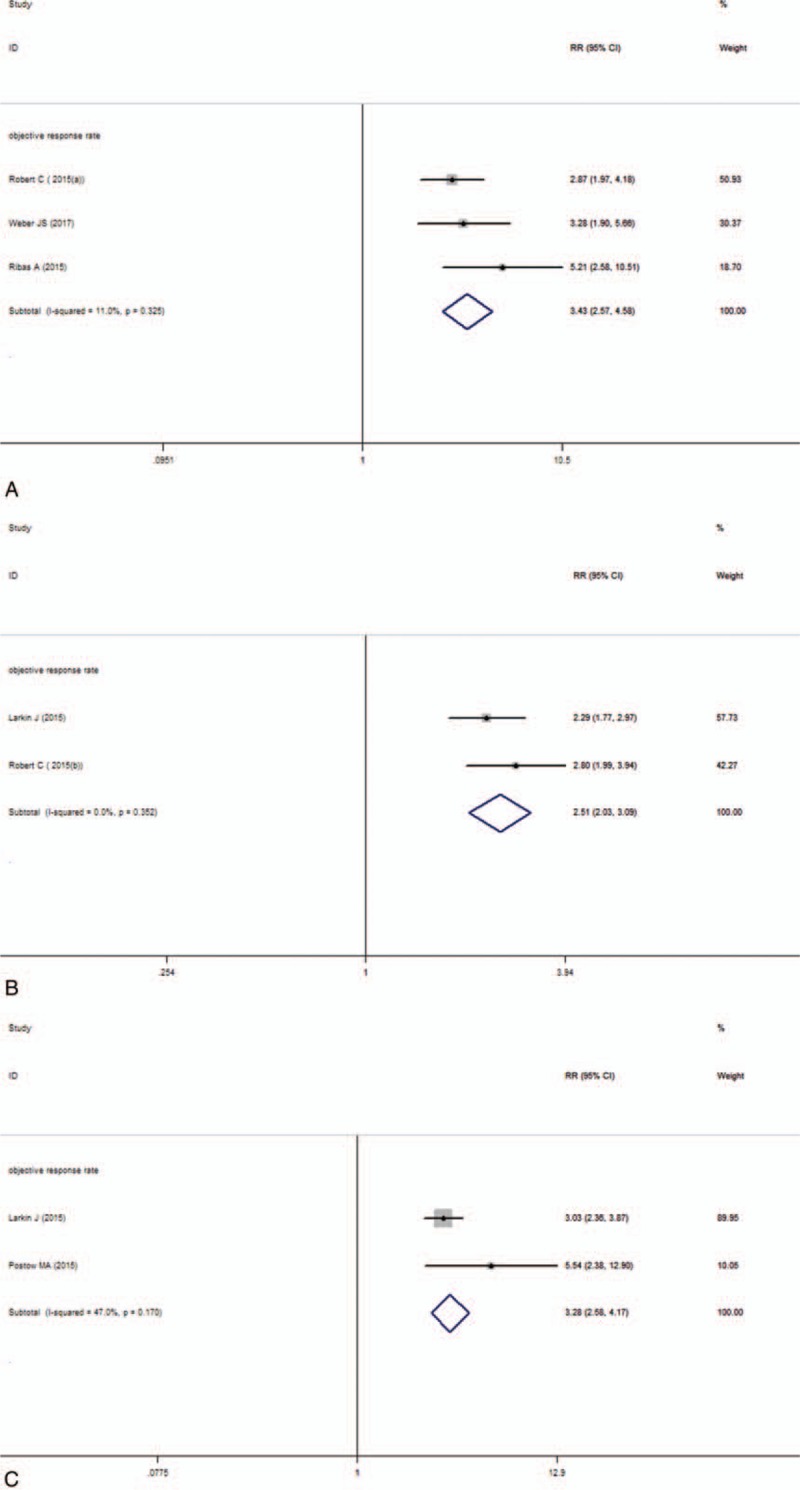
Forest plot of the objective response analysis. A, Anti-PD-1 versus chemotherapy. B, Anti-PD-1 versus ipilimumab. C, Nivolumab-plus-ipilimumab versus ipilimumab.
Figure 3.
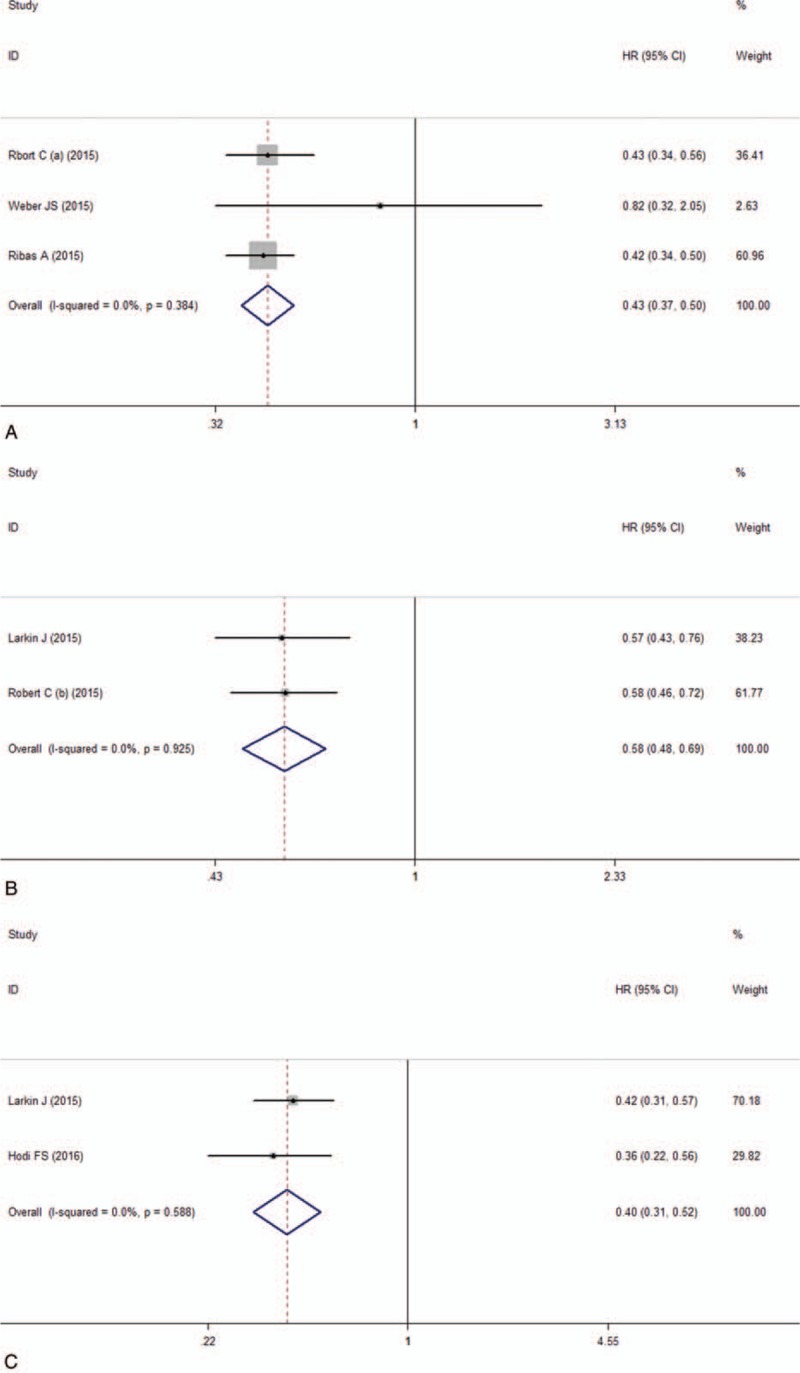
Forest plot of the hazard ratio of the median PFS analysis. A, Anti-PD-1 versus chemotherapy. B, Anti-PD-1 versus ipilimumab. C, Nivolumab-plus-ipilimumab versus ipilimumab.
3.1.2. Anti-PD-1 (nivolumab or pembrolizumab) versus anti-CTLA-4 (ipilimumab)
Between the anti-PD-1 and the ipilimumab group, the pooled RR of ORR was 2.51 (95% CI: 2.03–3.09, I2 = 0, P = .352; Fig. 2B). It suggested that patients were more likely to respond to anti-PD-1 treatment than ipilimumab and anti-PD-1 can improve the objective response rate. For the median PFS, the pooled HR was 0.58 (95% CI: 0.50–0.66, I2 = 0, P = .925; Fig. 3B). The result showed that the patients treated with anti-PD-1 can significantly improve the PFS than the ipilimumab group. For 1-year OS, the pooled RR was 1.37 (95% CI: 1.08–1.74, I2 = 85.8%, P = .008; Fig. 4A) and suggested that anti-PD-1 treatment improved the 1-year OS than ipilimumab treatment. There is heterogeneity between these 2 studies and the random effect model was used.
Figure 4.
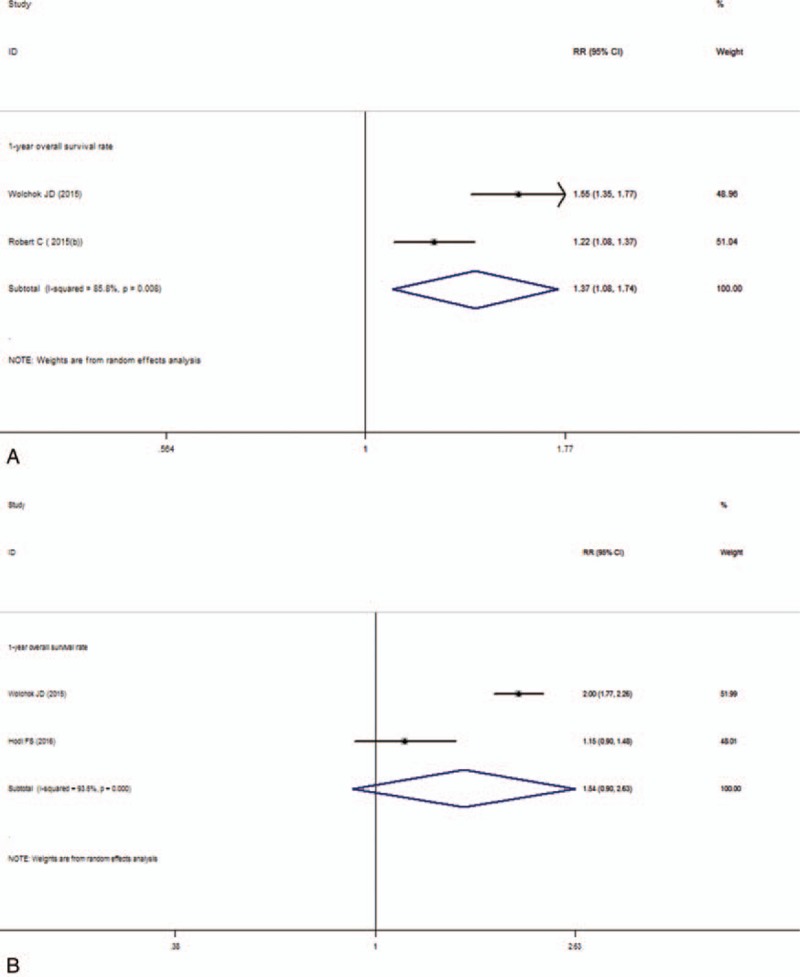
Forest plot of the overall survival rate analysis. A, Anti-PD-1 versus ipilimumab. B, Nivolumab-plus-ipilimumab versus ipilimumab.
3.1.3. Nivolumab-plus-ipilimumab versus ipilimumab
Compared nivolumab-plus-ipilimumab treatment with ipilimumab, the pooled RR of ORR was 3.28 (95% CI: 2.58–4.17, I2 = 47%, P = .170; Fig. 2C). The result suggested that nivolumab-plus-ipilimumab treatment had a clear significant advantage over the ipilimumab monotherapy. The median PFS analysis of this subgroup showed that the pooled HR was 0.41 (95% CI: 0.30–0.52, I2 = 0, P = .588; Fig. 3C). Similarly, the result also showed significantly advantage for the nivolumab-plus-ipilimumab group than the ipilimumab group and nivolumab-plus-ipilimumab can improve the PFS than the ipilimumab alone. The pooled RR of 1-year OS was 1.54 (95% CI: 0.90–2.63, I2 = 93.5%, P = .000; Fig. 4B) and there is heterogeneity between these 2 studies and the random effect model was used. The result indicated that there is no statistically difference between nivolumab-plus-ipilimumab treatment and ipilimumab treatment.
3.2. Adverse events
3.2.1. Anti-PD-1 (nivolumab or pembrolizumab) versus chemotherapy
In this subgroup, the OR of the overall incidence of the treatment-related adverse events and the discontinuation treatment adverse events were 0.38 (95% CI: 0.22–0.67, I2 = 69.5%, P = .038) and 0.70 (95% CI: 0.32–1.54, I2 = 49.7%, P = .137), respectively (Fig. 5A). There is slightly heterogeneity among the studies and random effect model was used. The analysis showed that the risk for the overall incidence of adverse events of grade 3 to 4 of anti-PD-1 treatment compared with chemotherapy was decreased. The most frequent adverse events were fatigue, diarrhea, and vomiting in the anti-PD-1 group and fatigue, anaemia, and vomiting in the chemotherapy group. According to the organ category in the subgroups, there is no statistically significant difference on the incidence of most adverse events between the anti-PD-1 group and the chemotherapy group except nausea and fatigue. The incidence of nausea and fatigue was decreased in the anti-PD-1 group than in the chemotherapy group. The pooled OR of the adverse events analyzed according to organ category in this subgroup is presented in Figure 6A.
Figure 5.

Forest plot of the overall incidence of the treatment-related adverse events and the discontinuation treatment adverse events analysis. A, Anti-PD-1 versus chemotherapy. B, Anti-PD-1 versus ipilimumab. C, Nivolumab-plus-ipilimumab versus ipilimumab.
Figure 6.
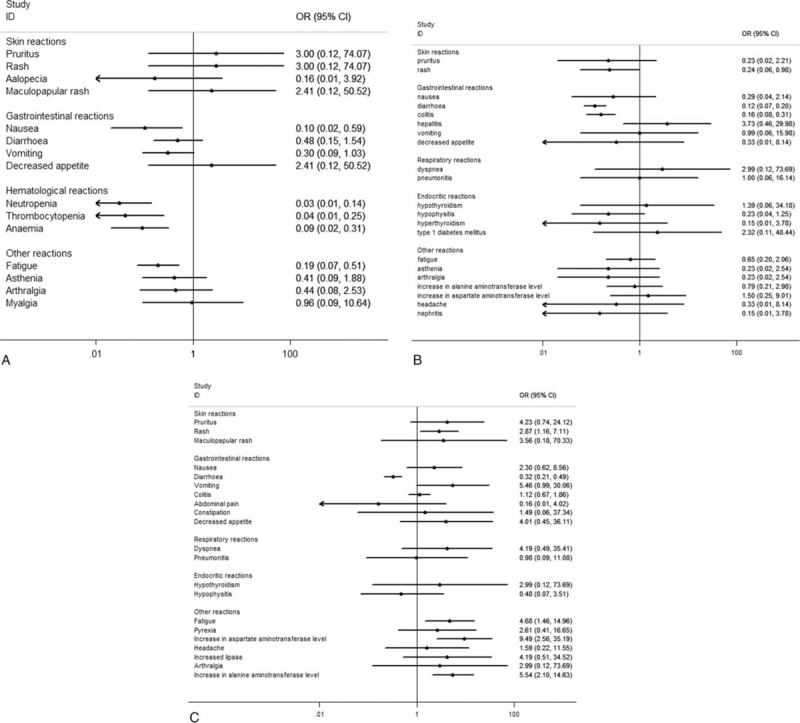
Forest plot of the select incidence of the adverse events according to the organ category. A, Anti-PD-1 versus chemotherapy. B, Anti-PD-1 versus ipilimumab. C, Nivolumab-plus-ipilimumab versus ipilimumab.
3.2.2. Anti-PD-1 (nivolumab or pembrolizumab) versus ipilimumab
In the subgroup of anti-PD-1 versus ipilimumab, the OR of the treatment-related adverse events and the discontinuation treatment adverse events were 0.52 (95% CI: 0.40–0.69, I2 = 0, P = .917) and 0.41 (95% CI: 0.28–0.61, I2 = 48.5%, P = .164), respectively (Fig. 5B). These results showed that the risk for the overall incidence of adverse events of grade 3 to 4 was decreased in the anti-PD-1 group compared with the ipilimumab group. The most frequent treatment-related adverse events of grade 3to 4 were diarrhea, colitis, and hepatic toxicity (elevated alanine aminotransferase and elevated aspartate aminotransferase) in the anti-PD-1 group, the same as the ipilimumab group. According to the pooled OR of the adverse events analysis, there is statistically significant decrease of the incidence of diarrhea and colitis in the anti-PD-1 group than in the ipilimumab group. As for the incidence of other adverse events there was no difference between the anti-PD-1 and the ipilimumab group (Fig. 6B).
3.2.3. Nivolumab-plus-ipilimumab versus ipilimumab
In this subgroup, the pooled OR of the treatment-related adverse events and the discontinuation treatment adverse events were 3.32 (95% CI: 2.44–4.52, I2 = 0, P = .729) and 2.94 (95% CI: 2.00–4.31, I2 = 0, P = .422), respectively (Fig. 5C). These results indicated an increased risk for high-grade adverse events for nivolumab-plus-ipilimumab group compared with the ipilimumab monotherapy group. The most frequent treatment-related adverse events of grade 3 to 4 were colitis, diarrhea, and hepatic toxicity (elevated alanine aminotransferase and elevated aspartate aminotransferase) in the nivolumab-plus-ipilimumab group and diarrhea, colitis, and nausea in the ipilimumab group. According to the pooled OR of the adverse events analysis, the incidence of diarrhea was decreased but rash, fatigue, and hepatic toxicity increased in the nivolumab-plus-ipilimumab group than in the ipilimumab group (Fig. 6C).
4. Discussion
The efficacy of nivolumab/pembrolizumab and nivolumab-plus-ipilimumab in the treatment of advanced melanoma were estimated in this meta-analysis. The pooled RR of ORR and HR of PFS showed that nivolumab/pembrolizumab and nivolumab-plus-ipilimumab treatment had respectively improved objective response rate and prolonged PFS compared with chemotherapy and ipililumab treatment alone. The pooled RR of 1-year OS showed that nivolumab/pembrolizumab treatment could improve 1-year OS compared with ipililumab treatment. But between nivolumab-plus-ipilimumab treatment and ipilimumab treatment, there is no statistically difference on 1-year OS.
The novel immunotherapy agents targeting specific immune regulatory checkpoints have improved the potential of cancer immunotherapy to obtain long-lasting antitumor responses in patients with different cancers. As a high selectivity for immune suppressive inhibitory T-cell receptor, using antibodies to block the PD-1 would be expected to have higher antitumor activity with lower adverse effects compared with CTLA-4 blockade because of greater specificity for tumor antigen-specific T cells and less effects on autoreactive T cells.[37]
Our analysis compared nivolumab/pembrolizumab treatment with chemotherapy or ipilimumab as control group respectively and revealed that there is a significantly higher ORR in the anti-PD-1 treatment group in advanced melanoma patients. In our study, we also compared nivolumab-plus-ipilimumab treatment with ipilimumab monotherapy. The similar result, a significantly higher ORR, was also observed in the nivolumab-plus-ipilimumab group than the ipilimumab group. These results are highly consistent with the results of many previous individual studies. In addition, anti-PD-1 monotherapy and nivolumab-plus-ipilimumab treatment resulted in a significantly improvement of the median PFS compared with chemotherapy or ipilimumab treatment in patients with metastatic melanoma. These results indicated that anti-PD-1, nivolumab, and pembrolizumab, can significantly increase ORR and PFS compared with chemotherapy or ipilimumab treatment. Compared with ipilimumab monotherapy, nivolumab-plus-ipilimumab treatment also increased ORR and PFS. These results indicated that the prospect of anti-PD-1 immunotherapy is promising. The pooled RR of 1-year OS showed that anti-PD-1 treatment can increase 1-year OS for patients compared with ipililumab treatment. But there is no statistically difference between nivolumab-plus-ipilimumab treatment and ipilimumab treatment. From the point of view of the results mentioned above, anti-PD-1 immunotherapy should be a promising treatment option for metastatic melanoma patients regardless of who had not previously received treatment or progressed after anti-CTLA-4 or BRAF inhibitor treatment.
Treatment benefits and risks are equally important to patients, and the efficacy and safety of a drug are equally important in clinical trials. In this meta-analysis, grade 3 to 4 adverse effects were analyzed to evaluate the safety. The RCTs included in this meta-analysis reported that anti-PD-1 treatment resulted in fewer treatment-related adverse events than the chemotherapy or the ipilimumab treatment group. Our pooled analysis showed that the risk of grade 3 to 4 adverse events decreased in anti-PD-1 treatment compared with chemotherapy or ipilimumab treatment. However, in the subgroup of nivolumab-plus-ipilimumab versus ipilimumab, the risk of overall incidence of treatment-related adverse events of grade 3 to 4 was higher in the combination group than in the ipilimumab monotherapy group. A randomized, phase 2 trial[38] assessed the safety and efficacy of sequential administration of nivolumab followed by ipilimumab or the reverse sequence and showed that nivolumab followed by ipilimumab improved efficacy outcomes but increased overall frequency of adverse events compared with the reverse sequence. These results suggest that combination therapy of nivolumab and ipilimumab appears to be a clinical beneficial option and the sequential administration should be considered. Even though the risk of overall incidence increased, these adverse events were generally manageable with established treatment guidelines according to the RCTs[22,24] included in this subgroup. The results suggested that combination therapy of nivolumab and ipilimumab has a manageable safety profile. For patients who received the combination of nivolumab-plus-ipilimumab regimen, it is also observed that the onset of most adverse events occurred during the combination phase rather than the maintenance phase (nivolumab monotherapy).[24] It indicated that the combination of nivolumab-plus-ipilimumab increased the incidence of serious adverse events. The most common adverse events observed with nivolumab-plus-ipilimumab therapy are fatigue, rash, and hepatic toxicity. Therefore, when standardizing an anti–PD-1 combined with anti-CTLA-4 therapy regimen for metastatic melanoma, additional combinations of drugs to prevent or treat these adverse events should be considered. In addition, because of the cost of expensive, the cost-utility analysis of the combination of nivolumab-plus-ipilimumab therapy should be addressed.
Some limitations in our meta-analysis should be mentioned. First, our results were based on unadjusted analysis, more accurate outcomes would result from adjustments for other confounders such as gender, age, BRAF mutation status, PD-L1 status, prior systemic therapy, and so on. Second, the small number of included trials for each subgroup make the outcomes more prone to be influenced by a potential publication bias. Because of the limited number of studies for each subgroup analysis, we could not confidently assess publication bias or conduct a sensitivity analysis. Third, language of studies was limited to English, which may result in missing data from studies published in other languages. Finally, randomized controlled trials should have follow-up for longer time to appraise the long-term toxicity of anti-PD-1 and anti-PD-1 combined with anti-CTLA-4.
5. Conclusions
Our meta-analysis suggests that anti-PD-1 monotherapy and nivolumab-plus-ipilimumab combination therapy would be a promising approach for the treatment of advanced melanoma, regardless of the patients of untreated or after anti-CTLA-4 treatment, with significant improvement in ORR and PFS and fewer adverse events relative to chemotherapy or ipilimumab treatments. Anti-PD-1 monotherapy could improve 1-year OS than ipilimumab. The nivolumab-plus-ipilimumab treatment could significantly improve ORR and PFS and increase adverse events, which could be managed, but did not increase 1-year OS significantly compared with ipilimumab monotherapy treatment. Because our analysis is based on a small number of included trials for each subgroup, the inherent limitations of included studies prevent us from reaching definitive conclusions. Future large-volume, well-designed RCTs with extensive follow-up are awaited to confirm and update the findings of this analysis.
Footnotes
Abbreviations: CI = 95% confidence intervals, CTLA-4 = cytotoxic T-lymphocyte antigen-4, HR = hazard ratio, OR = odds ratio, ORR= objective response rate, OS= overall survival, PD-1 = programmed cell death 1, PFS= median progression-free survival, RCTs= randomized controlled trials, RR= risk ratios.
This work was supported by National Natural Science Foundation of China (No. 81560426) and the program of Gansu Provincial Key Laboratory of Evidence Based Medicine and Clinical Translation (20150105).
The authors have no conflicts of interest to disclose.
References
- [1].Aris M, Barrio MM. Combining immunotherapy with oncogene-targeted therapy: a new road for melanoma treatment. Front Immunol 2015;6:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Faghfuri E, Faramarzi MA, Nikfar S, et al. Nivolumab and pembrolizumab as immune-modulating monoclonal antibodies targeting the PD-1 receptor to treat melanoma. Expert Rev Anticancer Ther 2015;15:981–93. [DOI] [PubMed] [Google Scholar]
- [3].Mashima E, Inoue A, Sakuragi Y, et al. Nivolumab in the treatment of malignant melanoma: review of the literature. Onco Targets Ther 2015;8:2045–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mahoney KM, Freeman GJ, McDermott DF. The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in melanoma clinical therapeutics. Clin Ther 2015;37:764–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Carosella ED, Ploussard G, LeMaoult J, et al. A systemic review of immunotherapy in urologic cancer: evolving roles for targeting of CTLA-4, PD-1/P D-L1, and HLA-G. Eur Urol 2015;68:267–79. [DOI] [PubMed] [Google Scholar]
- [6].Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infitrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA 2007;104:3360–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nakanishi J, Wada Y, Matsumoto K, et al. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol Immunother 2007;56:1173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hino R, Kabashima K, Kato Y, et al. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer 2010;116:1757–66. [DOI] [PubMed] [Google Scholar]
- [9].Massi D, Brusa D, Merelli B, et al. PD-L1 marks a subset of melanomas with a shorter overall survival and distinct genetic and morphological characteristics. Ann Oncol 2014;25:2433–42. [DOI] [PubMed] [Google Scholar]
- [10].Barbee MS, Ogunniyi A, Horvat TZ, et al. Current status and future directions of the immune checkpoint inhibitors ipilimumab, pembrolizumab, and nivolumab in oncology. Ann Pharmacother 2015;49:907–37. [DOI] [PubMed] [Google Scholar]
- [11].Niezgoda A, Niezgoda P, Czajkowski R. Novel approaches to treatment of advanced melanoma: a review on targeted therapy and immunotherapy. Biomed Res Int 2015;2015:851387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pardoll DM. The blockade of immunecheck points in cancer immunotherapy. Nat Rev Cancer 2012;12:252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517–26. [DOI] [PubMed] [Google Scholar]
- [15].Curran MA, Montalvo W, Yagita H, et al. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA 2010;107:4275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014;32:1020–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013;369:134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320–30. [DOI] [PubMed] [Google Scholar]
- [20].Weber JS, D Angelo S, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015;16:375–84. [DOI] [PubMed] [Google Scholar]
- [21].Weber J, Minor D, D Angelo S, et al. Overall survival in patients with advanced melanoma (MEL) who received nivolumab (NIVO) versus investigators choice chemotherapy (ICC) in the phase 3 CheckMate 037 trial. Pigment Cell Melanoma Res 2017;30:150. [Google Scholar]
- [22].Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. CheckMate 067: a phase III randomized double-blind study of nivolumab (NIVO) monotherapy or NIVO combined with ipilimumab (IPI) versus IPI monotherapy in previously untreated patients (pts) with advanced melanoma (MEL). Ann Oncol 2015;26:vi28. [Google Scholar]
- [24].Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015;372:2006–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hodi FS, Chesney J, Pavlick AC, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol 2016;17:1558–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015;372:2521–32. [DOI] [PubMed] [Google Scholar]
- [27].Long G, McNeill C, Schachter J, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival analysis of KEYNOTE-006. Asia-Pacific J Clin Oncol 2016;12:61. [Google Scholar]
- [28].Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015;16:908–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hamid O, Puzanov I, Dummer R, et al. Final overall survival for KEYNOTE-002: Pembrolizumab (pembro) versus investigator-choice chemotherapy (chemo) for ipilimumab (ipi)-refractory melanoma. Ann Oncol 2016;27(suppl 6):1107O.26940689 [Google Scholar]
- [30].Chen R, Peng PC, Wen B, et al. Anti-Programmed cell death (PD)-1 immunotherapy for malignant tumor: a systematic review and meta-analysis. Transl Oncol 2016;9:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yun S, Vincelette ND, Green MR, et al. Targeting immune checkpoints in unresectable metastatic cutaneous melanoma: a systematic review and meta-analysis of anti-CTLA-4 and anti-PD-1 agents trials. Cancer Med 2016;5:1481–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Jin C, Zhang X, Zhao K, et al. The efficacy and safety of nivolumab in the treatment of advanced melanoma: a meta-analysis of clinical trials. Onco Targets Ther 2016;9:1571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lin Z, Chen X, Li Z, et al. PD-1 antibody monotherapy for malignant melanoma: a systematic review and meta-analysis. PLoS One 2016;11:e0160485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Guan X, Wang H, Ma F, et al. The efficacy and safety of programmed cell death 1 and programmed cell death 1 ligand inhibitors for advanced melanoma: a meta-analysis of clinical trials following the PRISMA guidelines. Medicine 2016;95:e3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Tie Y, Ma X, Zhu C, et al. Safety and efficacy of nivolumab in the treatment of cancers: a meta-analysis of 27 prospective clinical trials. Int J Cancer 2017;140:948–58. [DOI] [PubMed] [Google Scholar]
- [36].Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0. The Cochrane Collaboration. Updated March 2011. [Google Scholar]
- [37].Homet Moreno B, Parisi G, Robert L, et al. Anti-PD-1 therapy in melanoma. Semin Oncol 2015;42:466–73. [DOI] [PubMed] [Google Scholar]
- [38].Weber JF, Gibney G, Sullivan RJ, et al. Sequential administration of nivolumab and ipilimumab with a planned switch in patients with advanced melanoma (CheckMate 064): an open-label, randomised, phase 2 trial. Lancet Oncol 2016;17:943–55. [DOI] [PMC free article] [PubMed] [Google Scholar]


