Abstract
To establish a risk scoring system for predicting locoregional recurrence (LRR) and explore the potential value of radiotherapy in T1 to T2 node-negative breast cancer patients treated with mastectomy. From January 2001 to February 2008, a total of 353 node-negative T1 to T2 breast cancer cases treated with mastectomy without adjuvant radiotherapy were retrospectively analyzed. Preliminary screening of the prognostic factors was accomplished by Kaplan–Meier univariate analysis, and survival curves between different groups were compared by log-rank test. Risk factors were determined using Cox proportional hazards model. A categorical risk scoring system was generated according to the Cox model, weighing the relative importance of each risk variable. Median follow-up was 115.7 months (range, 1.2–238.4 months). The overall 5-year locoregional recurrence-free survival (LRFS) was 89.8% (95% confidence interval [CI] = 86.7%–92.9%). Chest wall (53.8%) was found to be the most common site of LRR, followed by supraclavicular nodes (48.7%). Age ≤40 years, primary tumor size ≥4.5 cm and number of nodes resected ≤10 were found to be independent factors for poor prognosis of LRR. Two risk stratifications based on the scoring system were subsequently obtained. The 5-year LRFS was 91.6% (95% CI = 88.5%–94.7%) with low risk (score <2) and 75.7% (95% CI = 61.8%–89.6%) with high risk (score ≥2), respectively (χ2 = 7.544, P = .006). In addition, significant differences in overall survival (P = .045) and disease-free survival (P = .019) were presented between them. Patients with T1-2N0M0 breast cancer achieved favorable prognosis in general. Those with risk factors, including age ≤40 years, primary tumor size ≥4.5 cm and number of nodes resected ≤10, were at higher risk of LRR. The established scoring system could help to distinguish the subgroups that might potentially benefit from postoperative radiotherapy.
Keywords: breast cancer, locoregional recurrence, prognostic factors, scoring system
1. Introduction
Early breast cancer patients usually achieve a favorable prognosis. Yet, a large proportion fail in the modern treatment system. Several studies have revealed that nearly 20% of locoregional recurrence (LRR) rates occurred in T1-2N0 patients with variable risk factors undergoing mastectomy, as compared to the 5.2% incidence described in the entire T1-2N0 population.[1–3] Thus, recurrence of early stage breast cancer remains a research hotspot.
At present, consensus statements reached by different guidelines recommend postmastectomy radiation therapy (PMRT) for patients with T3 to T4 tumors, ≥4 lymph nodes positive (LN+), 1 to 3 LN+ with risk factors and pectoral muscle or surgical margins invasion. For pT1-2N0 breast cancer patients, PMRT is typically spared unless mastectomy margins are proved positive. Nevertheless, several investigators have suggested that PMRT should be considered if patients with T1-2N0 disease have risk factors (such as tumor size ≥2 cm, close margins, premenopausal status, lymphovascular invasion, triple-negative, etc.),[2,4,5] of which risk was comparable to or even higher than that with 1 to 3 positive nodes. Therefore, identifying the high-risk subgroup from pT1-2N0 patients that may potentially benefit from postoperative radiotherapy is a common goal of several researchers.
This retrospective analysis is aimed at establishing a risk scoring system for early breast cancer patients treated with mastectomy through assessment of each independent risk factor. The distinction between different risk stratifications based on the scoring system might provide further evidence for individualized therapy.
2. Methods and materials
2.1. Patients
From January 2001 to February 2008, a total of 353 lymph-node-negative (LN−) invasive breast cancer patients undergoing either radical mastectomy or modified radical mastectomy without adjuvant radiotherapy at our institute were identified through the breast cancer database. Of these patients, 161 had T1 disease and 192 had T2 disease according to the seventh edition of the American Joint Committee on Cancer criteria. Patients with neoplastic spread elsewhere in the body at the first detection of breast cancer were excluded. Our study group had neither neoadjuvant therapy nor history of other primary malignancies.
Variables used for analysis, including age, pathological tumor size, menopausal status, primary tumor location, nipple involvement, number of nodes removed, systemic therapy, pathological characteristics such as histological type, lymphovascular invasion, estrogen receptor (ER), progesterone receptor (PR), human epidermalgrowth factor receptor-2 (HER-2), and Ki-67 status, were obtained from electronic medical records. Expressions of ER and PR in primary breast cancer were ascertained by immunohistochemistry (IHC). ER or PR negative was defined as <1% immunoreactive cells, in accordance with recent guidelines. HER-2 positive was defined as IHC (++ to +++), based on the relevant literature.[6] This study was approved by the Ethics Committee of Fujian Cancer Hospital (YKT2016-021-01), in accordance with the Helsinki Declaration.
2.2. Treatments
A total of 226 cases received adjuvant chemotherapy regimens (anthracycline or taxane) ≤4 cycles, and 127 cases >4 cycles. Median cycle was 3 (range, 0–7). Patients with ER or PR+ took at least 5-year adjuvant endocrine therapy with tamoxifen or aromatase inhibitors unless contraindicated or intolerant.
2.3. Follow-up
Follow-up data including the local and (or) regional recurrence, distant metastasis and survival of the patients were recorded from medical records and telephone interviews from the date of diagnosis to February 2015 or the date of death. After exclusion of 2 patients who were lost to follow-up, a total of 353 patients with complete data were evaluated.
2.4. Statistical analysis
The observation endpoints included overall survival (OS) (defined as the survival time from surgery to death from any cause), locoregional recurrence-free survival (LRFS) (defined as the time from surgery to pathologically confirmed LRR), and disease-free survival (DFS) (defined as the time from surgery to recurrence, distant metastasis, or death). LRR occurring simultaneously with distant metastasis was considered as an LRR event, but patients who had distant metastasis before LRR were excluded.
The prognostic factors were screened by Kaplan–Meier univariate analysis, and the survival curves were compared using log-rank test. In particular, the cutoff point of tumor size affecting survival was determined by Cutoff Finder application.[7] Total risk scores were initially derived using Cox proportional hazards model according to beta regression coefficient, after dividing T1-2N0 breast cancer cases into different groups (score, 0–4). All statistical analyses were performed by IBM SPSS version 19.0, Chicago, IL. Any difference was considered to be statistically significant if P was <.05.
3. Results
3.1. Survival and relapse features
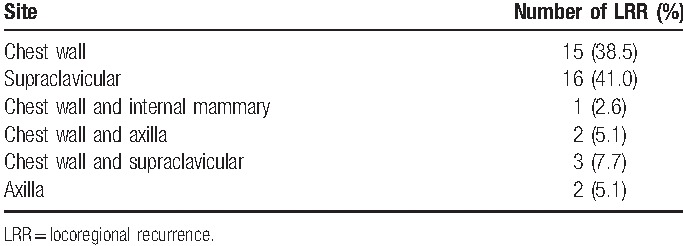
Median follow-up was 115.7 months (range, 1.2–238.4 months). A total of 49 patients died during the follow-up period, of which 4 died of causes unrelated to tumors and 45 died of breast cancer. The disease progressed in 74 cases, of which 35 developed distant metastasis. LRR was observed in 39 patients, of which 21 had isolated LRR and 18 had both LRR and distant metastasis. Chest wall was found to be the most common site of LRR (21/39, 53.8%), followed by supraclavicular nodes (19/39, 48.7%). Overall prevalence and location of LRR are outlined in Table 1.
Table 1.
Prevalence and location of locoregional recurrence over the entire follow-up period.
3.2. Effect of local recurrence on survival
The 5-year LRFS, DFS, and OS for entire cohort were 89.8% (95% confidence intervals [CI] = 86.7%–92.9%), 81.0% (95% CI = 76.9%–85.1%), and 90.0% (95% CI = 86.9%–93.1%), respectively. The 5-year OS of patients with LRR was 61.3% (95% CI = 44.3%–74.5%) versus 93.5% (95% CI = 90.8%–96.2%) without LRR, with statistically significant difference (χ2 = 93.384, P = .000) (Fig. 1).
Figure 1.
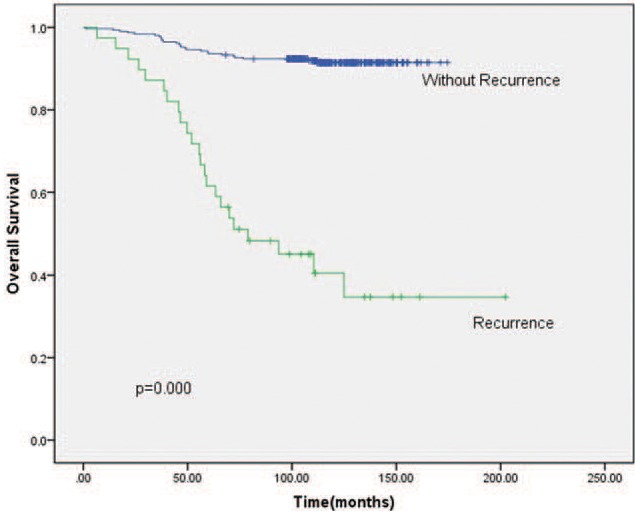
Kaplan–Meier OS (overall survival) of patients with or without recurrence. The 5-year OS of patients with LRR (locoregional recurrence) versus without LRR showed statistically significant difference.
3.3. Effect of tumor size on survival
The results of cutoff point determination for primary tumor size indicated that 4.5 cm was the optimal point, which was supported by multiple methods of Cutoff Finder. The details are shown in Figures 2 and 3. The 5-year LRFS (65.0% vs 91.3%), DFS (57.9% vs 82.4%), and OS (72.8% vs 91.0%) were significantly different between ≥4.5-cm group and <4.5-cm group (P = .000, .016, and .019, respectively), as seen in Figure 4.
Figure 2.
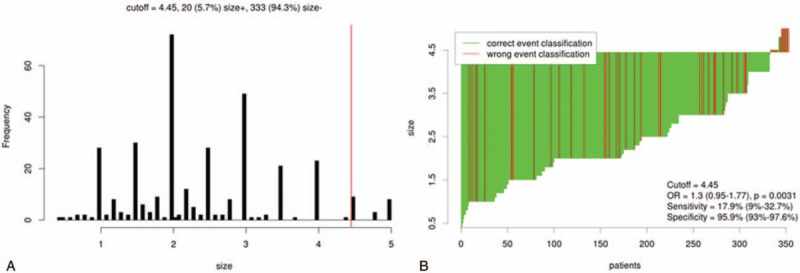
Distribution based cutoff optimization (independent of outcome and survival data) in the T1-2N0M0 breast cancer data. (A) Histograms of primary tumor size in 353 node-negative T1 to T2 breast cancer cases. Vertical line (red line) designated the optimal cutoffs derived from the survival-based model. (B) Waterfall Plot of the optimal dichotomization. The classification using tumor size status and the optimal cutoff was compared to the event of locoregional recurrence result.
Figure 3.
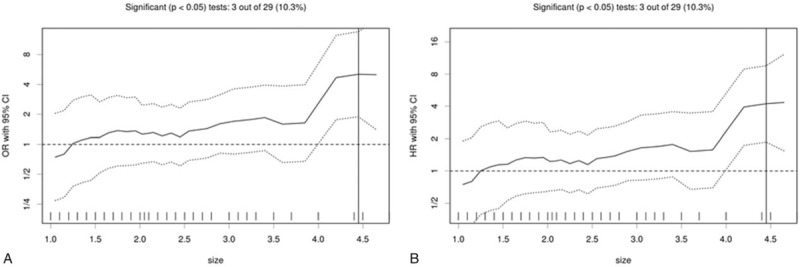
Cutoff optimization by correlation with survival in the T1-2N0M0 breast cancer data. (A) The odds ratio including 95% confidence interval (CI) is plotted in dependence of the cutoff. A vertical line designates the dichotomization showing the most significant correlation with locoregional recurrence (LRR). (B) The hazard ratio including 95% CI is plotted in dependence of the cutoff. A vertical line designates the dichotomization showing the most significant correlation with LRR. The distribution of tumor size in the 353 tumors is shown as a rug plot at the bottom of the figures.
Figure 4.
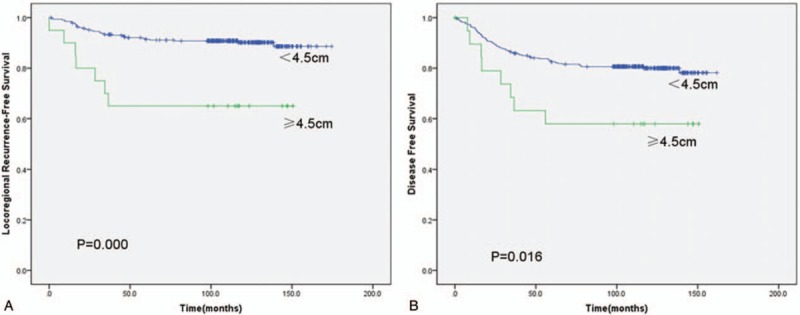
(A) Kaplan–Meier locoregional recurrence-free survival of patients with tumor size <4.5 versus ≥4.5 cm. (B) Kaplan–Meier disease-free survival of patients with tumor size <4.5 versus ≥ 4.5 cm.
3.4. Univariate analysis
In univariate statistical analysis (Table 2), age ≤40 years (P = .023), primary tumor size ≥4.5 cm (P = .000), and number of nodes resected ≤10 (P = .012) were found to be prognostic factors of LRFS in patients with early breast cancer after radical mastectomy. Chemotherapy, tumor size, and number of nodes resected were prognostic factors of DFS (P = .020, .016, and .000, respectively). Tumor size, number of nodes resected, and molecular subtypes were prognostic factors of OS (P = .019, .001, and .017, respectively). However, menstrual status, lymphovascular invasion, primary tumor location, pathology, history of benign breast disease, ER status, chemotherapy cycle, expression of HER-2, endocrine therapy, and nipple involvement had no effect on 5-year LRFS, DFS, and OS in the whole group (P > .05). The effects of age and number of nodes resected on survival are shown in Figures 5 and 6.
Table 2.
5-Year locoregional recurrence-free survival, disease-free survival, overall survival with regard to patient, tumor, and treatment characteristics.
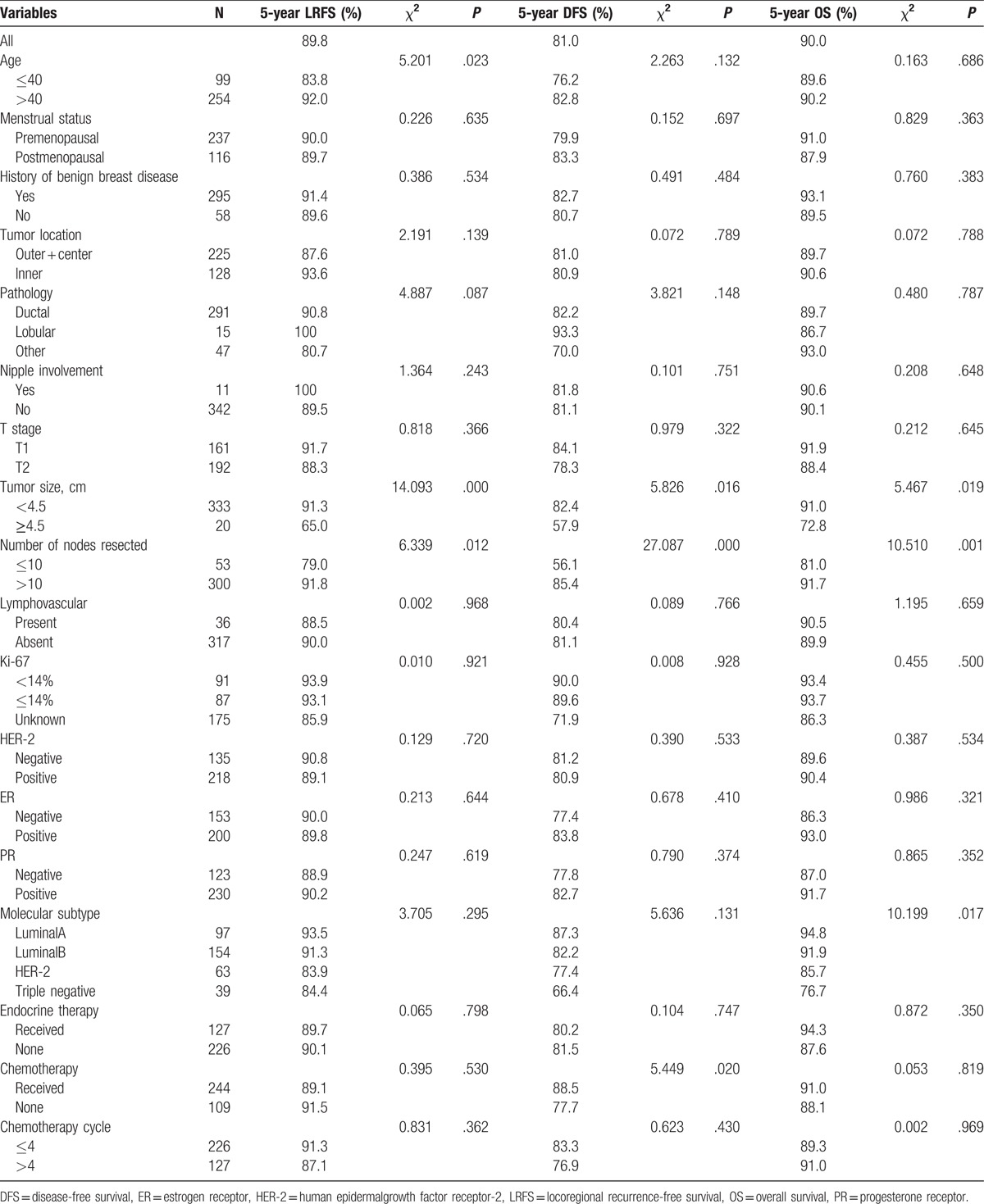
Figure 5.
(A) Kaplan–Meier locoregional recurrence-free survival of patients with age >40 versus ≤40 years. (B) Kaplan–Meier disease-free survival of patients with age >40 versus ≤ 40 years.
Figure 6.
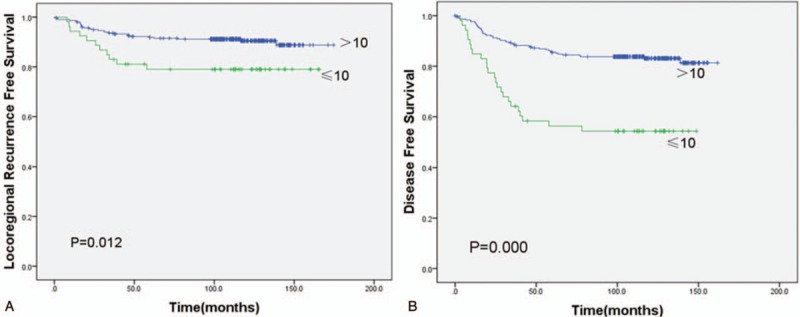
(A) Kaplan–Meier locoregional recurrence-free survival of patients with number of nodes resected >10 versus ≤10. (B) Kaplan–Meier disease-free survival of patients with number of nodes resected >10 versus ≤10.
3.5. Multivariate analysis with Cox proportional hazards model
By incorporating the LRFS-relevant factors into Cox proportional hazards model and using the likelihood ratio test, we found that age ≤40 years (hazard ratio [HR] = 1.936, 95% CI = 1.023–3.644, P = .042), primary tumor size ≥4.5 cm (HR = 4.007, 95% CI = 1.761–9.115, P = .001), and number of nodes resected ≤10 (HR = 2.052, 95% CI = 1.013–4.157, P = .046) were independent factors for poor prognosis of LRR (Table 3).
Table 3.
Multivariate analysis of risk factors for locoregional recurrence-free survival.
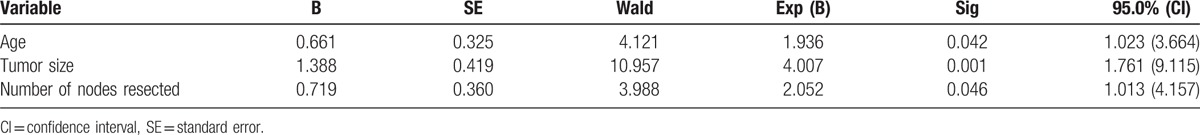
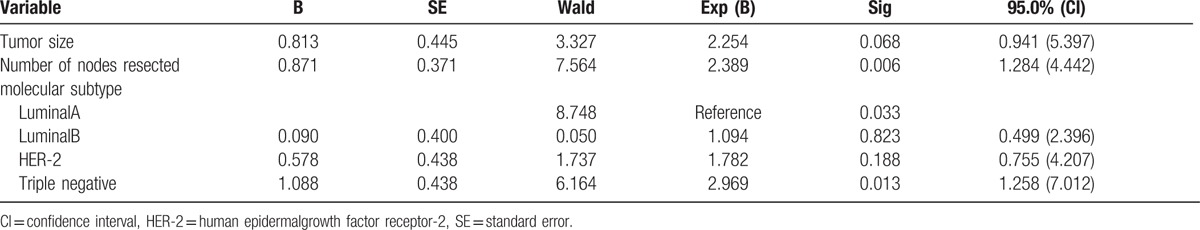
Furthermore, tumor size (HR = 2.249, 95% CI = 1.077–4.698, P = .031) and number of nodes resected (HR = 3.089, 95% CI = 1.885–5.062, P = .000) were identified as independent prognostic variables of DFS. And among those variables affecting OS, number of nodes resected (HR = 2.389, 95% CI = 1.284–4.442, P = .006) and molecular subtype were independent prognostic factors, based on the multivariate analysis. Triple negative status (HR = 2.969, 95% CI = 1.258–7.012, P = .013) was recognized as a poor prognostic indicator with respect to OS. The results are shown in Tables 4 and 5.
Table 4.
Multivariate analysis of risk factors for disease-free survival.
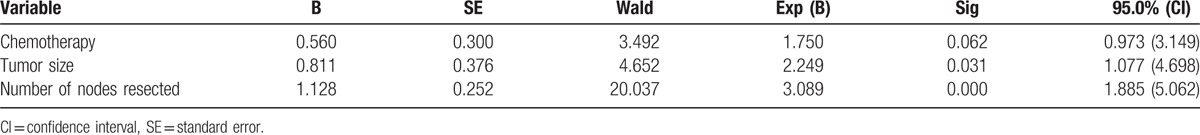
Table 5.
Multivariate analysis of risk factors for overall survival.
3.6. Establishment of risk scoring system and comparison of prognosis based on risk stratifications
Weighing each risk variable (to express its relative importance) by beta regression coefficient and Exp (B) (derived by Cox model), age ≤40 years, primary tumor size ≥4.5 cm and number of nodes resected ≤10 were assigned values of 1, 2, and 1, respectively. As shown in Table 6, the total score was derived by addition of the individual scores of all significant parameters, and 2 risk stratifications were consequently defined (low risk: total score <2, high risk: total score ≥2).
Table 6.
Risk scoring system.
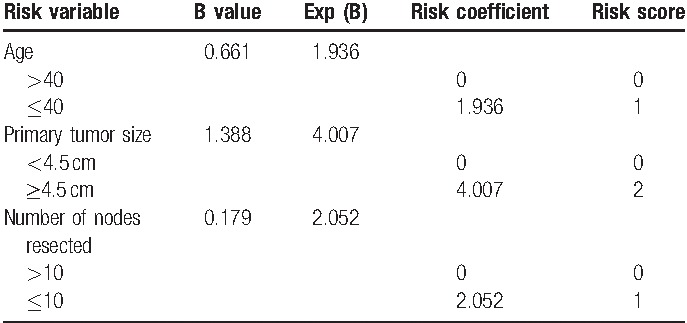
Comparisons of prognosis based on risk stratification were as follows: 316 cases with low risk (score <2), 37 cases with high risk (score ≥2). The 5-year LRFS was 91.6% (95% CI = 88.5%–94.7%) with low risk and 75.7% (95% CI = 61.8%–89.6%) with high risk, respectively (χ2 = 7.544, P = .006). In addition, significant differences in OS (P = .045) and DFS (P = .019) were seen between them (Fig. 7).
Figure 7.
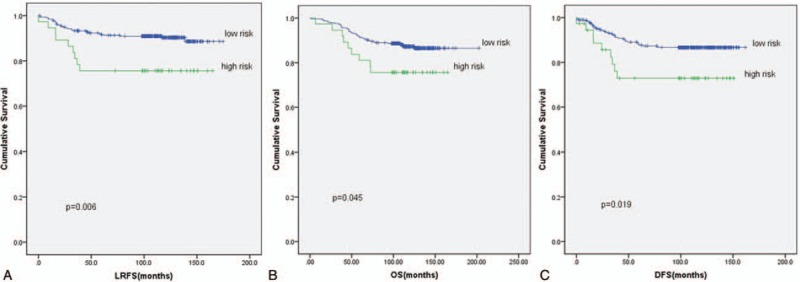
(A) Kaplan–Meier locoregional recurrence-free survival of patients with low risk versus high risk. (B) Kaplan–Meier overall survival of patients with low risk versus high risk. (C) Kaplan–Meier disease-free survival of patients with low risk versus high risk.
4. Discussion
This is one of the few studies focusing on the potential value of PMRT for pT1-2N0 patients with multiple risk factors. The study has identified age ≤40 years, primary tumor size ≥4.5 cm and number of nodes resected ≤10 as independent risk factors of LRR among pT1-2N0 patients. In addition, risk stratifications based on the scoring system revealed 5-year LRFS of 91.6% (95% CI = 88.5%–94.7%) with low risk (score <2) and 75.7% (95% CI = 61.8%–89.6%) with high risk (score ≥2), respectively (χ2 = 7.544, P = .006). This outcome might help to screen high-risk pT1-2N0 patients who could benefit from PMRT.
Previous meta-analysis had shown that the OS rate can remarkably benefit from the reduction of LRR rate for early breast cancer.[8] Therefore, the local control has become critical for preventing treatment failures. An overview of 36 randomized trials from Early Breast Cancer Trialists’ Collaborative Group has reported a highly statistically significant 2/3s reduction in LRR for early breast cancer patients with PMRT (6.7% vs 19.6%, P < .001).[9] In addition, a few trials have demonstrated that subgroups of pT1-T2N0 patients with specific prognostic factors were at higher risk of LRR.[1,3] The 10-year LRR for this subgroup with multiple poor risk factors was about 14% or even higher than those with 1 to 3 nodes metastasis.[10] But there is still no consensus on the risk factors of LRR for pT1-T2N0 patients. Herein, we tried to establish a new scoring system to identify the risk subgroup.
Primary tumor size is thought to be associated with increased LRR rates in LN−breast cancer. But the cutoff point of the tumor size in multiple investigations is variable, including 4, 3, 2.5, and 2 cm, and so on, and patients with larger size tumors showed worse prognosis.[11–14] Katz et al[10] reported that 10-year LRR risk for patients with primary tumor size >4 cm was >20%. Similar results were found in our study. Furthermore, 4.5 cm was found to be the cutoff point of tumor size. The results had shown that the 5-year LRFS for patients with tumor size <4.5 cm was better than those with ≥4.5 cm (91.3% vs 65.0%, P = .000).
Number of lymph node dissection has prognostic value for breast cancer patients. Insufficient lymph node dissection in T1-T2N0-1M0 stage cases might underestimate the postoperative lymph node stage.[15–17] Some researchers believed that the number of lymph node dissection <6 was associated with a poor prognosis,[18] while extensive and excessive dissection might increase the risk of subcutaneous dropsy, lymphorrhagia, upper limb lymphedema, and other postoperative complications. However, there is no consensus on the number of dissected lymph nodes. Katz et al[10] stated that LRR was much higher for patients with axillary lymph node dissection number <10 than those ≥10 (24% vs 11%, P <.02). Salama et al[19] also reported 10% to 15% decreased DFS rate for axillary lymph node dissection number <10 than those with more extensive dissection. In our study, the 5-year LRFS for patients with axillary lymph node dissection number ≤10 was lower than those >10 (79.0% vs 91.8%, P = .012), which is consistent with previous studies.
With the onset age of breast cancer trending younger, cancer invasiveness in young patients is stronger than in older.[20] Jwa et al[21] reported 307 patients with pT1-2N0 breast cancer and found that LRR for age <50 years was 11.4 times as high as those aged ≥50 years. Sharma et al[16] also reported similar results in recent years. A total of 1019 patients with early breast cancer were analyzed. Age was found to be the only independent adverse factor (HR = 2.41, 95% CI = 1.28–3.56, P = .004), and the 10-year LRR for age ≤40 years was significant higher than those aged >40 years (11.3% vs 1.5%, P < .001). Multivariate analysis in our study also showed that age was the prognostic factor of LRFS and significantly higher risk of relapse was observed in age ≤40 years subgroup (83.8% vs 92.0%, P = .023).
Some researchers believed that the molecular subtype might be a prognostic factor for LRR.[22] Liu et al[23] analyzed prognostic factors in axillary LN−patients and showed that the molecular subtype was an independent prognostic factor for LRR. HER-2 overexpression and triple-negative subtype had a poor prognosis, while luminal A and B is better. Gonzalez-Angulo et al[24] also indicated that the 5-year LRFS significantly differed between HER-2 positive subset and negative subset in T1abN0M0 patients (77.1% vs 93.7%, P = .002). Herein, we have shown that molecular subtype had little effect on the 5-year LRFS (P = .295) and DFS (P = .131), while 5-year OS was significantly different between different subgroups (P = .017). The 5-year OS of triple-negative type was 76.7%, which was the worst. So, our results also suggested molecular subtype as an important prognostic factor.
Synthetically, T1-2N0 breast cancer patients were heterogeneous. Therefore, identification of higher risk LRR subgroup would be important for postoperative treatment options. While a few investigators had attempted to predict the recurrence risk after surgery by combining various prognostic factors,[1,25] no consensus was reached. In addition, most previous researchers had tried to distinguish low-risk from high-risk subgroup by simply adding the number of independent risk factors together, which might ignore the relative importance of each risk factor. Our study not only identified independent risk factors for LRR but also assigned a value to each risk variable according to its relative importance, which made the risk scoring system more effective. Based on our scoring system, the 5-year LRFS was significantly different between the low-risk and high-risk subgroup (91.6% vs 75.7%, P = .006).
Similar to other retrospective studies, there are some limitations in this study. As a single-center retrospective clinical research, we have enrolled all 353 eligible cases in our institution and evaluated them as thoroughly as possible. But the more comprehensive study was still restricted by the limitation of samples. Besides selection bias of case and treatment method, accurately addressing the expression of Ki-67 as well as others is difficult when some Ki-67 expression data is missing.[26–28] Furthermore, it will be more convincing if prospective trials are designed to verify the benefits of our risk scoring system. Longer follow-up is also necessary to evaluate the value of PMRT for the high-risk subgroup.
In conclusion, we have evaluated long-term survival outcomes and identified risk factors affecting prognosis in cases with T1-2N0M0 breast cancer. In addition, a new scoring system was established to identify the higher risk LRR subgroup. Further verification of this scoring system might not only ensure that the high-risk subgroups have access to effective postoperative radiotherapy but also help the low-risk patients to avoid unnecessary radiation and financial burden.
Footnotes
Abbreviations: CI = confidence interval, DFS = disease-free survival, ER = estrogen receptor, HER-2 = human epidermalgrowth factor receptor-2, HR = hazard ratio, IHC = immunohistochemistry, LN− = lymph nodes negative, LN+ = lymph nodes positive, LRFS = locoregional recurrence-free survival, LRR = locoregional recurrence, OS = overall survival, PMRT = postmastectomy radiation therapy, PR = progesterone receptor.
J-lL and X-yL have contributed equally to the article.
Funding/support: This study was supported by the Fujian Province Natural Science Foundation (no. 2016J01437), the Fujian Medical Innovation Project (no. 2015-CX-8), the Key Clinical Specialty Discipline Construction Program of Fujian, PR China, and the National Clinical Key Specialty Construction Program.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Abi-Raad R, Boutrus R, Wang R, et al. Patterns and risk factors of locoregional recurrence in T1–T2 node negative breast cancer patients treated with mastectomy: implications for postmastectomy radiotherapy. Int J Radiat Oncol Biol Phys 2011;81:e151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jagsi R, Raad RA, Goldberg S, et al. Locoregional recurrence rates and prognostic factors for failure in node-negative patients treated with mastectomy: implications for postmastectomy radiation. Int J Radiat Oncol Biol Phys 2005;62:1035–9. [DOI] [PubMed] [Google Scholar]
- [3].Truong PT, Lesperance M, Culhaci A, et al. Patient subsets with T1–T2, node-negative breast cancer at high locoregional recurrence risk after mastectomy. Int J Radiat Oncol Biol Phys 2005;62:175–82. [DOI] [PubMed] [Google Scholar]
- [4].Abdulkarim BS, Cuartero J, Hanson J, et al. Increased risk of locoregional recurrence for women with T1-2N0 triple-negative breast cancer treated with modified radical mastectomy without adjuvant radiation therapy compared with breast-conserving therapy. J Clin Oncol 2011;29:2852–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pierce LJ. The use of radiotherapy after mastectomy: a review of the literature. J Clin Oncol 2005;23:1706–17. [DOI] [PubMed] [Google Scholar]
- [6].Wang SL, Li YX, Song YW, et al. Prognostic value of estrogen receptor, progesterone receptor and human epidermal growth factor receptor-2 in node positive breast cancer patients treated by mastectomy. Zhonghua zhong liu za zhi 2010;32:520–5. [PubMed] [Google Scholar]
- [7].Budczies J, Klauschen F, Sinn BV, et al. Cutoff Finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS One 2012;7:e51862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366:2087–106. [DOI] [PubMed] [Google Scholar]
- [9].EBCTCG. Effects of radiotherapy and surgery in early breast cancer. An overview of the randomized trials. Early Breast Cancer Trialists’ Collaborative Group. N Engl J Med 1995;333:1444–55. [DOI] [PubMed] [Google Scholar]
- [10].Katz A, Strom EA, Buchholz TA, et al. Locoregional recurrence patterns after mastectomy and doxorubicin-based chemotherapy: implications for postoperative irradiation. J Clin Oncol 2000;18:2817–27. [DOI] [PubMed] [Google Scholar]
- [11].Bollet MA, Kirova YM, Fourquet A, et al. Prognostic factors for local recurrence following breast-conserving treatment in young women. Expert Rev Anticancer Ther 2010;10:1215–27. [DOI] [PubMed] [Google Scholar]
- [12].Gurleyik G, Aker F, Aktekin A, et al. Tumor characteristics influencing non-sentinel lymph node involvement in clinically node negative patients with breast cancer. J Breast Cancer 2011;14:124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Quiet CA, Ferguson DJ, Weichselbaum RR, et al. Natural history of node-negative breast cancer: a study of 826 patients with long-term follow-up. J Clin Oncol 1995;13:1144–51. [DOI] [PubMed] [Google Scholar]
- [14].Rosen PP, Groshen S, Kinne DW. Prognosis in T2N0M0 stage I breast carcinoma: a 20-year follow-up study. J Clin Oncol 1991;9:1650–61. [DOI] [PubMed] [Google Scholar]
- [15].Iyer RV, Hanlon A, Fowble B, et al. Accuracy of the extent of axillary nodal positivity related to primary tumor size, number of involved nodes, and number of nodes examined. Int J Radiat Oncol Biol Phys 2000;47:1177–83. [DOI] [PubMed] [Google Scholar]
- [16].Sharma R, Bedrosian I, Lucci A, et al. Present-day locoregional control in patients with t1 or t2 breast cancer with 0 and 1 to 3 positive lymph nodes after mastectomy without radiotherapy. Ann Surg Oncol 2010;17:2899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wu SG, Wang Y, Zhou J, et al. Number of negative lymph nodes should be considered for incorporation into staging for breast cancer. Am J Cancer Res 2015;5:844–53. [PMC free article] [PubMed] [Google Scholar]
- [18].Cancello G, Maisonneuve P, Rotmensz N, et al. Prognosis in women with small (T1mic,T1a,T1b) node-negative operable breast cancer by immunohistochemically selected subtypes. Breast Cancer Res Treat 2011;127:713–20. [DOI] [PubMed] [Google Scholar]
- [19].Salama JK, Heimann R, Lin F, et al. Does the number of lymph nodes examined in patients with lymph node-negative breast carcinoma have prognostic significance? Cancer 2005;103:664–71. [DOI] [PubMed] [Google Scholar]
- [20].Colleoni M, Rotmensz N, Robertson C, et al. Very young women (<35 years) with operable breast cancer: features of disease at presentation. Ann Oncol 2002;13:273–9. [DOI] [PubMed] [Google Scholar]
- [21].Jwa E, Shin KH, Lim HW, et al. Identification of risk factors for locoregional recurrence in breast cancer patients with nodal stage N0 and N1: who could benefit from post-mastectomy radiotherapy? PLoS one 2015;10:e0145463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Metzger-Filho O, Sun Z, Viale G, et al. Patterns of Recurrence and outcome according to breast cancer subtypes in lymph node-negative disease: results from international breast cancer study group trials VIII and IX. J Clin Oncol 2013;31:3083–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Liu X, Guan Y, Zhang W, et al. Predictors of recurrence in breast cancer subtypes with negative lymph node in a Chinese population. Int J Clin Exp Pathol 2014;7:3202–12. [PMC free article] [PubMed] [Google Scholar]
- [24].Gonzalez-Angulo AM, Litton JK, Broglio KR, et al. High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumors 1 cm or smaller. J Clin Oncol 2009;27:5700–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hastings J, Iganej S, Huang C, et al. Risk factors for locoregional recurrence after mastectomy in stage T1 N0 breast cancer. Am J Clin Oncol 2014;37:486–91. [DOI] [PubMed] [Google Scholar]
- [26].Kontzoglou K, Palla V, Karaolanis G, et al. Correlation between Ki67 and breast cancer prognosis. Oncology 2013;84:219–25. [DOI] [PubMed] [Google Scholar]
- [27].Petrelli F, Viale G, Cabiddu M, et al. Prognostic value of different cut-off levels of Ki-67 in breast cancer: a systematic review and meta-analysis of 64,196 patients. Breast Cancer Res Treat 2015;153:477–91. [DOI] [PubMed] [Google Scholar]
- [28].Yerushalmi R, Woods R, Ravdin PM, et al. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol 2010;11:174–83. [DOI] [PubMed] [Google Scholar]


