Abstract
To estimate the effect of hepatitis C virus (HCV) coinfection on the development of complications and progression of human immunodeficiency virus (HIV) disease among HIV-infected elite controllers.
Single-center retrospective cohort. Kaplan–Meier methods, prevalence ratios, and Cox proportional-hazards models were used.
In all, 55 HIV-infected elite controllers were included in this study. Among them, 45% were HIV/HCV coinfected and 55% were HIV mono-infected. Median follow-up time for the cohort was 11 years. Twenty-five patients experienced a complication and 16 lost elite controller status during the study period. HCV coinfected patients were 4.78 times (95% confidence interval 1.50–15.28) more likely to develop complications compared with HIV mono-infected patients. There was no association between HCV coinfection status and loss of elite control (hazard ratio 0.75, 95% confidence interval 0.27–2.06).
Hepatitis C virus coinfection was significantly associated with the risk of complications even after controlling for sex, injecting drug use, and older age. HCV coinfected patients had higher levels of cellular activation while also having similar levels of lipopolysaccharide and soluble CD14. HCV coinfection was not associated with loss of elite controller status. Taken together, this suggests that HCV coinfection does not directly affect HIV replication dynamics or natural history, but that it may act synergistically with HIV to produce a greater number of associated complications. Continued follow-up will be needed to determine whether HCV cure through the use of direct-acting antivirals among HIV/HCV coinfected elite controllers will make the risk for complications among these patients similar to their HIV mono-infected counterparts.
Keywords: complication, elite controllers, epidemiology, hepatitis C infection, HIV
1. Introduction
Approximately 20% of all people infected with human immunodeficiency virus (HIV) in the United States are coinfected with hepatitis C virus (HCV). The proportion of HCV coinfection is as high as 93% among HIV-infected patients, with injection drug use as their primary risk factor.[1] The impact of HCV infection on the course of HIV infection appears to be mild and transient, with an early delay in CD4 reconstitution after starting highly active antiretroviral therapy (HAART), which then disappears with time.[2–5] Elite controllers are a unique subpopulation of HIV-infected patients capable of naturally suppressing HIV-1 to undetectable levels without therapy.[6–10] Little data exist about the effect of HCV infection on HIV-1 elite controllers, who have survived decades without the complications of HIV/AIDS. HCV coinfection has been shown to be associated with increased immune activation (CD38+ HLA-DR_+ CD8 cells).[11] Recently, increased hospitalization rates amongst elite controllers when compared with other medically controlled HIV patients have been reported,[12] and other studies have noted increased hospitalization rates and utilization in HIV/HCV coinfected patient versus HIV mono-infected patients.[13,14]
The purpose of this study was to estimate the effect of HCV coinfection among HIV-infected elite controllers on the risk of complications and the risk of disease progression compared with HIV mono-infected individuals. Our hypothesis was that individuals coinfected with HCV would be at increased risk for the development of complications and loss of elite controller status compared with HIV mono-infected individuals.
2. Methods
We conducted a retrospective cohort study of the elite controller data to estimate the relationship between HCV coinfection and the development of complications and HIV disease progression. This study was approved by the Institutional Review Board of the University of Maryland, Baltimore. Complications were defined as the development of cancer, cardiovascular events (cerebrovascular accident, myocardial infraction, or diagnosis of coronary artery disease), cirrhosis, organ failure (liver, kidney, or heart), or death after enrollment in the cohort. All outcomes were ascertained through medical chart review. For inclusion in the original cohort, patients had to be confirmed HIV-1-positive by Western blot and proviral DNA. Elite controller status was defined as viral loads <400 copies/mL for a 2-year period without the use of antiretroviral therapy.[1,10,15]
Disease progression (loss of elite controller status) was defined as 2 consecutive viral loads >400 copies/mL in a 2-year period or initiation of HAART. HCV infection was defined as being polymerase chain reaction (PCR)-positive for HCV.
All data were initially investigated in univariate analyses to determine the distribution, frequency, and amount of missing data. Continuous variables were assessed for the linearity of their relationship with the outcomes by first categorizing them into quartiles and then plotting the beta estimates. Variables that did not demonstrate a linear relationship were modeled as categorical variables. The unadjusted association between HCV coinfection and both predefined outcomes were investigated using Kaplan–Meier survival curves and Cox proportional-hazards models with HCV coinfection as the only predictor variable in the exploratory analysis. Potential confounders were assessed in stratified analysis. Baseline covariates that changed the crude hazard ratio (HR) for the association between HCV coinfection and the outcomes by more than 10%, or were associated with the outcome at an alpha level of 0.2 or less were further investigated in multivariable Cox models. The proportional-hazards assumption was tested by adding an interaction term of the covariates with time in the multivariable model. Markers of immune activation were not collected at baseline; therefore the association between markers of immune activation and the development of complications and loss of elite controller status were estimated using prevalence ratios. To be included in the study, patients must have had a cellular immune activation measurement documented. A sensitivity analysis was conducted comparing patients excluded from the study with patients included to assess whether they systematically differed by HCV coinfection, development of a complication, or loss of elite controller status. One patient coinfected with HCV experienced cirrhosis and liver failure. The final model was run including and excluding the patient.
3. Results
3.1. HCV coinfection and the development of complications
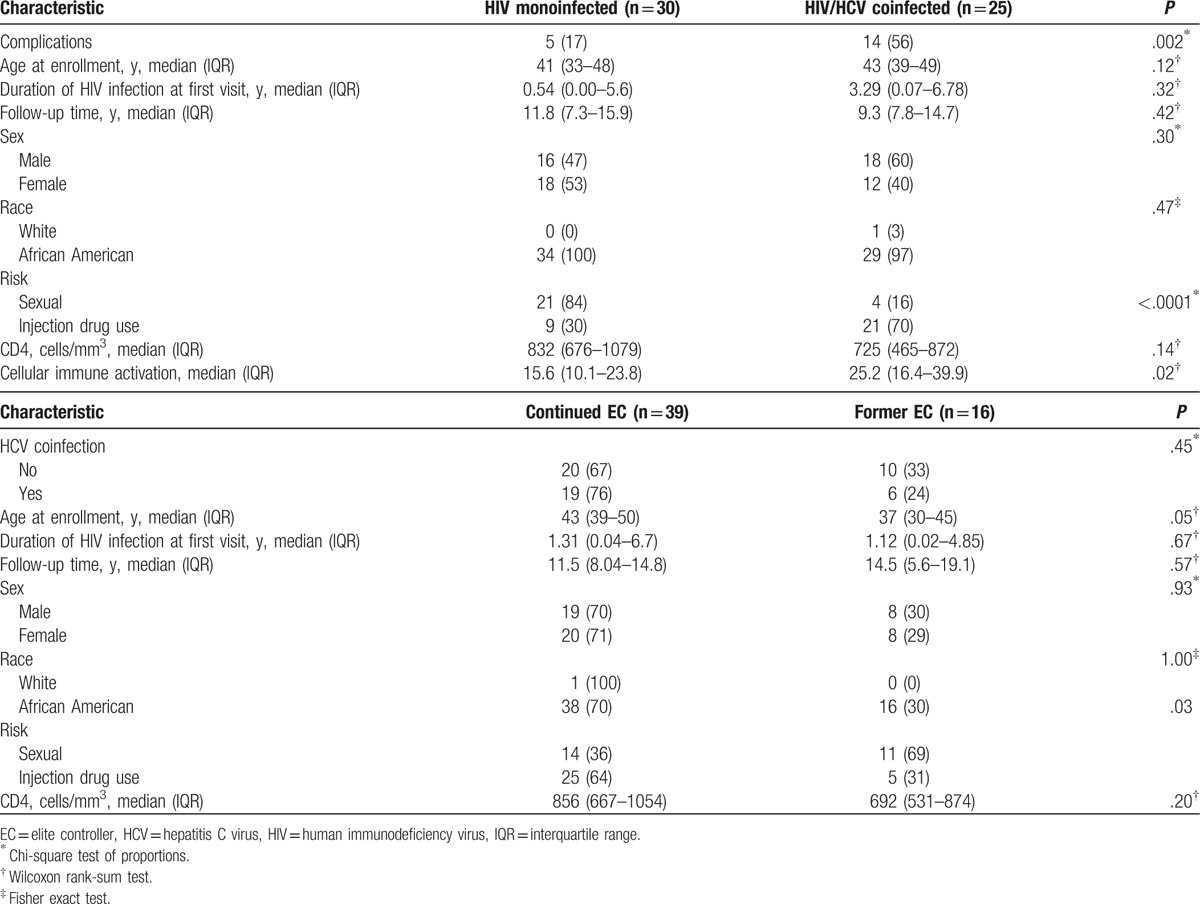
A total of 64 elite controller patients have been enrolled in the natural viral suppressor (NVS) cohort since 2004. We excluded 9 patients because they lacked information related to cellular immune activation for a final study sample of 55. Of the study sample, 25 (45%) patients were HIV/HCV coinfected and 30 (55%) were HIV monoinfected (Table 1). Median follow-up time from first clinic visit was 11.8 years (interquartile range (IQR) 7.3–15.9] among HIV monoinfected patients and 10.7 years (8–15.9) among HCV coinfected patients. Complications were more prevalent in those coinfected with HCV (56% vs 17%; P = .002). HCV coinfected patients had higher levels of cellular immune activation (proportion of HLADR+CD38+CD8+ T cells) compared with HIV monoinfected patients (25.2 vs 15.55; P = .02). Soluble CD14 (sCD14) and lipopolysaccharide (LPS) were not significantly different among HCV coinfected patients compared with HIV monoinfected patients (sCD14 2.6 [1.9–4.4] vs 2.2 [1.5–3.0]; P = .17; LPS 0.19 [0.15–0.28] vs 0.17 [0.12–0.22]; P = .24, respectively) (data not shown). HCV coinfected patients tended to be slightly older than HIV monoinfected patients and present with lower CD4 cell counts; however, no statistically significant differences were observed in these baseline factors or in other baseline variables.
Table 1.
Baseline characteristics of a cohort of elite controllers in Baltimore, Maryland, by HCV coinfection status and loss of elite controller (EC) status (n = 55).
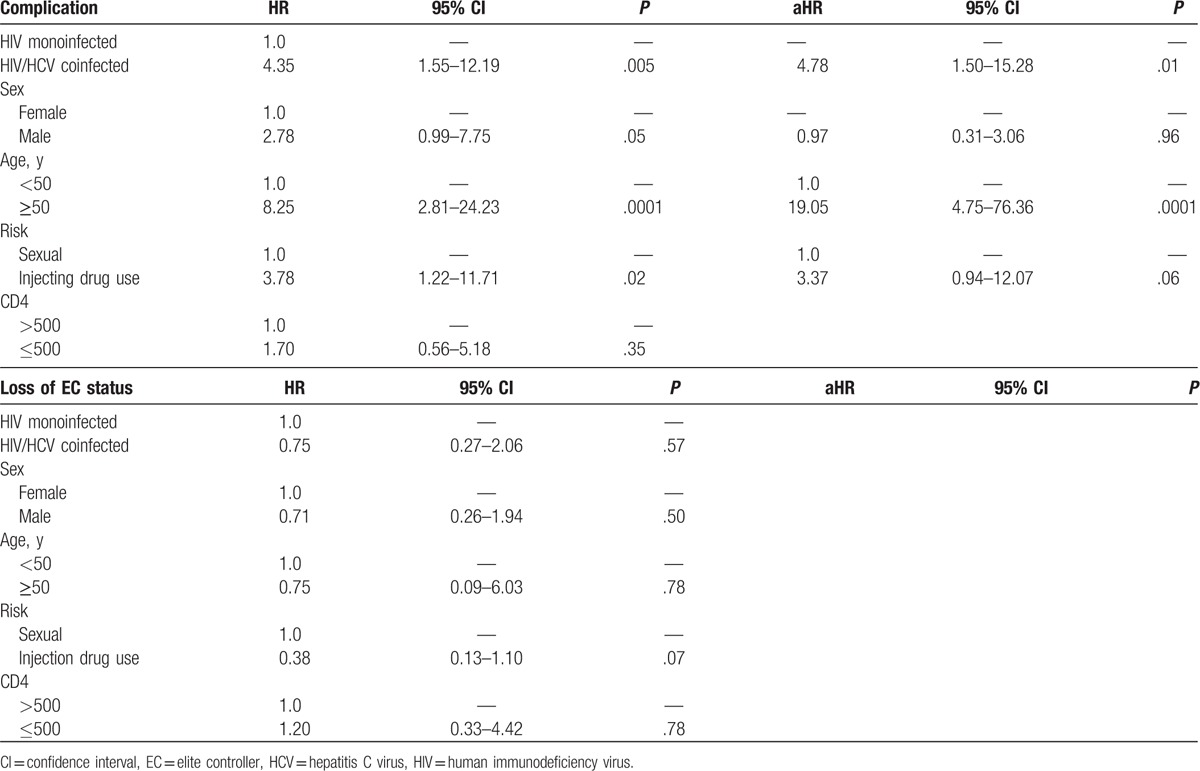
Of the patients who developed complications, 8 developed cancer, 5 developed organ failure, 4 developed cardiovascular complications, 1 developed cirrhosis, and 1 died after enrollment without one of the afore mentioned complications. HCV coinfected patients developed complications at a significantly faster rate compared with HIV monoinfected patients (P = .002) (Fig. 1A). Patients coinfected with HCV were 4 times more likely to develop a complication compared with HIV monoinfected patients (Table 2). After controlling for sex, injecting drug use, and age, HCV coinfected patients were 4.78 times more likely to develop complications compared with HIV monoinfected patients.
Figure 1.
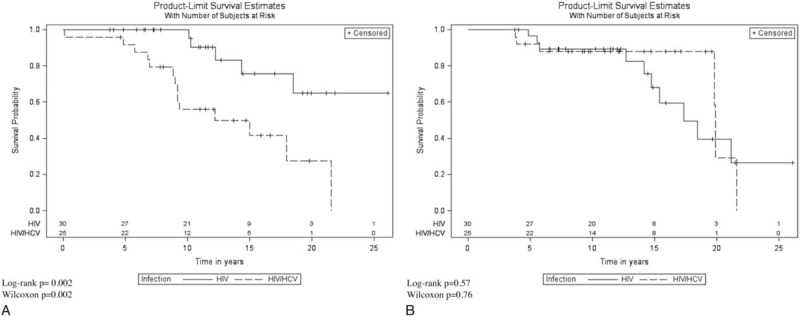
(A) Kaplan–Meier curve for time to development of a complication by HCV coinfection status. (B) Time to loss of natural viral suppressor status by HCV coinfection status. HCV = hepatitis C virus.
Table 2.
Unadjusted hazard ratios (HRs) and adjusted hazard ratios (aHRs) for the development of first complication or loss of elite controller status by HCV coinfection among a cohort of elite controllers in Baltimore, Maryland (n = 55).
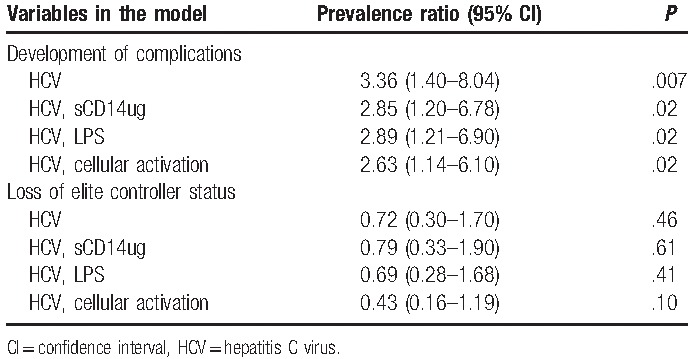
The prevalence of HCV coinfection was 3.36 times higher (P = .007) among patients who developed complications compared with patients who did not develop complications (Table 3). After individually controlling for markers of immune activation, the prevalence of HCV coinfection remained significantly higher among patients who developed complications compared with those who did not. The prevalence of HCV coinfection among patients who developed complications was 2.85 (1.20–6.78) times higher after controlling for sCD14, 2.89 (1.21–6,90) times higher after controlling for LPS, and 2.63 (1.14–6.10) times higher after controlling for cellular activation compared with patients who did not develop complications.
Table 3.
Prevalence ratios for the association between HCV coinfection and the development of complications, and HCV coinfection and loss of elite controller status controlling for immune activation (n = 55).
3.2. HCV coinfection and loss of elite control
From the time of first presentation for care to the end of the study period, 16 patients lost elite controller status (29%) (Table 1). There was moderate evidence that patients who lost their elite controller status were younger than patients who maintained elite controller status and were more likely to have sexual contact as their primary risk factor. There were no significant differences in other baseline characteristics between those who maintained elite controller status compared with those who lost viral control. There was some evidence that patients who lost elite controller status had higher levels of cellular immune activation (Table 3).
Time to loss of NVS status did not differ significantly by HCV coinfection status (P = .57) (Fig. 1B). There was no difference in the hazard for loss of elite controller status when comparing HCV coinfected patients to HIV monoinfected patients (HR 0.75, 95% confidence interval [CI] 0.27–2.06, P = .57) (Table 2). None of the baseline characteristics changed the crude association between HCV coinfection and loss of elite controller status or improved model fit. The prevalence of HCV coinfection status did not differ between patients who maintained elite controller status compared with those who lost elite controller status. After individually controlling for markers of immune activation, the prevalence of HCV coinfection status was not significantly different among those who lost elite controller status compared with those who maintained it (Table 3).
3.3. HCV viral profile among coinfected patients
The median log HCV viral load of coinfected patients at baseline was 6.27 (IQR 5.63–6.54). Twenty-four of the HCV coinfected patients had genotype 1a or 1b, and 1 had 3a. There was no difference in the median log HCV viral load among HCV coinfected patients who developed a complication or who lost their NVS status compared with the coinfected patients who did not develop a complication or maintained natural viral suppression (data not shown).
3.4. Sensitivity analysis
The 9 patients excluded from analysis due to missing immune activation levels did not differ significantly from patients included with regards to the prevalence of HCV coinfection, incidence of complications, or loss of elite controller status (data not shown). The crude and adjusted estimates of effect did not significantly differ when the patient with liver failure was excluded (data not shown). All results presented include the patient with liver failure.
4. Discussion
We compared the rate of development of a first complication in HCV coinfected versus HIV monoinfected individuals in an NVS cohort with a median follow-up of 11 years. There was a significantly higher cumulative incidence of complications among HCV coinfected patients compared with HIV monoinfected patients, and a significantly increased hazard for their development. Previous studies have revealed increased evidence of coronary atherosclerosis in elite controllers.[12,16] Crowell et al[12] compared morbidity rates and causes between elite controllers and immunologically intact persons with medically controlled HIV; a significantly higher rate of hospitalization was detected, with cardiovascular hospitalization being the most common. Two other studies reported no increased hospitalization among elite controllers[17]; however, both of these were conducted on a relatively small number of patients, in a younger population,[17] and HCV coinfection was not investigated. However, several other studies have noted increased hospitalization rates in HIV/HCV coinfected patients compared with HIV monoinfected patients, although elite controllers were not specifically studied.[13,14] In the present study, the incidence of complications was higher in patients coinfected with HCV, and complications were significantly more likely to occur in HCV coinfected patients.
The HIV virus continues to replicate in elite controllers even in the absence of a detectable viral load, leading to a chronic inflammatory state.[18,19] Elite controllers experience chronic low-grade inflammation and immune activation during the process of suppressing viremia to levels prominently higher than those receiving HAART therapy.[20] HAART therapy in elite controllers has led to decreased T-cell activation,[18,19,21] and increased CD4 cell counts in some, but not all, patients.[22,23] Various factors contribute to increased immune activation in elite controllers, consisting of persistent low-level viremia[24] and persistent immune response,[25] which may play a pathophysiologic role in higher hospitalization for several conditions including cardiovascular events.[12] Increased levels of D-dimer and soluble tissue factorin elite controllers have been detected as compared with both HIV-seronegative and HAART-controlled HIV patients.[20]
In this study, HCV coinfection was significantly associated with the risk of complications, even after controlling for CD4+ T-cell level, sex, and older age. Chronic inflammation is thought to be associated with CD4+ T-cell depletion and higher levels of immune activation.[21,26] Similarly, HCV coinfection remained significantly associated with a higher prevalence of complications when individual immune activation markers were controlled for. This study found that HCV coinfected patients had higher levels of cellular activation while also having similar levels of LPS and soluble CD14.[21,26]
We had previously found that among elite controllers, HCV coinfection was associated with lower mean CD4 cell count, CD4%, and CD4/CD8.[11] However, with analysis of more years of data and adjusting for differences based on sex, we no longer see this correlation. In addition, in this study, HCV coinfection is not associated with loss of elite controller status. Taken together, this suggests that HCV coinfection does not directly affect HIV replication dynamics or natural history, but that it may act synergistically with HIV to produce a greater number of associated complications. Because some complications (cardiovascular) could be decades in the making, it remains to be seen whether HCV therapy and/or HAART treatment could significantly affect this outcome. Anecdotally, we have several patients in this cohort who have had continued complications (stroke and carcinoma) despite successful HCV eradication. Other complications such as development of carcinoma may be more readily amenable to more rapid risk reduction with antiviral therapy. Further studies with long periods of follow-up will be needed to address these questions.
Our study has several limitations. Not all patients had measures of immune activation documented in their study records and were therefore excluded introducing the potential for selection bias. However, when we compared the available baseline covariates, and also the distribution of exposure and outcomes between patients included and excluded from the study, we found them to be similar indicating that had they been included our inferences would most likely not have been affected. The measures of immune activation were not assessed at enrollment into the study cohort, and we therefore cannot make temporal inferences of a causal nature nor could we control for them in the multivariable Cox models. We used prevalence rate ratios (PRRs) to estimate the associations of interest as risk was our parameter of interest. We chose PRRs as both the prevalence of HCV coinfection and the outcomes (complications and loss of elite controller status) were fairly common (>10%), and prevalence odds ratios (ORs) would be further away from the null, thus potentially overestimating the strength of the associations of interest.[27]
Continued follow-up will be needed to determine whether HCV cure through the use of direct-acting antivirals among HIV/HCV coinfected elite controllers will make the risk for complications among these patients similar to their HIV monoinfected counterparts. Further studies will also be needed to determine the effects of antiretroviral therapy in this group of patients coupled to its risk/benefit ratio. Whereas HIV patients coinfected with HCV are at significantly increased risk for the development of complications, HCV coinfection does not appear to impact the likelihood of HIV disease progression in this population.
Acknowledgment
The authors would like to thank Dr John Sorkin for his important contributions to the statistical methods used in this study.
Footnotes
Abbreviations: HAART = highly active antiretroviral therapy, HCV = hepatitis C virus, HIV = human immunodeficiency virus, PCR = polymerase chain reaction.
Funding: At the time of the study, KAS was supported by The Building Interdisciplinary Research Careers in Women's Health (BIRCWH) program award (NIH K12 HD43489-13). MMS was supported by R01AI110259-01A1 and 1I01BX002358-01A1. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
References
- [1].Solomon SS, Srikrishnan AK, Mehta SH, et al. High prevalence of HIV, HIV/hepatitis C virus co-infection and risk behaviors among IDUs in Chennai, India: a cause for concern. J Acquir Immune Defic Syndr 2008;49:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Law WP, Duncombe CJ, Mahanontharit A, et al. Impact of viral hepatitis co-infection on response to antiretroviral therapy and HIV disease progression in the HIV-NAT cohort. AIDS 2004;18:1169–77. [DOI] [PubMed] [Google Scholar]
- [3].Macias J, Pineda JA, Lozano F, et al. Impaired recovery of CD4+ cell counts following highly active antiretroviral therapy in drug-naive patients coinfected with human immunodeficiency virus and hepatitis C virus. Eur J Clin Microbiol Infect Dis 2003;22:675–80. [DOI] [PubMed] [Google Scholar]
- [4].Santin M, Mestre M, Shaw E, et al. Impact of hepatitis C virus coinfection on immune restoration during successful antiretroviral therapy in chronic human immunodeficiency virus type 1 disease. Eur J Clin Microbiol Infect Dis 2008;27:65–73. [DOI] [PubMed] [Google Scholar]
- [5].Seminari E, Tinelli C, Ravasi G, et al. Hepatitis C infection on immune recovery in HIV-positive patients on successful HAART: the role of genotype 3. Curr HIV Res 2010;8:186–93. [DOI] [PubMed] [Google Scholar]
- [6].Sajadi MM, Constantine NT, Mann DL, et al. Epidemiologic characteristics and natural history of HIV-1 natural viral suppressors. J Acquir Immune Defic Syndr 2009;50:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lambotte O, Boufassa F, Madec Y, et al. HIV controllers: a homogeneous group of HIV-1-infected patients with spontaneous control of viral replication. Clin Infect Dis 2005;41:1053–6. [DOI] [PubMed] [Google Scholar]
- [8].Pereyra F, Addo MM, Kaufmann DE, et al. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J Infect Dis 2008;197:563–71. [DOI] [PubMed] [Google Scholar]
- [9].Bailey JR, Lassen KG, Yang HC, et al. Neutralizing antibodies do not mediate suppression of human immunodeficiency virus type 1 in elite suppressors or selection of plasma virus variants in patients on highly active antiretroviral therapy. J Virol 2006;80:4758–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sajadi MM, Heredia A, Le N, et al. HIV-1 natural viral suppressors: control of viral replication in the absence of therapy. AIDS 2007;21:517–9. [DOI] [PubMed] [Google Scholar]
- [11].Sajadi MM, Pulijala R, Redfield RR, et al. Chronic immune activation and decreased CD4 counts associated with hepatitis C infection in HIV-1 natural viral suppressors. AIDS (London, England) 2012;26:1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Crowell TA, Gebo KA, Blankson JN, et al. Hospitalization rates and reasons among HIV elite controllers and persons with medically controlled HIV infection. J Infect Dis 2015;211:1692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Crowell TA, Gebo KA, Balagopal A, et al. Impact of hepatitis coinfection on hospitalization rates and causes in a multicenter cohort of persons living with HIV. J Acquir Immune Defic Syndr 2014;65:429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Katrak S, Park LP, Woods C, et al. Patterns of healthcare utilization among veterans infected with hepatitis C virus (HCV) and human immunodeficiency virus (HIV) and coinfected with HIV/HCV: unique burdens of disease. Open Forum Infect Dis 2016;3:ofw173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sajadi MM, Shakeri N, Talwani R, et al. Hepatitis C infection in HIV-1 natural viral suppressors. AIDS (London, England) 2010;24:1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pereyra F, Lo J, Triant VA, et al. Increased coronary atherosclerosis and immune activation in HIV-1 elite controllers. AIDS (London, England) 2012;26:2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Okulicz JF, Marconi VC, Landrum ML, et al. Clinical outcomes of elite controllers, viremic controllers, and long-term nonprogressors in the US Department of Defense HIV natural history study. J Infect Dis 2009;200:1714–23. [DOI] [PubMed] [Google Scholar]
- [18].Hatano H, Yukl SA, Ferre AL, et al. Prospective antiretroviral treatment of asymptomatic, HIV-1 infected controllers. PLoS Pathog 2013;9:e1003691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chun T-W, Justement JS, Murray D, et al. Effect of antiretroviral therapy on HIV reservoirs in elite controllers. J Infect Dis 2013;208:1443–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Krishnan S, Wilson EM, Sheikh V, et al. Evidence for innate immune system activation in HIV type 1-infected elite controllers. J Infect Dis 2014;209:931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sedaghat AR, Rastegar DA, O’Connell KA, et al. T cell dynamics and the response to HAART in a cohort of HIV-1-infected elite suppressors. Clin Infect Dis 2009;49:1763–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Boufassa F, Lechenadec J, Meyer L, et al. Blunted response to combination antiretroviral therapy in HIV elite controllers: an international HIV controller collaboration. PloS One 2014;9: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Okulicz JF, Grandits GA, Weintrob AC, et al. CD4 T cell count reconstitution in HIV controllers after highly active antiretroviral therapy. Clin Infect Dis 2010;50:1187–91. [DOI] [PubMed] [Google Scholar]
- [24].Hatano H, Delwart EL, Norris PJ, et al. Evidence for persistent low-level viremia in individuals who control human immunodeficiency virus in the absence of antiretroviral therapy. J Virol 2009;83:329–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hersperger AR, Migueles SA, Betts MR, et al. Qualitative features of the HIV-specific CD8+ T cell response associated with immunologic control. Curr Opin HIV AIDS 2011;6:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sousa AE, Carneiro J, Meier-Schellersheim M, et al. CD4 T cell depletion is linked directly to immune activation in the pathogenesis of HIV-1 and HIV-2 but only indirectly to the viral load. J Immunol 2002;169:3400–6. [DOI] [PubMed] [Google Scholar]
- [27].Zocchetti C, Consonni D, Bertazzi PA. Relationship between prevalence rate ratios and odds ratios in cross-sectional studies. Int J Epidemiol 1997;26:220–3. [DOI] [PubMed] [Google Scholar]


