Abstract
Type 2 diabetes mellitus (T2DM) is a long-term metabolic disorder. It is characterized by hyperglycemia, insulin resistance (IR), and relative impairment in insulin secretion. IR plays a major role in the pathogenesis of T2DM. Many previous studies have investigated the relationship between estrogen, androgen, and obesity, but few focused on the relationship between sex hormones, abnormal lipid metabolism, and IR. The goal for the present study was to identify the association of IR with sex hormone, abnormal lipid metabolism in type 2 diabetes, and impaired glucose tolerance (IGT) patients.
In total 13,400 participants were analyzed based on the results of the glucose tolerance test. Using a cross-sectional study, we showed the relationship between IR and the level of sex hormones among 3 different glucose tolerance states: normal control people, IGT, and T2DM patients. We also analyzed the relationship between IR and abnormal lipid metabolism.
Significantly, luteinizing, progesterone, estradiol, prolactin, and follicle-stimulating hormone levels decreased in T2DM and IGT patients compared with those in normal control people. The association between IR and lipid metabolism disorders in T2DM and IGT patients was also observed.
Our clinical findings may offer new insights into understanding the mechanism of metabolic disorders and in new therapeutic methods for the treatment of the prevalence of type 2 diabetes.
Keywords: abnormal lipid metabolism, cross-sectional study, IGT, insulin resistance, sex hormone, T2DM
1. Introduction
Diabetes is a major public health concern. It was reported that about 347 million people worldwide were suffering from diabetes in 2008. The incidence of diabetes would rapidly increase and almost double by 2030.[1] The faster increase (about 50%) in the near future would occur in developing counties.[1] As the largest developing country, China has the largest number of individuals with diabetes in the world. More than 90% of diabetic patients suffer from type 2 diabetes mellitus (T2DM).[2] In 2014, the prevalence of T2DM was estimated at 9.32% among Chinese population aged 18 to 79 years, representing an estimated 96.3 million people.[3]
T2DM is a long-term metabolic disorder that is characterized by high blood sugar, insulin resistance (IR), and relative lack of insulin. IR is a primary pathogenic feature of T2DM and an independent risk factor for cardiovascular disease (CVD).[4,5] It is known that endocrine disruptors play an important part in the incidence of metabolic diseases. The pancreatic beta-cell is a target of estrogens,[6] which plays an important role in blood glucose homeostasis. 17β-estradiol (E2) levels above or below the physiological range may promote IR and type 2 diabetes. Serum testosterone levels have also been studied in populations of men attending hospital-based diabetes clinics, with estimates that 30% to 50% of men with T2DM have low testosterone.[7] All these indicated the association between sex steroids and glucose metabolism. Therefore, understanding the mechanisms through which IR evolves is critical for improving strategies in the prevention and treatment of diabetic.
IR has long been associated with obesity and the disorder of lipids and hormones.[8–10] However, few studies have comprehensively collected and analyzed the clinical data from a single hospital to assess the associations of various clinical parameters in T2DM patients. Here, we performed a cross-sectional study of the comparison between disturbed lipid metabolism and the sexual hormone state in healthy people, patients with impaired glucose, and diabetic patients in China. Hence, the aim of the study was to investigate the association of IR with lipid metabolism and sexual hormone at different glucose states among T2DM, prediabetic patients, and normal control people to offer new insights into the treatment of diabetes.
2. Methods
A total of 13,400 patients were enrolled at Beijing Health Center from January 2016 to August 2016 in China. A standard questionnaire was used to collect information about the health status, medication history, and lifestyles of participants. Each subject signed an agreement of participation in this study that was approved by the ethics committee of the Beijing Hospital.
The cross-sectional data from healthy subjects have no diabetes. Depending on blood glucose levels, patients were divided into 3 groups: control group with normal blood glucose levels, impaired glucose tolerance (IGT) group, and diabetes group. After oral glucose tolerance test (OGTT), the glucose tolerance data were obtained; meanwhile, the basic clinical indicators, such as body fat rate, sex hormones, urinary protein, thyroid function, were determined. During physical examinations, the height, weight, and BMI of all the participants were measured. High-density lipoprotein (HDL), low-density lipoprotein (LDL), triglyceride (TG), total triiodothyronine 3 (TT3), and total thyroxine 4 (TT4) were measured by colorimetric enzymatic assays using an autoanalyzer (Hitachi 7170). Fasting insulin, fasting blood glucose, luteinizing hormone (LH), progesterone, prolactin, testosterone, estradiol, and follicle stimulating hormone (FSH) levels were measured. IR was estimated by using the homeostasis model assessment of IR (HOMA-IR) (FINS (uIU/mL) × FBG (mmol/L)/22.5).
2.1. Statistical analysis
Normally distributed variables were expressed as mean ± standard deviation, while variables with a skewed distribution were expressed as medians, along with the upper and lower quartiles. Two-tailed results with P < .05 were considered statistically significant. Multiple liner analysis was used to analysis the correlations between parameters and IR in groups at different ages and gender. All statistical analyses were performed with SPSS 17.0 software (SPSS Inc, Chicago, IL) and Graph Pad Prism software 7.0.
3. Results
In total 13,400 were analyzed in the study excluding those who have missing data. Based on the results of the glucose tolerance test, 10,908 participants (81.4%) with normal blood glucose were grouped into control, 1203 participants (9.0%) into IGT group, and 1289 (9.6%) into diabetes group.
3.1. Clinical characteristics of the groups
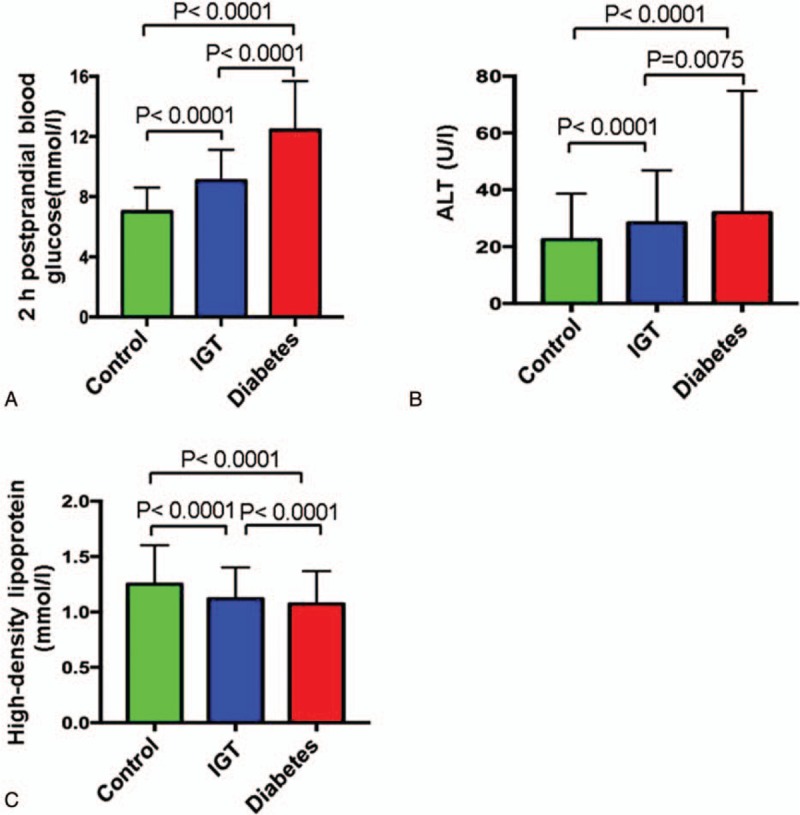
Clinical characteristics of 13,400 participants are shown in Table 1. The mean age and BMI of the participants were 49.50 ± 9.69 years old and 25.24 ± 3.46 kg/m2. The fasting blood glucose level in the diabetic group was increased by 43.9% compared with the control group (Fig. 1A). Compared with the control group, the levels of fasting insulin and 2 hours postprandial insulin in the IGT group were increased by 34.24% and 21.54%, respectively. The low-density lipoprotein levels in all the 3 groups were not significantly different (data not shown). However, the levels of high-density lipoprotein in diabetic patients compared with IGT patients were significantly decreased (Fig. 1B and C). Strikingly, the levels of luteinizing hormone, progesterone, estradiol, follicle stimulating, and prolactin in both the diabetic and IGT groups are lower than that in the control group (Fig. 2A–D). However, testosterone levels in diabetic and IGT patients were respectively higher than those in the control group (Fig. 2D). To investigate what factors will affect the IR index of the people with IGT or normal glucose tolerance, we performed a further regression analysis to see the association between these parameters and IR (Tables 2–6) in general and in different groups based on age and gender. Notably, we found that in general BMI (P < .05), body fat percentage (P = .044), HbA1c (P < .05), LDL (P < .05), cholesterol (CHOL) (P < .05), TG (P < .05), HDL (P < .05), ALT (P < .05), progesterone (P = .022), testosterone (P < .05), TT4 (P = .006), free triiodothyronine 3 (FT3) (P < .05), free thyroxine 4 (FT4) (P < .05) are associated with IR. For women with IGT under 40, body fat percentage (P = .016), HbA1c (P < .05), ALT (P < .05), LDL (P = .031), TPOAb (P = .025) are associated with IR. For women in IGT older than 40, HbA1c (P < .05), ALT (P < .05), FT4 (P = .004) are associated with IR. For men in IGT under 40, HbA1c (P = .024), TG (P = .012), ALT (P = .010) are associated with IR.
Table 1.
Clinical characteristics of 13,400 participants.
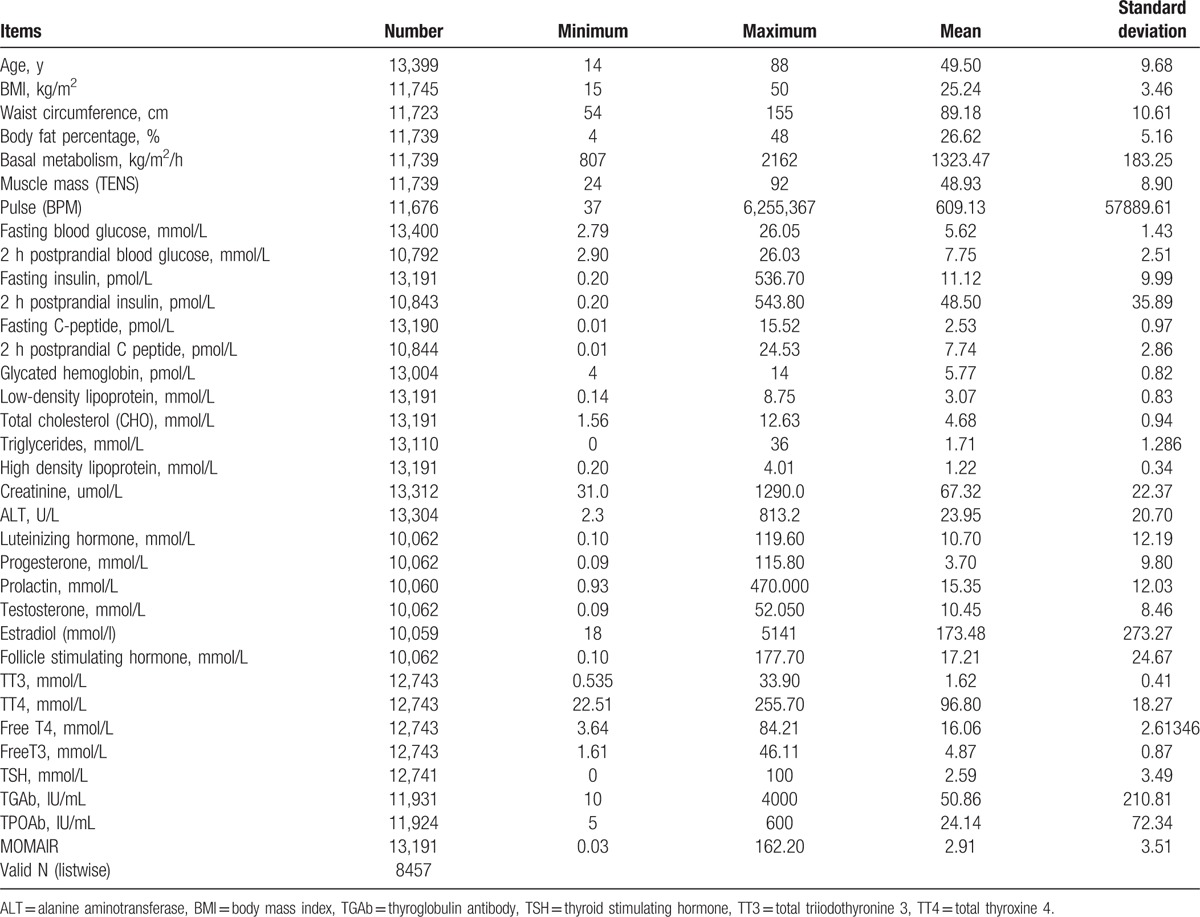
Figure 1.
Comparisons of blood biochemistry tests among 3 groups. A, Two hours postprandial blood glucose. B, Alanine aminotransferase (ALT). C, High-density of lipoprotein.
Figure 2.
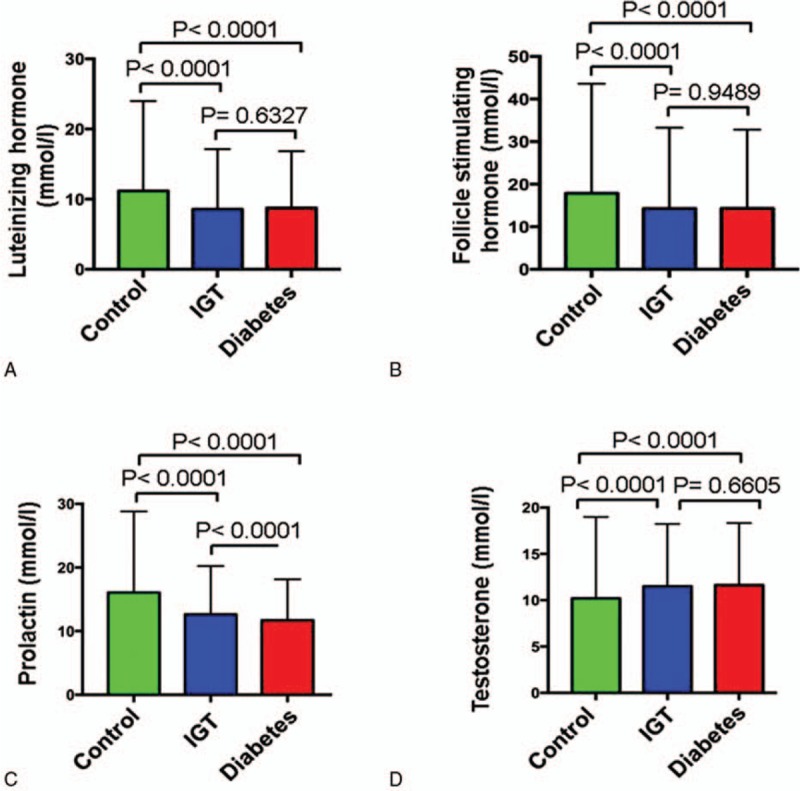
Comparisons of hormone levels among 3 groups. A, Luteinizing hormone. B, Follicle stimulating progesterone. C, Prolactin. D, Testosterone.
Table 2.
Multiple linear regression analysis showed that BMI, body fat percentage, HbA1c, LDL, CHOL, TG, HDL, ALT, progesterone, testosterone, TT4, FT3, and FT4 are significantly associated with IR.
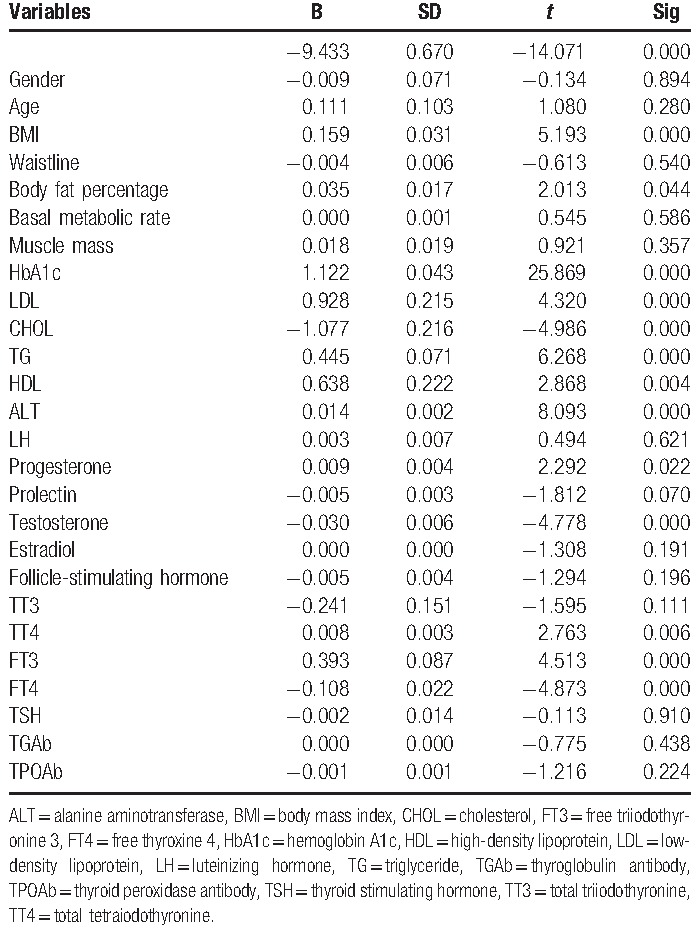
Table 6.
Multiple linear regression analysis showed that for men in IGT more than 40, BMI, HbA1c, LDL, CHOL, TG, ALT, progesterone,TT4, and FT4 are significantly associated with IR.
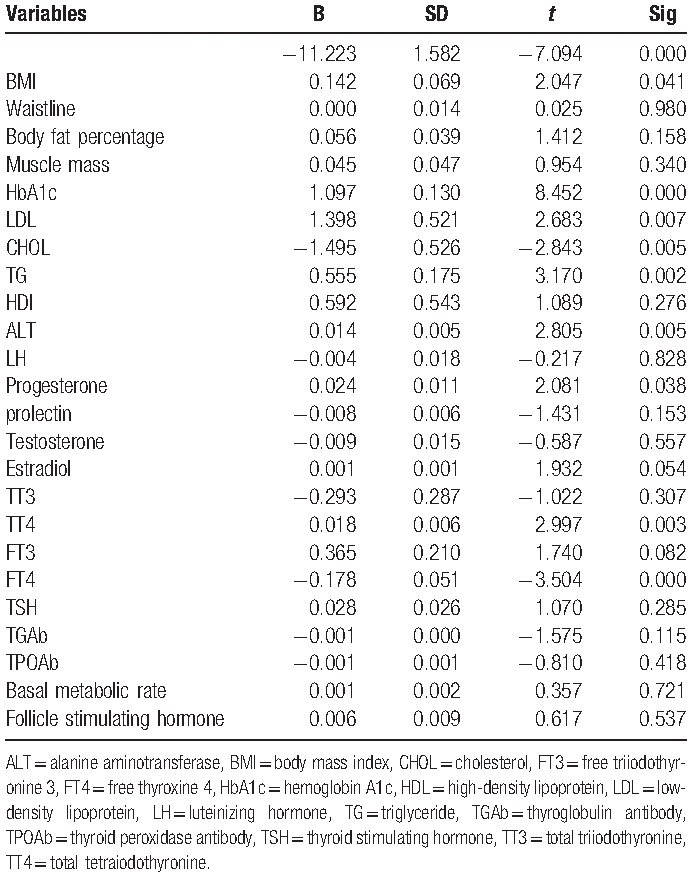
Table 3.
Multiple linear regression analysis showed that for women with IGT under 40, Body fat percentage, HbA1c, ALT, LDL, and TPOAb are significantly associated with IR.
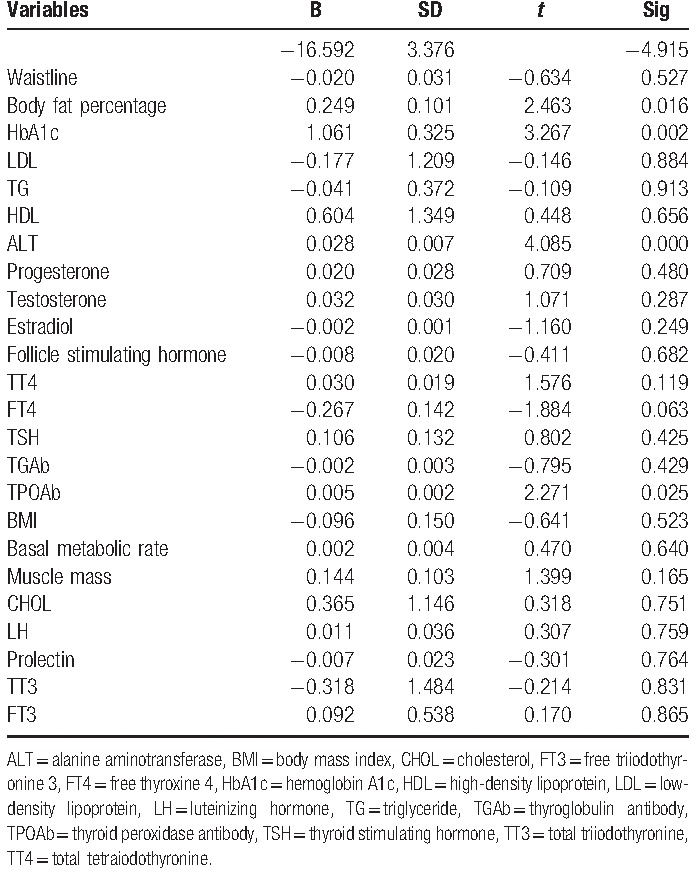
Table 4.
Multiple linear regression analysis showed that for women in IGT older than 40, HbA1c, ALT, and FT4 are significantly associated with IR.
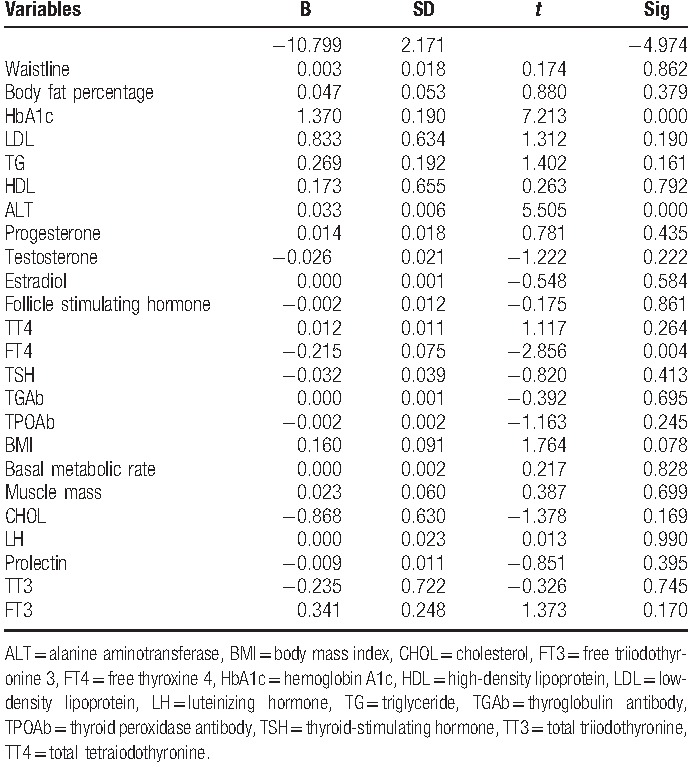
Table 5.
Multiple linear regression analysis showed that for men in IGT under 40, HbA1c, TG, and ALT are significantly associated with IR.
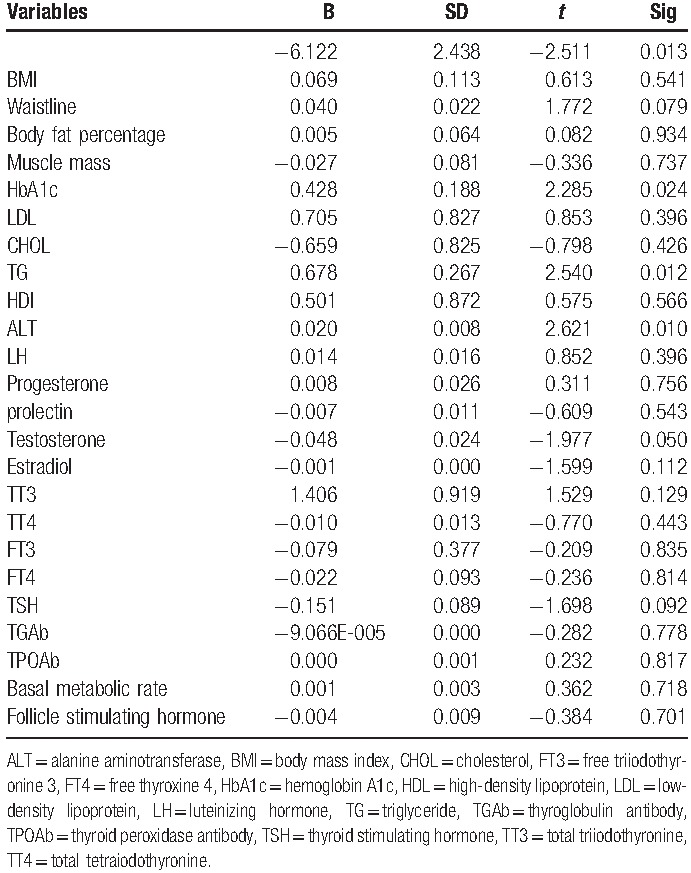
For men in IGT more than 40, BMI (P = .041), HbA1c (P < .05), LDL (P = .007), CHOL (P = .005), TG (P = .002), ALT (P = .005), progesterone (P = .038), TT4 (P = .003), FT4 (P < .05) are associated with IR.
3.2. Insulin, HbA1c, and HOMA-IR increased in both IGT and diabetes groups
The levels of hemoglobin A1c (HbA1c) in both IGT and diabetes groups were significantly higher than those in control group (Fig. 3A). The homeostatic model assessment (HOMA) is a method used to quantify IR and β-cell function, thus, to be an IR index. Significantly, the HMOMA-IR in the diabetes and IGT groups were 62.08% and 46.99% higher than that in control group (Fig. 3B).
Figure 3.
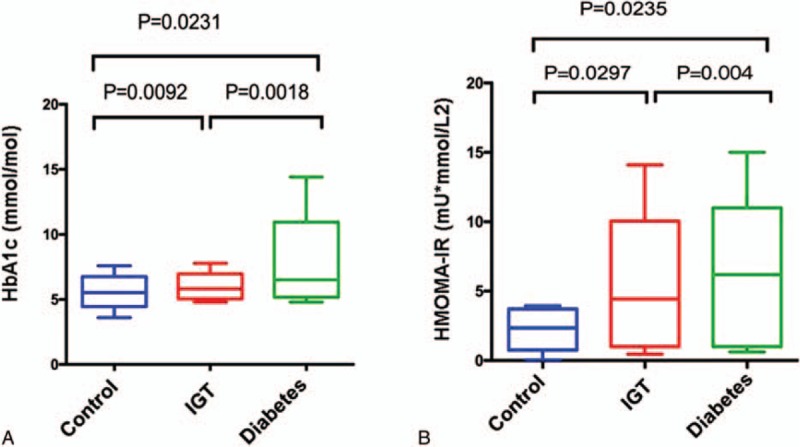
Comparisons of HbA1c (A) and HOMA-IR (B) among 3 groups. HbA1c = hemoglobin A1c, HOMA-IR = homeostasis model assessment of insulin resistance.
3.3. Analysis of IR and abnormal lipid metabolism in T2DM and IGT groups
The total amount of fatty substances, CHOL and TG in diabetes and IGT groups were higher than that in control group (Fig. 4). TG in diabetes and IGT groups showed a 34.71% and 25.12% increase, respectively, compared with control group (Fig. 4B). However, HDL in diabetic and IGT groups had a 14.10% and 6.40% decrease, respectively, compared with control group (Fig. 4C). The results indicated that IR and abnormal lipid metabolism appeared only in IGT and diabetes groups. Significantly, compared with control group, IGT and diabetes groups showed higher TT3, TT4, FT3, and FT4 levels (which is in accordance with the lipid level), indicating the disturbance in the metabolic state (Fig. 5).
Figure 4.
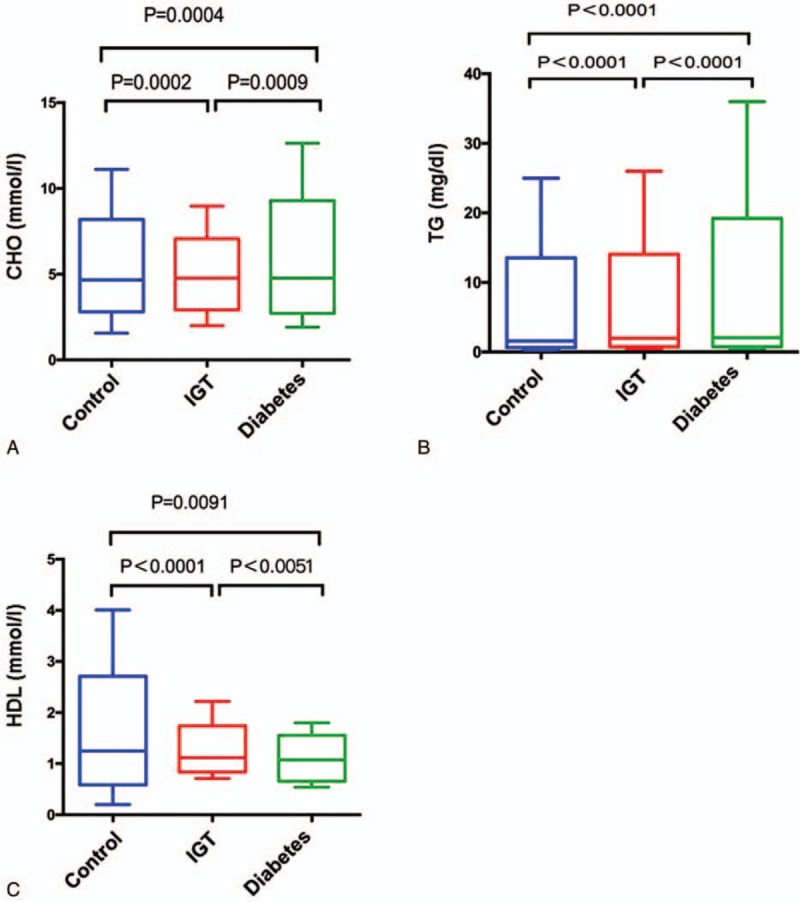
Comparisons of blood CHOL (A), TG (B), and HDL (C) levels among 3 groups. CHOL = cholesterol, HDL = high-density lipoprotein, TG = triglyceride.
Figure 5.
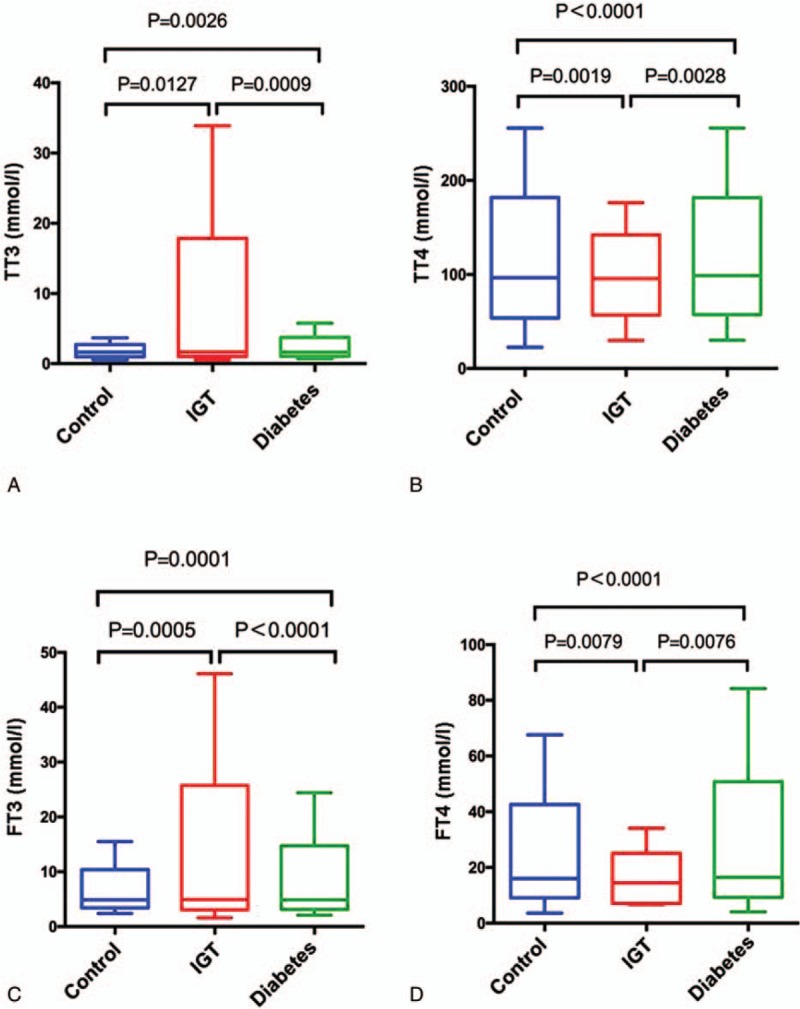
Comparisons of TT3 (A), TT4 (B), FT3 (C), and FT4 (D) levels among 3 groups. FT3 = free triiodothyronine 3, FT4 = free thyroxine 4, TT3 = total triiodothyronine 3, TT4 = total thyroxine 4.
3.4. Metabolism disorder is associated with sex hormone disorders in T2DM and IGT patients
It was reported that levels of plasma testerone were significantly lower in men with diabetics than in man without diabetics. Sex hormone-binding globulin (SHBG) gene polymorphisms may cause a high risk of T2DM through its impact on testosterone and estradiol levels. A prospective cohort study[11] also demonstrated that higher SHBG and lower calculated free testosterone (cFT) levels were risk factors for all causes of mortality in men with type 2 diabetes mellitus, which could be influenced by the SHBG, a carrier protein of testosterone. Both progesterone and estradiol levels were significantly decreased in diabetes mellitus and IGT patients compared with normal control participants (Figs. 2 and 6). Progesterone levels decreased around 55.84% and 53.74%, respectively, in diabetes and IGT groups. Estradiol levels decreased around 39.70% and 42.62% in diabetes and IGT groups, respectively.
Figure 6.
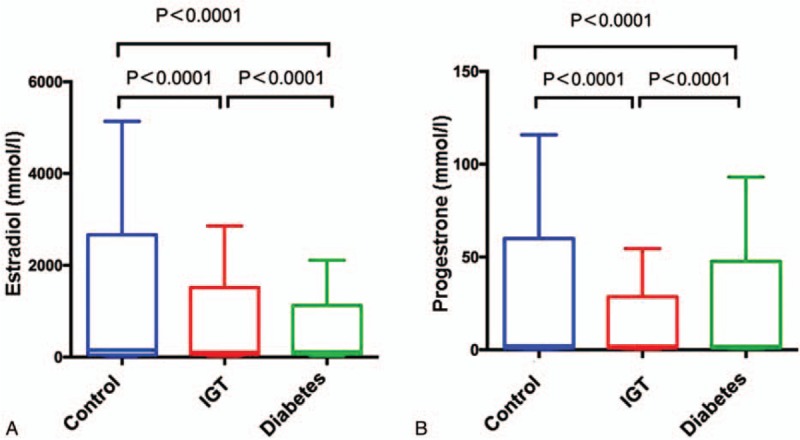
Comparisons of estradiol (A) and progesterone (B) levels among 3 groups.
4. Discussion
IR is closely associated with diabetes mellitus, thus, the homeostatic model assessment was accounted for IR (HOMA-IR), which is a function of the product of fasting insulin and glucose levels.[12] We used levels of fasting insulin, 2 hours postprandial insulin, and HOMA-IR to estimate IR in all the participants. HbA1c and fasting blood glucose were also used to evaluate the state of glucose metabolism. Patients with insulin resistance require increasing levels of insulin to maintain normal glucose levels and are likely to progress to type 2 diabetes mellitus.[13] The IGT group consists of prediabetic patients. In our study, we observed significantly higher blood glucose and hyperinsulinemia in both T2DM and IGT patients compared with the normal control group, indicating that disorder of glucose metabolism occurred in the T2DM and prediabetic patients.
A common feature of type 2 diabetes is the dysregulation of lipid metabolism. In our study, we found that diabetes mellitus patients and prediabetic patients with IGT showed significantly increasing amounts of fatty substances, CHOL, and TG with, however, a dramatically decreasing amount of HDL compared with normal control people. This indicates that the association of IR and abnormal lipid metabolism occurred in T2DM and prediabetic patients.
One effect of insulin resistance is enhanced glucose output from the liver. In contrast, triglyceride synthesis and very-low-density lipoproteins (VLDL) release are enhanced. The intracellular accumulation of lipids triggers activation of novel protein kinases C with subsequent impairments in insulin signaling. In the liver, insulin normally stimulates glucose uptake, glucose oxidation, and glycogen synthesis; suppresses gluconeogenesis; and stimulates triglyceride synthesis and secretion via VLDLs.[14] And conversely, studies reported that reduction of free fatty acid (FFA) levels represented a selective target for modulating insulin resistance.[15]
We also found that the levels of TT3, TT4, FT3, and FT4 in T2DM and IGT patients were significantly higher than those in normal control people. This indicated that disturbance of the lipid metabolism status occurred in T2DM and IGT patients, since the levels of TT3, TT4, FT3, and FT4 were in accordance with lipid levels in patients.
Several groups of researchers have studied the association between sex hormone and glucose metabolism. These studies have divided subjects by genders, and showed that testosterone was a protective factor for men while estradiol was a protective factor for women in type 2 diabetic patients.[10] It has also been reported that testosterone levels were lower in men with metabolic syndrome and type 2 diabetes mellitus, and the report also predicted the onset of this adverse metabolic status.[16] Several animal models were used to investigate the mechanism.[17] The study showed that SHBG gene polymorphisms could be the cause of high risk factors in T2DM patients through their impact on testosterone and estradiol levels.[14] A prospective cohort study also demonstrated that higher levels of SHBG and lower levels of cFT were risk factors in the main cause of mortality in men with type 2 diabetes mellitus. This could be influenced by the SHBG, which is the carrier protein of testosterone.[18]
Our results showed both progesterone and estradiol were significantly decreased in T2DM and IGT patients, which is consistent with the results in previous studies.[5,11] Meanwhile, Baradaran et al[19] found an inverse correlation between serum estradiol concentration and carotid atherosclerosis by using ultrasonography to evaluate the intimamedia thickness (IMT) in men with type 2 diabetes mellitus.
Whether hormone replacement therapy (HRT) could decrease the effects of insulin resistance? Different studies have resulted in different conclusions. Fukui et al[20] showed that estrogen and progesteron could decrease insulin sensitivity, while studies from other groups showed that there was no influence on the insulin sensitivity.[21,22] The population, type, and route of administration of the hormone therapy, as well as the method used for measuring insulin sensitivity, may explain the controversial results of estrogen replacement on glucose metabolism. Bitoska et al[23] studied 40 women in natural menopause with type 2 diabetes. They showed that HRT can decrease HOMA-IR, fasting plasma glucose (FPG), and insulinemia in type 2 diabetes.[23] This indicates that HRT is associated with a statistically significant increase of insulin sensitivity. To better understand the association between sex hormone, insulin resistance, and glucose homeostasis in diabetic patients, larger clinical prospective trials would be needed. The study of lipid-lowering, hypoglycemic drug sensitivities of patients with different levels of sex hormones could be a follow-up study.
Footnotes
Abbreviations: ALT = alanine aminotransferase, BMI = body mass index, cFT = calculated free testosterone, CHOL = cholesterol, CVD = cardiovascular disease, FBG = fasting blood glucose, FFA = free fatty acid, FINS = fasting insulin, FPG = fasting plasma glucose, FSH = follicle stimulating hormone, HbA1c = hemoglobin A1c, HDL = high-density lipoprotein, HOMA = homeostatic model assessment, HOMA-IR = homeostasis model assessment of insulin resistance, HRT = hormone replacement therapy, IGT = impaired glucose tolerance, IMT = intima-media thickness, IR = insulin resistance, LDL = low-density lipoprotein, LH = luteinizing hormone, OGTT = oral glucose tolerance test, SHBG = sex hormone-binding globulin, T2DM = type 2 diabetes mellitus, TG = triglyceride, TGAb = thyroglobulin antibody, TPOAb = thyroid peroxidase antibody, TSH = thyroid stimulating hormone, TT3 = total triiodothyronine 3, TT4 = total thyroxine 4, VLDL = very-low-density lipoproteins.
The authors have no conflicts of interest to disclose.
References
- [1].Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev 2013;93:137–88. [DOI] [PubMed] [Google Scholar]
- [2].Walatara KN, Athiththan LV, Hettiaratchi UK, et al. Effect of demographic status and lifestyle habits on glycaemic levels in apparently healthy subjects: a cross-sectional study. J Diabetes Res 2016;2016:5240503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].D A. International diabetes federation. Press Release, Cape Town, South Africa 2006; 4. [Google Scholar]
- [4].Patel TP, Rawal K, Bagchi AK, et al. Insulin resistance: an additional risk factor in the pathogenesis of cardiovascular disease in type 2 diabetes. Heart Fail Rev 2016;21:11–23. [DOI] [PubMed] [Google Scholar]
- [5].Laakso M. Is insulin resistance a feature of or a primary risk factor for cardiovascular disease? Curr Diab Rep 2015;15:105. [DOI] [PubMed] [Google Scholar]
- [6].Nadal A, Alonso-Magdalena P, Soriano S, et al. The pancreatic beta-cell as a target of estrogens and xenoestrogens: implications for blood glucose homeostasis and diabetes. Mol Cell Endocrinol 2009;304:63–8. [DOI] [PubMed] [Google Scholar]
- [7].Liu RT, Chung MS, Wang PW, et al. The prevalence and predictors of androgen deficiency in Taiwanese men with type 2 diabetes. Urology 2013;82:124–9. [DOI] [PubMed] [Google Scholar]
- [8].Juang PS, Peng S, Allehmazedeh K, et al. Testosterone with dutasteride, but not anastrazole, improves insulin sensitivity in young obese men: a randomized controlled trial. J Sex Med 2014;11:563–73. [DOI] [PubMed] [Google Scholar]
- [9].Chao J, Rubinow KB, Kratz M, et al. Short-term estrogen withdrawal increases adiposity in healthy men. J Clin Endocrinol Metab 2016;101:3724–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Allan CA. Sex steroids and glucose metabolism. Asian J Androl 2014;16:232–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Feigerlova E, Saulnier PJ, Gourdy P, et al. Sex hormone levels are not associated with progression of renal disease in male patients with T2DM. Diabetes Metab 2016;43:140–5. [DOI] [PubMed] [Google Scholar]
- [12].Song Y, Manson JE, Tinker L, et al. Insulin sensitivity and insulin secretion determined by homeostasis model assessment and risk of diabetes in a multiethnic cohort of women: the Women's Health Initiative Observational Study. Diabetes Care 2007;30:1747–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- [14].Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet 2010;375:2267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Montecucco F, Steffens S, Mach F. Insulin resistance: a proinflammatory state mediated by lipid-induced signaling dysfunction and involved in atherosclerotic plaque instability. Mediators Inflamm 2008;2008:767623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zuo Z, Wu T, Lin M, et al. Chronic exposure to tributyltin chloride induces pancreatic islet cell apoptosis and disrupts glucose homeostasis in male mice. Environ Sci Technol 2014;48:5179–86. [DOI] [PubMed] [Google Scholar]
- [17].Tint AN, Hoermann R, Wong H, et al. Association of sex hormone-binding globulin and free testosterone with mortality in men with type 2 diabetes mellitus. Eur J Endocrinol 2016;174:59–68. [DOI] [PubMed] [Google Scholar]
- [18].Shin JY, Park EK, Park BJ, et al. High-normal glucose levels in non-diabetic and pre-diabetic men are associated with decreased testosterone levels. Korean J Fam Med 2012;33:152–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Baradaran N, Ahmadi H, Salem S, et al. The protective effect of diabetes mellitus against prostate cancer: role of sex hormones. Prostate 2009;69:1744–50. [DOI] [PubMed] [Google Scholar]
- [20].Fukui M, Kitagawa Y, Kamiuchi K, et al. Association between serum estradiol concentrations and carotid atherosclerosis in men with type 2 diabetes mellitus. Metabolism 2008;57:285–9. [DOI] [PubMed] [Google Scholar]
- [21].Sites CK, Brochu M, Tchernof A, et al. Relationship between hormone replacement therapy use with body fat distribution and insulin sensitivity in obese postmenopausal women. Metabolism 2001;50:835–40. [DOI] [PubMed] [Google Scholar]
- [22].Brussaard HE, Gevers Leuven JA, Frolich M, et al. Short-term oestrogen replacement therapy improves insulin resistance, lipids and fibrinolysis in postmenopausal women with NIDDM. Diabetologia 1997;40:843–9. [DOI] [PubMed] [Google Scholar]
- [23].Bitoska I, Krstevska B, Milenkovic T, et al. Effects of hormone replacement therapy on insulin resistance in postmenopausal diabetic women. Open Access Maced J Med Sci 2016;4:83–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


