Abstract
Background:
Autologous hematopoietic stem cell transplantation (HSCT) has been proposed for patients with refractory Crohn's disease (CD), but it is associated with mortality and adverse events; the balance between risks and benefits becomes significantly important in the therapy. The aim of the study was to assess the efficacy and safety of autologous HSCT therapy for refractory CD.
Methods:
We conducted a comprehensive search of PubMed, Embase, the Cochrane library, and Web of Science from inception to February 2017. The pooled estimate rates for efficacy and safety of refractory CD was performed by meta-analysis and reported according to the standard Cochrane guidelines and the PRISMA statement.
Results:
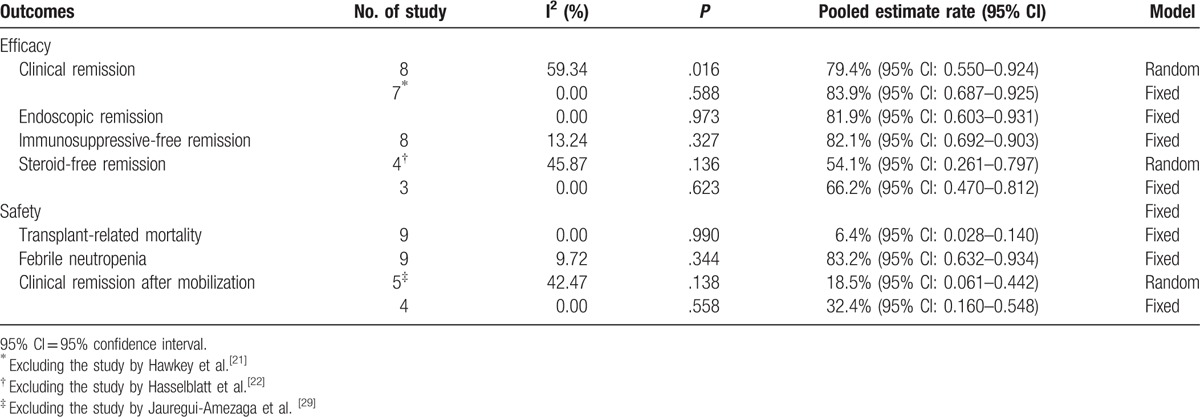
Four prospective uncontrolled cohort studies, 4 prospective case series, and 1 randomized controlled trial (RCT) were included. Autologous HSCT had a high rate of clinical and endoscopic remission in refractory CD [79.4%, 95% confidence interval (95% CI): 0.550–0.924; 81.9%, 95% CI: 0.603–0.931, respectively]. In the case of safety, it had a relatively high incidence rate of transplant-related mortality (6.4%, 95% CI: 0.028–0.140). A significant association was observed between autologous HSCT and the incidence of febrile neutropenia (83.2%, 95% CI: 0.632–0.934). About 18.5% (95% CI: 0.061–0.442) of patients with refractory CD reached clinical remission at mobilization phase. Besides, 82.1% (95% CI: 0.692–0.903) and 54.1% (95% CI: 0.261–0.797) patients with refractory CD could achieve immunosuppressive-free and steroid-free remission for at least 12 months after the therapy.
Conclusion:
Autologous HSCT could be a complicated treatment with relatively high mortality and significantly high efficacy for refractory CD, which should be used with caution. However, more RCTs of larger samples using refined and standardized protocols and longer period of follow-up time are needed to further assess the outcomes of autologous HSCT therapy.
Keywords: autologous, Crohn's disease, hematopoietic stem cell transplantation, inflammatory bowel disease, meta-analysis, refractory, stem cell therapy
1. Introduction
Crohn's disease (CD) is one of the 2 main disease categories of inflammatory bowel disease (IBD), which is characterized by chronic and relapsing intestinal inflammation and noncaseating granulomas. Although its mechanism has been believed that the interaction of environmental factors, including intestinal microbiota, with the host immune system in genetically susceptible individuals may be an important cause, the specific mechanism of the initiation of this disease is still incompletely unclear[1–3]; as a result, these therapies fail to completely improve symptoms and reduce the inflammatory process fundamentally. Moreover, a large proportion of patients with CD would be refractory to conventional medications (generally aminosalicylate anti-inflammatory drugs, corticosteroids, and perhaps biologics) within a reasonable time.[4,5] Surgery may be required for refractory patients if necessary; however, it cannot cure CD. It involves removing the diseased part of the intestine and rejoining the healthy ends, but the disease tends to recur after surgery.[6,7] Once refractory CD develops, patients would be at the risk of no more drugs to be managed.
In 1998, Lopez-Cubero et al[8] investigated hematopoietic stem cell transplantation (HSCT) in CD from the experience described in autoimmune diseases. Four of 5 patients followed up of 4.5 to 15.3 years had remained free of CD, suggesting that host immune dysregulation of CD can be corrected by HSCT. Subsequently, HSCT has emerged as a promising therapy, especially for poorly responsive CD patients to conventional treatments.[9,10] HSCT is the transplantation of multipotent hematopoietic stem cells (HSCs), which can be categorized into autologous (the patient's own stem cells), allogeneic (the stem cells come from a donor), or syngeneic (from an identical twin) according to the source of HSC.[11,12] Allogeneic HSCT is reserved for patients with life-threatening diseases, such as multiple myeloma or leukemia, due to its complicated and dangerous procedure with many possible complications not only for the recipients, including veno-occlusive disease, mucositis, infections (sepsis), graft-versus-host disease (GVHD), and the development of new malignancies, but also for the donors, including drug risks (filgrastim), access risks (jugular/subclavian/femoral veins are used), and certain severe adverse events (AEs, pulmonary edema/deep vein thrombosis, splenic rupture, and myocardial infarction).
Compared with allogeneic HSCT with above risks, especially for refractory CD, autologous HSCT would exempt the recipient from certain disease of donor associated with a susceptibility gene,[13] which can successfully avoid the problems on HLA-match.
Since the 1990s, there have been several case reports or case series documenting improvements of CD when undergoing HSCT for hematologic malignancies,[8,14–16] followed by clinical trials investigating efficacy and safety of autologous HSCT in refractory CD.[2,3,8] However, given the previous studies of a small sample size, the apparent advantages of autologous HSCT would be challenged. Besides, the mortality and other AEs of autologous HSCT need a comprehensive investigation. Therefore, the aim of this meta-analysis was to assess the efficacy and safety of autologous HSCT for refractory CD.
2. Methods
2.1. Search strategy
The systematic review and meta-analysis were performed and reported according to the standard Cochrane guidelines and the PRISMA statement.[17,18] We conducted electronic searches without language restriction of PubMed, Embase, the Cochrane library, and Web of Science from inception to February 2017. The search strategy of this study was a combination of “autologous” AND (“hematopoietic stem cell” OR “haematopoietic stem cell”) AND (“transplantation” OR “transplant” OR “therapy”) AND “refractory” AND (“Crohn's disease” OR “Crohn's disease”). Manual searches of reference lists from relevant papers were also conducted to identify additional studies that may have been missed in the database search. Using the Endnote X7 constructed the library of studies and automatically excluded the duplicates.
2.2. Inclusion and exclusion criteria
A Population and Intervention (regarded as modified PICOS) question was designed to determine the strict inclusion and exclusion criteria before the literature search. Studies were selected on the basis of the following inclusion criteria: human studies; included patients with refractory CD; HSCTs were used for treatment of refractory CD; and efficacy and AEs were reported. The studies that were published as case reports, reviews, letters, or conference abstracts were excluded.
2.3. Data extraction
Two reviewers (Xiao Qiu and Jue-Rong Feng) extracted data independently from the included studies using standardized data extraction forms. Data included author; year and country of publication; type of study; sample size; age of the patient; conditioning regimen; procedure of mobilization and CD34+ cell selection; efficacy outcomes and AEs; prognosis; and time of follow-up. Discrepancies in eligibility or data extraction were resolved through discussion, and rereview of the studies and consultation with one other author when necessary.
2.4. Statistical analysis
To assess autologous HSCT, the primary outcomes of efficacy in this study were the pooled estimate proportions of clinical and endoscopic remission after autologous HSCT for refractory CD patients. Secondary outcomes of efficacy included steroid-free and immunosuppressive-free remission. The primary outcome of safety was transplant-related mortality (TRM). Secondary outcomes of safety were febrile neutropenia and other relevant AEs. In addition, to assess the mobilization phase of autologous HSCT, the outcome of efficacy was the pooled estimate proportion of clinical remission, and the outcomes of safety were AEs.
We estimated aforementioned outcomes under the fixed and random effects model. Heterogeneity was assessed by Cochran Q test and Chi-square test.[19] More specifically, the I2 statistic was used to estimate inconsistency in meta-analyses, which indicates the inconsistency between studies due to heterogeneity rather than chance.[19] In the Q test, a P value of <.1 was deemed statistically significant. The I2 method was used to assess for degree of heterogeneity, with a score discrimination of 0% to 40%, 30% to 60%, 50% to 90%, and 75% to 100% consistent with low, moderate, substantial, and considerable heterogeneity, respectively.[20] In cases of moderate or high heterogeneity, each study was rereviewed to identify whether any discrepancy could be identified, and sensitivity analysis was performed by excluding 1 study at a time to reflect influence of individual study on pooled proportion. All statistical analyses were performed using Comprehensive Meta-analysis (Biostat, Englewood, NJ).
Meta-analysis is a systematic review based on previous studies and the ethical approval is not necessary.
3. Results
3.1. Search results
The initial search strategy yielded 82 abstracts for review, of which 70 studies were selected for a detailed review and 9 studies met the inclusion criteria involving 97 adult patients (Fig. 1). This included 4 prospective uncontrolled cohort studies and 4 prospective case series. In addition, 1 randomized controlled trial (RCT) was identified and included in this review. No meta-analyses of autologous HSCT for refractory CD were identified. The characteristics of each included study are presented in Table 1. [21–29]
Figure 1.
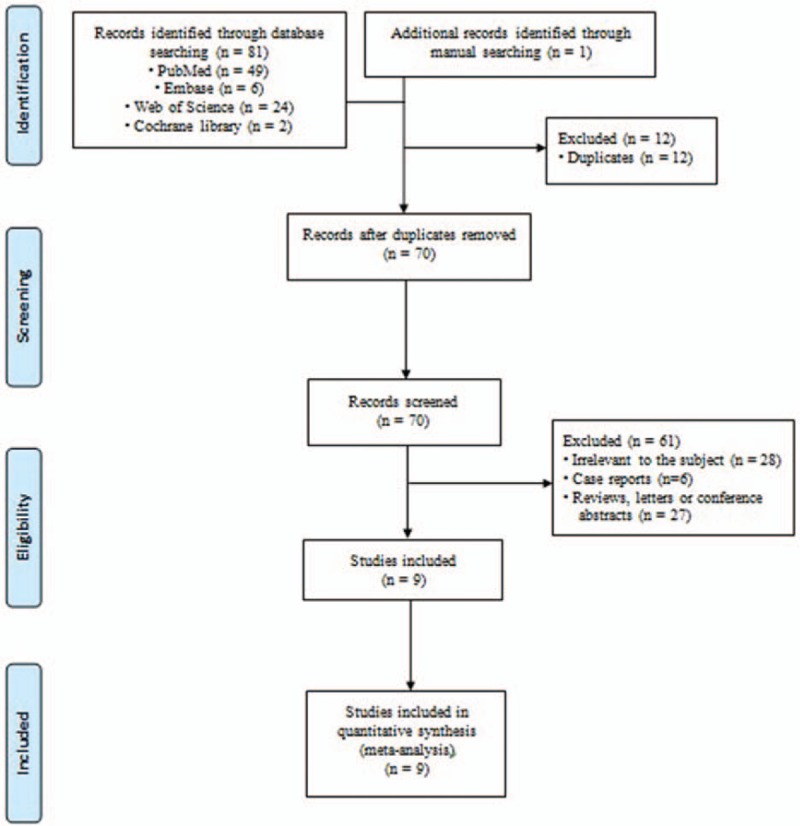
Flow chart of the selection of studies for inclusion in the meta-analysis.
Table 1.
Characteristics of the included studies.
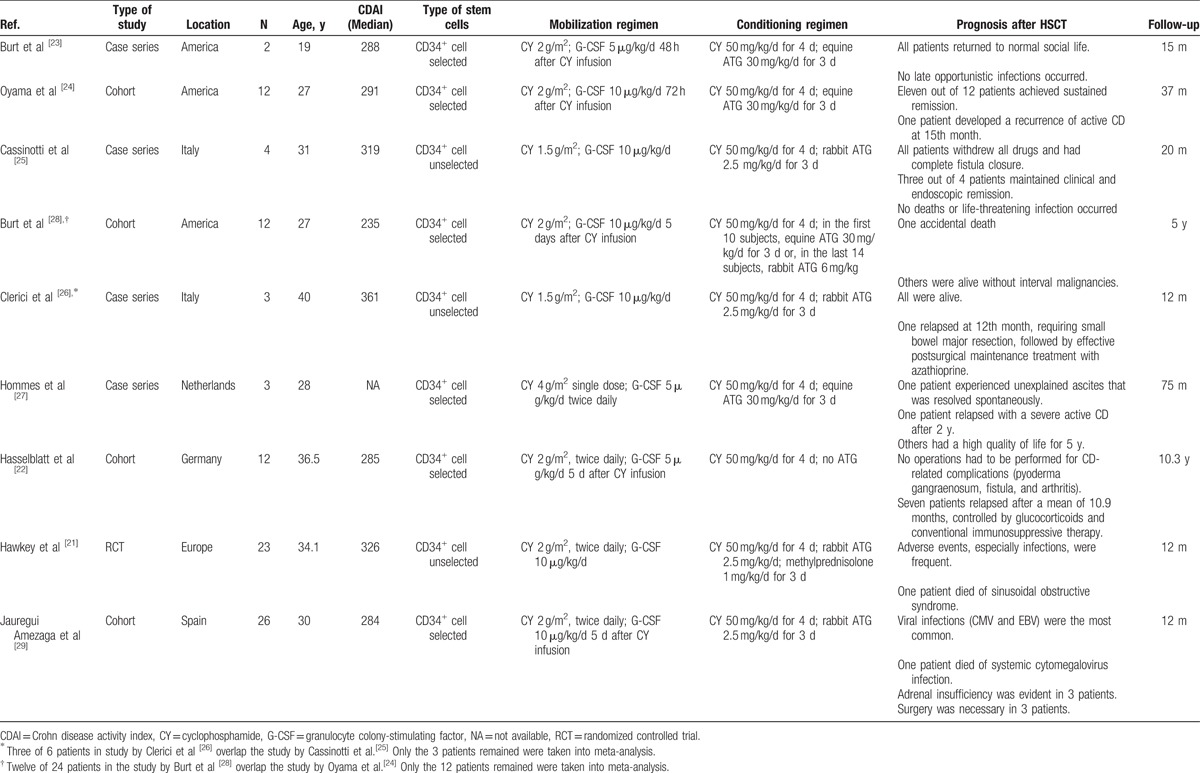
3.2. Efficacy of autologous HSCT therapy for refractory CD
Efficacy was regarded as the following outcomes: clinical remission, endoscopic remission, steroid-free, and immunosuppressive-free remission after autologous HSCT.
3.2.1. Primary outcomes—clinical remission
Clinical remission was defined by a CDAI <150 at the end of treatment. Clinical remission was reported in 8 trials involving 68 patients. The pooled estimate rate of clinical remission was 79.4% [95% confidence interval (95% CI): 0.550–0.924] (Fig. 2). There was a moderate heterogeneity between studies (P = .016, I2 = 59.34%).
Figure 2.
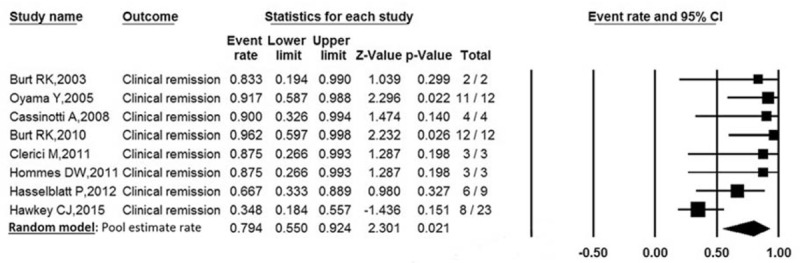
Forest plot of pooled estimate rate for clinical remission after autologous HSCT.
Sensitivity analyses found that it could eliminate heterogeneity between studies by excluding the 1 study by Hawkey et al.[21] The inconsistency may exist due to this study design of randomized clinical trial. After removing the study, the pooled estimate rate was 83.9% (95% CI: 0.687–0.925, P = .588, I2 = 0%).
3.2.2. Primary outcomes—endoscopic remission
Endoscopic remission was defined as endoscopic improvement or complete absence of mucosal lesions at the end of treatment. Endoscopic remission was reported in 5 trials involving 19 patients. The pooled estimate rate for endoscopic remission was 81.9% (95% CI: 0.603–0.931) (Fig. 3). There was no heterogeneity between studies (P = .973, I2 = 0%).
Figure 3.
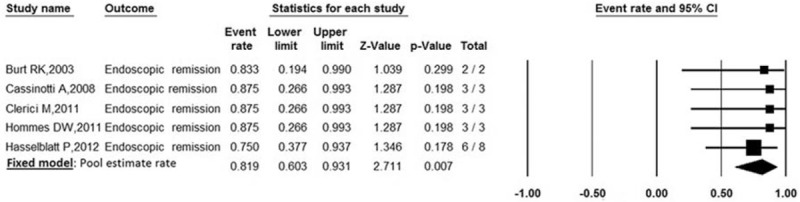
Forest plot of pooled estimate rate for endoscopic remission after autologous HSCT.
3.2.3. Secondary outcomes—steroid-free remission
Steroid-free remission was defined as CD patients maintaining remission without corticosteroids. Steroid-free remission was reported in 4 trials involving 37 patients. The pooled estimate rate was 54.1% (95% CI: 0.261–0.797) (Table 2). There was a moderate heterogeneity between studies (P = .136, I2 = 45.87%).
Table 2.
Pooled effects of autologous hematopoietic stem cell therapy for refractory Crohn's disease by meta-analysis.
Sensitivity analyses found that it could eliminate heterogeneity between studies by excluding the study by Hasselblatt et al.[22] Stem cell mobilization and a follow-up of 10.3 years from the study were obviously different from other studies. After removing the study, the pooled estimate rate was 66.2% (95% CI: 0.470–0.812, P = .623, I2 = 0%).
3.2.4. Secondary outcomes - immunosuppressive-free remission
Immunosuppressive-free remission was defined as CD patients maintaining remission without immunosuppressive drugs. Immunosuppressive-free remission was reported in 8 trials involving 68 patients. The pooled estimate rate was 82.1% (95% CI: 0.692–0.903) (Table 2). There was a low heterogeneity between studies (P = .327, I2 = 13.24%).
3.3. Safety of autologous HSCT therapy for refractory CD
AEs during autologous HSCT and follow-up period can be mainly documented, such as TRM, febrile neutropenia, infectious AEs, and other noninfectious AEs.
3.3.1. Primary outcomes—TRM
Data on TRM were available for all included trials involving 89 patients. The pooled estimate rate for TRM was 6.4% (95% CI: 0.028–0.140) (Fig. 4). Significant homogeneity was observed among the studies (P = .990, I2 = 0%).
Figure 4.
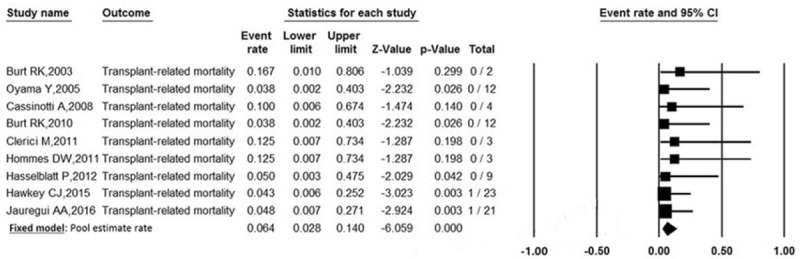
Forest plot of pooled estimate rate for TRM after autologous HSCT.
3.3.2. Secondary outcomes—febrile neutropenia
The most common AE is febrile neutropenia, which was reported in all studies. However, data on febrile neutropenia were available in 4 trials involving 34 patients. The pooled estimate rate was 83.2% (95% CI: 0.632–0.934) (Table 2). There was a low heterogeneity between studies (P = .344, I2 = 9.72%).
3.3.3. Secondary outcomes—other AEs
In addition to TRM and febrile neutropenia, 6 of 9 studies reported no unexpected severe AEs or serious life-threatening complications.[22–27] Infection was frequent during autologous HSCT or the first year after transplantation. As far as virus infection is concerned, Epstein–Barr virus, cytomegalovirus, and BK virus were reported to occur in the first year after transplantation.[21,25,28,29] In addition, there were some other infectious AEs documenting in several studies, such as pneumonia, bronchitis, and urinary tract infection. Among noninfectious AEs, allergic reaction to ATG (fever and hypotension) was reported in 2 studies. Besides, 2 studies reported acute renal failure, one of which was related to septic shock.[24,29] Included studies also documented that gastrointestinal symptoms, such as nausea, vomiting, diarrhea, and abdominal pain, were more often in patients.
3.4. Efficacy of mobilization phase in autologous HSCT
Available data did not allow us to assess the endoscopic and medication-free remission after mobilization. Data on clinical remission were available in 5 trials involving 48 patients. The pooled estimate rate was 18.5% (95% CI: 0.061–0.442) (Table 2). There was a moderate heterogeneity between studies (P = .138, I2 = 42.47%).
Sensitivity analyses found that it could eliminate heterogeneity between studies by excluding the study by Jauregui-Amezaga et al. [29] After removing the study, the pooled estimate rate was 32.4% (95%CI: 0.160–0.548, P = 0.558, I2 = 0%).
3.5. Safety of mobilization phase in autologous HSCT
Available data did not allow us to assess the safety of mobilization. The study by Oyama et al[24] reported no mobilization-related disease flares or infections. Minor AEs were common in another two studies, such as nausea, headache and arthralgia, one patient of which developed a transient deterioration of kidney function due to decreased oral intake because of nausea, which responded rapidly to intravenous fluids.[25,27] In addition, Jauregui-Amezaga et al[29] reported that 16 of 26 patients suffered febrile neutropenia. The RCT by Hawkey et al[21] reported 17 serious AEs in 11 of 23 patients, which mainly included infectious AEs and gastrointestinal AEs. It is encouraging that no death occurred during mobilization in all 9 studies.
4. Discussion
It was the first meta-analysis that comprehensively summarized the efficacy and safety of autologous HSCT for refractory CD. Our analysis assessed the efficacy of autologous HSCT primarily through clinical and endoscopic remission, then further through immunosuppressive-free and steroid-free remission. The study confirmed that autologous HSCT had a high rate of clinical and endoscopic remission in refractory CD, 82.1% and 54.1% patients could reach medication-free remission for at least 12 months after autologous HSCT, and their relapses were treated successfully with previous medication that they had been nonresponsive to, indicating that autologous HSCT is quite an attractive option in patients with refractory CD. However, in the case of safety, it had a relatively high incidence rate of TRM. Besides, a significant association was observed between autologous HSCT and the incidence of febrile neutropenia, which may be related to acute inflammatory reactions by a subset of patients to particular preparations of HSCs.[30] Interestingly, we found that 18.5% of patients with refractory CD received clinical remission at mobilization phase.
Autologous HSCT mainly includes 3 stages, including mobilization, conditioning, and transplantation phase. No data are available with respect to the mechanisms of autologous HSCT in refractory CD. This is partly because of the scarcity of studies performed until now. In theory, HSCT could “reset” the immune system by eliminating self-reactive T-lymphocytes and memory cells so as to allow the transplanted stem cells to develop into self-tolerant lymphocytes such as CD4+CD25+FoxP3+ T-reg cells.[14,31] There is also evidence that suppressing Th17 response contributes to a decrease in CD activity.[32] As a result of resetting the immune system, patients can achieve clinical remission with medications that they were previously unresponsive to.[33]
Importantly, patients with refractory CD could be in stage of medication-free remission. For instance, steroid-treated of long time is at a high risk of developing adrenal insufficiency. Interestingly, we found that 18.5% of patients with refractory CD received clinical remission at mobilization phase, which was associated with cyclophosphamide and granulocyte colony-stimulating factor (G-CSF) that can modulate T-cell and Th2 function.[34–41] Therefore, the stem cell mobilization by cyclophosphamide and G-CSF could help improve the efficacy of autologous HSCT to a certain extent, but could not be taken alone without autologous HSCT. Definitive evidence regarding the efficacy of mobilization will be provided by the prospective randomized multicenter ASTIC trial, determining whether there is a potential clinical benefit of mobilization followed by HSCT versus mobilization therapy alone.[42,43] In general, the stem cell mobilization phase was safe enough to permit patients to enter the conditioning and transplant phase.
Unfortunately, transplant-related death was seen in 2 patients, resulting from systemic cytomegalovirus infection and sinusoidal obstructive syndrome, respectively.[21,29] In addition, several AEs occurred during HSCT and follow-up period as well. First, in the case of TRM, we believed that the relatively high mortality may be partly due to the small sample size of clinical trials. Second, AEs and TRM may be specific to CD, as the immunity of patients with severe active CD may be already weakened by both the disease and long-term use of immunosuppressants, which could increase the toxicity that results from conditioning and transplantation.[44,45] Thus, the careful selection and screening of CD patients before transplantation is be of great importance, as it is associated with the outcomes of the patients to some extent. Furthermore, regimen-related AEs can be serious, mainly including bacterial and viral infections.[22,24,25,27,29,46] Immunosuppressive therapies during mobilization phase can also result in hepatotoxicity, pancreatitis, and pancytopenia.[28] Besides, patients who have been previously treated with steroid for long periods of time are at a high risk of developing adrenal insufficiency.[29] Therefore, it seems that the use of drug and the dosage in mobilization and conditioning regimens plays an important role in the mechanism of AEs and TRM. The mobilization regimen recommended by the European Group for Blood and Marrow Transplantation (EBMT) is cyclophosphamide (2–4 g/m2) along with uromitexan and careful hyperhydration, followed by administration of G-CSF (5–10 μg/kg).[46] The conditioning regimen used in HSCT varies considerably; however, a consensus has not yet been reached.
Among the 9 included studies, only 1 was a randomized controlled trail, which suggested that autologous HSCT, compared with conventional therapy, did not result in a statistically significant improvement in sustained disease remission at 1 year. It is important to note that the author chose a highly stringent primary end point requiring patients be off all immunosuppressive drugs, with CDAI less than 150, no active treatment, and free of active disease on imaging, which has not been used in any previous study, thus resulting in no statistically significant difference in sustained disease remission between HSCT and conventional therapy.[21,47–49] Taking this into account, we choose the individual components of the primary composite outcome for our analysis, such as CDAI<150, which is consistent with the majority of clinical trials. In general, even if RCTs are considered the best way for assessing a treatment effect, it is necessary to choose a meaningful end point for treatment group of patients with severe disease, so as to evaluate the clinical efficacy objectively. Moreover, we hold the opinion that patients who had continuing refractory CD treated with conventional medical therapy are not perfect controls, owing to a poor outcome despite its use. In addition, it is known to us all that transplant is a way of treating disease that a long-term follow-up period is needed, so it is inevitable that a large proportion of patients may withdraw from the group during such a long time, especially, the compliance of patients in control group can be poorer.
More recently, the study by DiNicola et al[50] suggested that minimizing toxicity of autologous HSCT therapy is recommended for future research. The protocols of therapy need to be further refined through the cooperation of gastrointestinal and hematological malignancy professionals, so as to incorporate safer therapy and achieve the right amount of immune “cease fire” to restore gut tolerance as a treatment for refractory CD, especially for those severe ones. Furthermore, the study indicated that the safety can possibly be increased with less toxic regimens of chemotherapy, as the purpose of the autologous HSCT therapy for CD is not full ablation.
Several limitations of our analysis should be mentioned. First, many studies published in conferences were available abstracts only. Moreover, a limited number of RCTs of long-term follow-up on autologous HSCT for refractory CD are available up to now. Second, AEs reporting was not standardized. Given the definitions of AEs varied among studies, it is difficult to pool these data for quantitative analysis further. Finally, included studies did not document the clinical behavior of CD (such as inflammatory, fistulizing, or stenotic disease) before and after autologous HSCT. Moreover, some but not all studies had considerable differences in autologous HSCT, such as stem cell mobilization, conditioning regimen, whether CD34+ cell selection, whether use of ATG and immunoablation (Table 1).
5. Conclusion
Our meta-analysis suggested that autologous HSCT could be a complicated treatment with relatively high mortality and significantly high efficacy for refractory CD that should be used with caution. However, more RCTs of larger samples using refined and standardized protocols and longer period of follow-up time are needed to further assess the outcomes of autologous HSCT therapy.
Acknowledgment
We acknowledge all clinical researchers of the selected studies and patients related to these studies.
Footnotes
Abbreviations: AE = adverse event, CD = Crohn's disease, HSCT = hematopoietic stem cell transplantation, IBD = inflammatory bowel disease, RCT = randomized controlled trial, TRM = transplant-related mortality.
XQ and JRF contributed equally to this work.
The authors declare that they have no competing interests.
References
- [1].Luo Y, de Lange KM, Jostins L, et al. Exploring the genetic architecture of inflammatory bowel disease by whole-genome sequencing identifies association at ADCY7. Nat Genet 2017;49:186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med 2009;361:2066–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bernstein CN, Fried M, Krabshuis JH, et al. World Gastroenterology Organization Practice Guidelines for the diagnosis and management of IBD in 2010. Inflamm Bowel Dis 2010;16:112–24. [DOI] [PubMed] [Google Scholar]
- [4].Rizzello F, Gionchetti P, Venturi A, et al. Review article: the management of refractory Crohn's disease. Aliment Pharmacol Ther 2002;16(Suppl 4):40–7. [DOI] [PubMed] [Google Scholar]
- [5].Ng SC, Chan FK, Sung JJ. Review article: the role of non-biological drugs in refractory inflammatory bowel disease. Aliment Pharmacol Ther 2011;33:417–27. [DOI] [PubMed] [Google Scholar]
- [6].Sachar DB. Patterns of postoperative recurrence in Crohn's disease. Scand J Gastroenterol Suppl 1990;172:35–8. [DOI] [PubMed] [Google Scholar]
- [7].Rutgeerts P, Goboes K, Peeters M, et al. Effect of faecal stream diversion on recurrence of Crohn's disease in the neoterminal ileum. Lancet 1991;338:771–4. [DOI] [PubMed] [Google Scholar]
- [8].Lopez-Cubero SO, Sullivan KM, McDonald GB. Course of Crohn's disease after allogeneic marrow transplantation. Gastroenterology 1998;114:433–40. [DOI] [PubMed] [Google Scholar]
- [9].Irhimeh MR, Cooney J. Management of inflammatory bowel disease using stem cell therapy. Curr Stem Cell Res Ther 2016;11:72–7. [DOI] [PubMed] [Google Scholar]
- [10].Hodson R. Inflammatory bowel disease. Nature 2016;540:S97. [DOI] [PubMed] [Google Scholar]
- [11].Felfly H, Haddad GG. Hematopoietic stem cells: potential new applications for translational medicine. J Stem Cells 2014;9:163–97. [PubMed] [Google Scholar]
- [12].Park B, Yoo KH, Kim C. Hematopoietic stem cell expansion and generation: the ways to make a breakthrough. Blood Res 2015;50:194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sonwalkar SA, James RM, Ahmad T, et al. Fulminant Crohn's colitis after allogeneic stem cell transplantation. Gut 2003;52:1518–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hawkey CJ, Hommes DW. Is stem cell therapy ready for prime time in treatment of inflammatory bowel diseases? Gastroenterology 2017;152:389–90. [DOI] [PubMed] [Google Scholar]
- [15].Kashyap A, Forman SJ. Autologous bone marrow transplantation for non-Hodgkin's lymphoma resulting in long-term remission of coincidental Crohn's disease. Br J Haematol 1998;103:651–2. [DOI] [PubMed] [Google Scholar]
- [16].Drakos PE, Nagler A, Or R. Case of Crohn's disease in bone marrow transplantation. Am J Hematol 1993;43:157–8. [DOI] [PubMed] [Google Scholar]
- [17].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9. w64. [DOI] [PubMed] [Google Scholar]
- [18].Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March, 2011]. The Cochrane Collaboration. Available at: www.cochrane-handbook.org. Accessed February 2, 2012. [Google Scholar]
- [19].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed) 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Deeks JJ, Higgins JP, Altman DG. On the behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 9: Analysing Data and Undertaking Meta-analyses. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]: John Wiley & Sons, Ltd; 2008. 243–96. [Google Scholar]
- [21].Hawkey CJ, Allez M, Clark MM, et al. Autologous hematopoetic stem cell transplantation for refractory Crohn's disease a randomized clinical trial. JAMA 2015;314:2524–34. [DOI] [PubMed] [Google Scholar]
- [22].Hasselblatt P, Drognitz K, Potthoff K, et al. Remission of refractory Crohn's disease by high-dose cyclophosphamide and autologous peripheral blood stem cell transplantation. Aliment Pharmacol Ther 2012;36:725–35. [DOI] [PubMed] [Google Scholar]
- [23].Burt RK, Traynor A, Oyama Y, et al. High-dose immune suppression and autologous hematopoietic stem cell transplantation in refractory Crohn's disease. Blood 2003;101:2064–6. [DOI] [PubMed] [Google Scholar]
- [24].Oyama Y, Craig RM, Traynor AE, et al. Autologous hematopoietic stem cell transplantation in patients with refractory Crohn's disease. Gastroenterology 2005;128:552–63. [DOI] [PubMed] [Google Scholar]
- [25].Cassinotti A, Annaloro C, Ardizzone S, et al. Autologous haematopoietic stem cell transplantation without CD34(+) cell selection in refractory Crohn's disease. Gut 2008;57:211–7. [DOI] [PubMed] [Google Scholar]
- [26].Clerici M, Cassinotti A, Onida F, et al. Immunomodulatory effects of unselected haematopoietic stem cells autotransplantation in refractory Crohn's disease. Dig Liver Dis 2011;43:946–52. [DOI] [PubMed] [Google Scholar]
- [27].Hommes DW, Duijvestein M, Zelinkova Z, et al. Long-term follow-up of autologous hematopoietic stem cell transplantation for severe refractory Crohn's disease. J Crohns Colitis 2011;5:543–9. [DOI] [PubMed] [Google Scholar]
- [28].Burt RK, Craig RM, Milanetti F, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in patients with severe anti-TNF refractory Crohn's disease: long-term follow-up. Blood 2010;116:6123–32. [DOI] [PubMed] [Google Scholar]
- [29].Jauregui-Amezaga A, Rovira M, Marin P, et al. Improving safety of autologous haematopoietic stem cell transplantation in patients with Crohn's disease. Gut 2016;65:1456–62. [DOI] [PubMed] [Google Scholar]
- [30].Hendrickson JE, Hillyer CD. Noninfectious serious hazards of transfusion. Anesth Analg 2009;108:759–69. [DOI] [PubMed] [Google Scholar]
- [31].Martinez-Montiel MD, Gomez-Gomez GJ, Flores AI. Therapy with stem cells in inflammatory bowel disease. World J Gastroenterol 2014;20:1211–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Martinez-Montiel Mdel P, Gomez-Gomez GJ, Flores AI. Therapy with stem cells in inflammatory bowel disease. World J Gastroenterol 2014;20:1211–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Darlington PJ, Touil T, Doucet JS, et al. Diminished Th17 (not Th1) responses underlie multiple sclerosis disease abrogation after hematopoietic stem cell transplantation. Ann Neurol 2013;73:341–54. [DOI] [PubMed] [Google Scholar]
- [34].Bingham S, Veale D, Fearon U, et al. High-dose cyclophosphamide with stem cell rescue for severe rheumatoid arthritis: short-term efficacy correlates with reduction of macroscopic and histologic synovitis. Arthritis Rheum 2002;46:837–9. [DOI] [PubMed] [Google Scholar]
- [35].Franzke A, Piao W, Lauber J, et al. G-CSF as immune regulator in T cells expressing the G-CSF receptor: implications for transplantation and autoimmune diseases. Blood 2003;102:734–9. [DOI] [PubMed] [Google Scholar]
- [36].Schmidt C, Wittig BM, Moser C, et al. Cyclophosphamide pulse therapy followed by azathioprine or methotrexate induces long-term remission in patients with steroid-refractory Crohn's disease. Aliment Pharmacol Ther 2006;24:343–50. [DOI] [PubMed] [Google Scholar]
- [37].Barta Z, Toth L, Zeher M. Pulse cyclophosphamide therapy for inflammatory bowel disease. World J Gastroenterol 2006;12:1278–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Stallmach A, Wittig BM, Moser C, et al. Safety and efficacy of intravenous pulse cyclophosphamide in acute steroid refractory inflammatory bowel disease. Gut 2003;52:377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Korzenik JR, Dieckgraefe BK. An open-labelled study of granulocyte colony-stimulating factor in the treatment of active Crohn's disease. Aliment Pharmacol Ther 2005;21:391–400. [DOI] [PubMed] [Google Scholar]
- [40].Georgoulias V, Papadakis E, Alexopoulos A, et al. Platinum-based and non-platinum-based chemotherapy in advanced non-small-cell lung cancer: a randomised multicentre trial. Lancet 2001;357:1478–84. [DOI] [PubMed] [Google Scholar]
- [41].Korzenik JR, Dieckgraefe BK, Valentine JF, et al. Sargramostim for active Crohn's disease. N Engl J Med 2005;352:2193–201. [DOI] [PubMed] [Google Scholar]
- [42].Labidi A, Serghini M, Ben Mustapha N, et al. Stem cell transplantation as rescue therapy for refractory Crohn's disease: a systematic review. Tunis Med 2014;92:655–9. [PubMed] [Google Scholar]
- [43].Hawkey CJ. Stem cell transplantation for Crohn's disease. Best Pract Res Clin Haematol 2004;17:317–25. [DOI] [PubMed] [Google Scholar]
- [44].Daikeler T, Tichelli A, Passweg J. Complications of autologous hematopoietic stem cell transplantation for patients with autoimmune diseases. Pediatr Res 2012;71:439–44. [DOI] [PubMed] [Google Scholar]
- [45].Swart JF, Delemarre EM, van Wijk F, et al. Haematopoietic stem cell transplantation for autoimmune diseases. Nat Rev Rheumatol 2017;13:244–56. [DOI] [PubMed] [Google Scholar]
- [46].Snowden JA, Saccardi R, Allez M, et al. Haematopoietic SCT in severe autoimmune diseases: updated guidelines of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant 2012;47:770–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hawkey CJ, Hommes DW. Is stem cell therapy ready for prime time in treatment of inflammatory bowel diseases? Gastroenterology 2017;152:389–97. e2. [DOI] [PubMed] [Google Scholar]
- [48].Hommes DW, Lacey PN. Stem cells: HSCT for Crohn's disease: work in progress or a bridge too far? Nat Rev Gastroenterol Hepatol 2016;13:128–30. [DOI] [PubMed] [Google Scholar]
- [49].Burt RK, Ruiz MA, Kaiser RL., Jr Stem cell transplantation for refractory Crohn's disease. JAMA 2016;315:2620. [DOI] [PubMed] [Google Scholar]
- [50].DiNicola CA, Zand A, Hommes DW. Autologous hematopoietic stem cells for refractory Crohn's disease. Expert Opin Biol Ther 2017;17:555–64. [DOI] [PubMed] [Google Scholar]


