Abstract
We analyzed the characteristics of patients with World Health Organization (WHO) grade III meningioma to identify factors that may predict tumor recurrence and overall survival (OS).
We retrospectively reviewed the patients diagnosed with WHO grade III meningioma who were surgically treated at our institute between 2008 and 2016. Survival outcome was assessed by Kaplan–Meier analysis. Cox regression analyses were performed to identify the prognostic factors associated with tumor recurrence and OS.
Forty-two patients were included. The mean follow-up time was 23.2 months (range 2–75 months). At the end of analysis, 30 patients were found with tumor recurrence. The 1-year, 3-year, and 5-year recurrence-free survival (RFS) were 51.6%, 33.9%, and 12.0%, respectively. At final follow-up, 23 patients were deceased, the 1-year, 3-year, and 5-year OS were 66.2%, 39.7%, and 35.8%, respectively. Twenty-eight newly diagnosed patients were included, and the 1-year, 3-year, and 5-year RFS were 63.5%, 44.3%, and 19.4%, respectively, and the 1-year, 3-year, and 5-year OS were 74.6%, 52.5%, and 46.7%, respectively. Extent of resection was the only factor associated with tumor recurrence and OS.
WHO grade III meningioma is rare, and difficult to manage with a high rate of recurrence and poor OS. Extent of resection is an independent prognostic factor related to tumor recurrence and OS. We could not confirm the usefulness of Ki-67. We suggest that more aggressive treatment, such as safety maximizing cytoreduction by surgery, would improve treatment outcomes.
Keywords: OS, prognostic factor, RFS, WHO grade III meningioma
1. Introduction
Meningioma arises from meninges of the brain and the spinal cord. It is the most common primary tumor and represents approximately one-third of central nervous system tumor in adult.[1] According to World Health Organization (WHO) classification, meningiomas are divided into 3 subtypes on the basis of histologic grading.[2] WHO grade III meningiomas account for 1% to 3% of intracranial meningiomas.[3] Compared with other subtypes, WHO grade III meningiomas show a great propensity for tumor recurrence and aggressive illness progression.[2]
The outcome of WHO grade III meningiomas is poor with a 5-year survival rate of 19.9% to 61% despite advances in treatment options.[4–9] The management for these patients is challenging. Recent studies demonstrate that patients would benefit from surgical resection.[4,6,7,10] Radiotherapy is also employed, but the efficiency is controversial.[11–16] A handful of studies report the use of chemotherapy for WHO grade III meningiomas, whereas the efficiency is limited.[17,18] Owing to the rarity of WHO grade III meningiomas, treatment strategies for these lesions have not reached an agreement. Further optimal management in the case of malignant meningioma is difficult to establish.
In our study, we analyze 42 patients diagnosed with WHO grade III meningioma, and try to elucidate the outcome and prognostic factors associated tumor recurrence and overall survival (OS) of patients with WHO grade III meningiomas.
2. Patients and methods
2.1. Patient selection
We retrospectively reviewed patients diagnosed with meningioma who were operated at the Department of Neurosurgery, West China Hospital of Sichuan University between 2008 and 2016. During the review period, a total of 3056 patients with meningioma were surgically treated. Of these patents, 2548 patients were identified as benign meningiomas, 463 patients were diagnosed with WHO grade II meningiomas and WHO grade III meningiomas were found in 45 patients. Pathologic reports and specimen of tumors were reviewed to confirm the diagnosis according 2007 WHO classification scheme. Three patients died of postoperation complications were excluded. Finally, 42 patients were included in this study. Our study was approved by our institute ethics board.
2.2. Parameters assessed
Data regarding patient age at surgery, sex, presenting symptom, history of previous surgery or previous radiotherapy, adjuvant radiotherapy, and salvage treatment after tumor recurrence were collected from inpatient and outpatient record. Previous surgery was defined as resection for meningiomas. Bone involvement was defined as hyperostosis, bone destruction, or bone infiltration on imaging, pathology reports, and surgery record. Tumor size was measured by maximum diameter of tumor on the basis of magnetic resonance imagings (MRI)s or surgery record. Ki-67 was extracted from pathologic reports. Tumor location was subdivided into 4 groups: convexity, skull-base, para-sagittal/para-sinus, and interventricular. The extent of resection was evaluated according to the Simpson grading scale by the use of the operative records. We defined Simpson grade I as complete resection, and Simpson grade II, III, and IV were classified as incomplete resection, no patient fell to Simpson grade V. Adjuvant radiotherapy (gamma knife or conventional radiotherapy) was given following surgical resection during study time without evidence of tumor recurrence. A redo surgery would be the first choice for patients who were found with tumor recurrence, and adjuvant radiotherapy was employed after redo surgery, but this is just an ideal strategy. If patients cannot tolerate or refuse a redo surgery, radiotherapy was suggested.
2.3. Patient follow-up
Patients were followed by neurosurgeons clinically, and MRIs and clinical evaluations were routinely obtained at 3, 6, and 12 months, and then once a year. We defined tumor recurrence as radiological evidence of tumor regrowth in cases of complete resection, or residual tumor progression in cases of incomplete resection. Recurrence-free survival (RFS) was calculated from the date of surgery to first radiological evidence of disease recurrence, or censored at the date of last follow-up in the absence of disease recurrence. OS was determined from the date of first surgery to the date of death (all causes), or last follow-up if the patient was still alive. If patients died, the cause was searched and quoted differently if related to the surgery or the progressing meningioma disease or not. A patient with no record for 1 year was considered lost to follow-up. Survival statistics were based on 2 different events: tumor recurrence and death. RFS and OS were calculated from the date of first surgery during the study time.
2.4. Statistics analysis
Quantitative variables were described using mean and range. Categorical variables were described using frequency and percentage. Survival outcome was assessed by the Kaplan-Meier method, and comparisons between groups were performed using log-rank tests. A P value <0.05 was regarded as statistically significant. Factors with a P < .05 on univariate analysis were incorporated into multivariate Cox proportional regression model. For the analysis, we considered both gamma knife and conventional radiotherapy equally. Statistical analysis was performed using IBM SPSS STATISTICS 22.0 (New York).
3. Results
3.1. Patients and tumor characteristics
A total of 42 patients diagnosed with WHO grade III meningioma were included (Table 1). The mean age was 50.2 years, (range 20–80 years). Fifty-two percent of the patients were female. The most common presenting symptom was headache, which occurred in 19 patients (45.2%), followed by epilepsy. Thirteen patients had a history of previous surgery, and 2 of them diagnosed as atypical meningioma progressed in WHO grade III meningioma during tumor recurrence. Previous radiotherapy (gamma knife and conventional radiotherapy) was employed in 8 patients, radiotherapy was performed in 7 patients as adjuvant treatment after resection for meningiomas, and only 1 patient selected radiotherapy as the primary treatment for meningioma. The most common location was convexity. Twelve patients (28.6%) achieved Simpson grade I resection (complete resection). Twenty-one patients received adjuvant radiotherapy after surgical resection. The mean Ki-67 index was 19.9% (range 5%–85%).
Table 1.
Patients characteristics.
At the end of analysis, 30 patients were found with tumor recurrence with a mean follow-up time of 23.2 months (Table 2). The 1-year, 3-year, and 5-year RFS were 51.6%, 33.9%, and 12.0%, respectively (Fig. 1A). The mean time to recurrence was 13.9 months (range 1–51 months). Of these recurrent cases, 13 patients were found with a history of previous surgery, and 7 patients had a history of previous radiotherapy, adjuvant radiotherapy was performed in 15 patients. Twelve patients with tumor recurrence selected a redo surgery, and adjuvant radiotherapy was employed in 7 of them. Temozolomide was selected as a salvage treatment in 1 patient after tumor recurrence.
Table 2.
Characteristics of recurrent patients.

Figure 1.
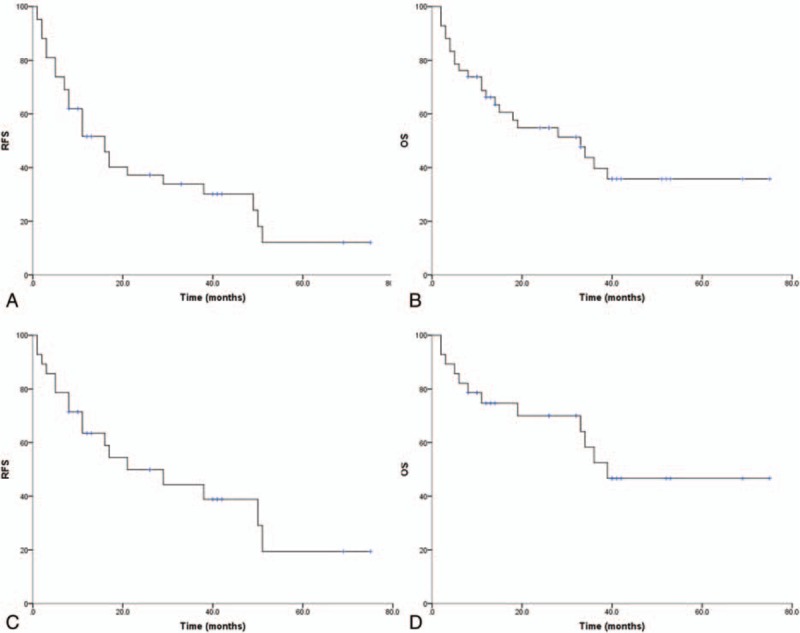
Survival curves. (A) Recurrence-free survival for all patients. (B) Overall survival for all patients. (C) Recurrence-free survival for newly diagnosed patients. (D) Overall survival for newly diagnosed patients.
At the last follow-up, 23 patients were deceased, and all of them died of primary disease. The 1-year, 3-year, and 5-year OS were 66.2%, 39.7%, and 35.8%, respectively (Fig. 1B).
Twenty-eight patients who were newly diagnosed with WHO grade III meningiomas were included in this study. Seventeen patients were found with tumor recurrence, and 12 patients died of tumor progression. The 1-year, 3-year, and 5-year RFS were 63.5%, 44.3%, and 19.4%, respectively (Fig. 1C). The 1-year, 3-year, and 5-year OS were 74.6%, 52.5%, and 46.7%, respectively (Fig. 1D).
3.2. Factors associated with tumor recurrence for all patients
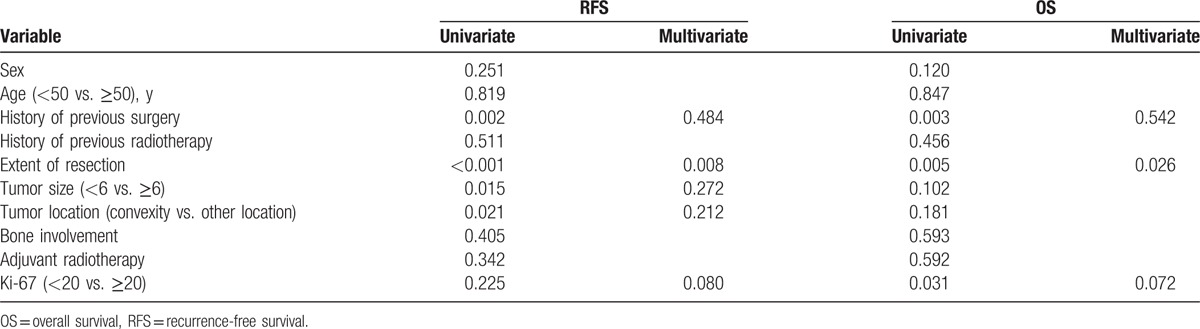
On univariate analysis, several prognostic factors were found. RFS was significantly superior in patients without history of previous surgery (Fig. 2A, P = .002). Compared with incomplete resection, patients with complete resection showed a significant better RFS (Fig. 2B, P < .001). A tumor size <6 cm was also related to a low recurrence rate (Fig. 2C, P = .015). Patients with a tumor located in convexity region had a lower recurrence rate than those with a tumor located in other regions (Fig. 2D, P = 0.021). Whereas, Ki-67 did not have an impact on RFS. Other variables including sex, age, history of previous radiotherapy, adjuvant radiotherapy, and bone involvement had no impact on RFS. We hypothesized that Ki-67 index associated with recurrence, and warranted subsequent multivariate Cox regression analysis. According to further multivariate COX regression analysis, extent of resection was the only factor associated with tumor recurrence (Table 3, P = .008).
Figure 2.
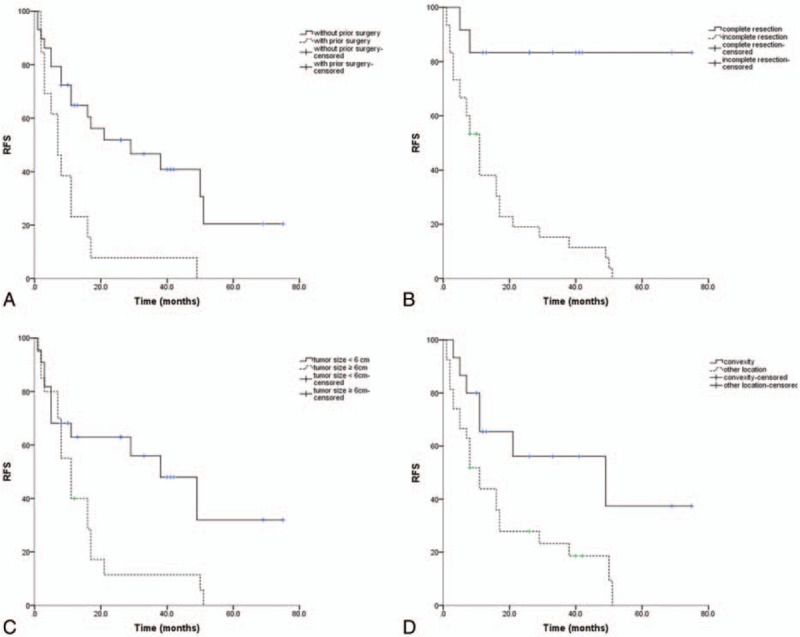
Recurrence-free survival curves for all patients. (A) Recurrence-free survival by history of prior surgery. (B) Recurrence-free survival by extent of resection. (C) Recurrence-free survival by tumor size. (D) Recurrence-free survival by tumor location.
Table 3.
Prognostic factors of RFS and OS for all patients.
3.3. Factors associated with OS for all patients
On univariate analysis, several prognostic factors were also found. A history of previous surgery was associated with worse OS (Fig. 3A, P = .003). Patients with tumors completely resected showed a superior OS than patients with incompletely resected (Fig. 3B, P = .005). A higher Ki-67 index was associated with worse OS (Fig. 3C, P = .031). On multivariate analysis, complete resection was also the only beneficial factor for OS (Table 3, P = .026).
Figure 3.
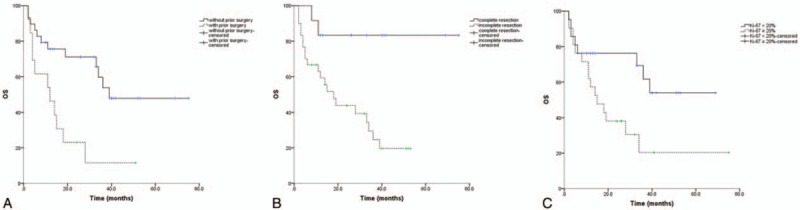
Overall survival curves for all patients. (A) Overall survival by history of prior history. (B) Overall survival by extent of resection. (D) Overall survival by Ki-67 index.
3.4. Factors associated with tumor recurrence and OS for newly diagnosed patients
In total, 28 newly diagnosed patients were included in this study (Table 4). On univariate analysis, extent of resection (Fig. 4A, P = .001), tumor size (Fig. 4B, P = .046), and tumor location (Fig. 4C, P = .005) have impact on RFS, complete resection predicted superior rate of OS in univariate analysis (Fig. 4D, P = 0.044), and patients with higher Ki-67 index did not demonstrate a worse RFS or OS. It suggested an association between Ki-67 index and RFS, OS that did not reach significance but did warrant subsequent multivariate Cox regression analysis. Further multivariate analysis revealed that extent of resection was associated with longer RFS (Table 4, P = .048) and better OS (Table 4, P = .031).
Table 4.
Prognostic factors of RFS and OS for newly diagnosed patients.
Figure 4.
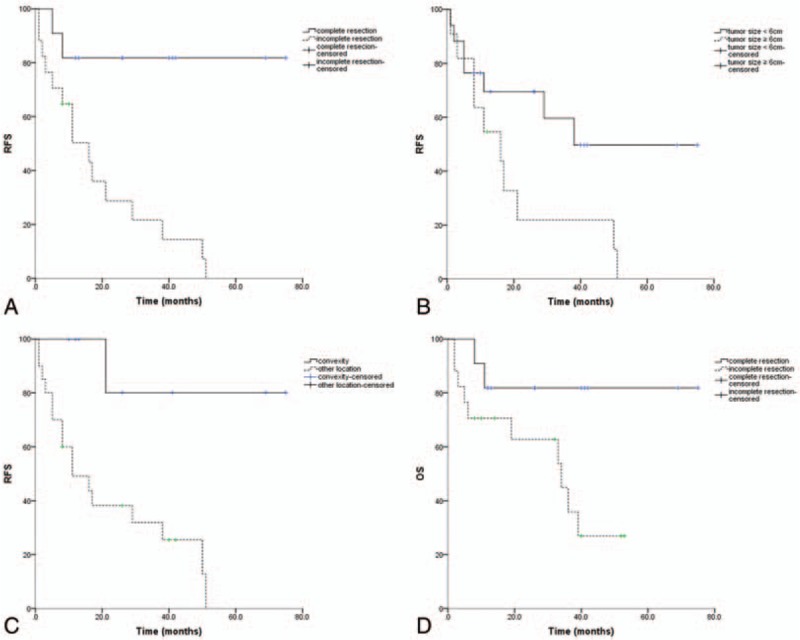
Survival curves for newly diagnosed patients. (A) Recurrence-free survival by extent of resection. (B) Recurrence-free survival by tumor size. (C) Recurrence-free survival by tumor location. (D) Overall survival by extent of resection.
4. Discussion
WHO grade III meningioma is a rare subtype of meningiomas with poor prognosis. Management of WHO grade III meningiomas is challenging for clinicians because of high recurrence rate and low survival rate. Owing to the rarity of WHO grade III meningiomas, only sparse studies regarding on WHO grade III meningiomas were carried out, and risk factors related to tumor recurrence and OS were poorly understood. In our study, we analyzed 42 patients diagnosed with WHO grade III meningiomas, elucidated the outcome and prognostic factors for tumor recurrence and OS of these patients. In our series, 22 patients were female; small size of this series may result in female preponderance. The mean age of the 42 patients was close to the mean age of patients with WHO grade I and II meningioma (50.2 vs. 52.2 years). Headache was the most common presenting symptom. Thirteen patients had a history of surgery, and 2 of them had a history of surgery for WHO grade II meningioma. Complete resection was achieved in 12 patients (28.6%). Twenty-one patients received adjuvant radiotherapy. At last following-up, 30 patients were found with tumor recurrence, and 23 patients died. The 1-year, 3-year, and 5-year RFS were 51.6%, 33.9%, and 12.0%, respectively. The 1-year, 3-year, and 5-year OS were 66.2%, 39.7%, 35.8%, respectively. Extent of resection was identified as independent prognostic factor associated with tumor recurrence and OS; Ki-67 index failed to predict tumor recurrence and OS. In total, 28 newly diagnosed patients were included, the 1-year, 3-year, and 5-year RFS were 63.5%, 44.3%, and 19.4%, respectively, and the 1-year, 3-year, and 5-year OS were 74.6%, 52.5%, and 46.7%, respectively. We analyzed the newly diagnosed patients separately, and extent of resection was the only prognostic factor related to RFS and OS.
4.1. Surgery
Since the seminal publication of Simpson in 1957, there is a general agreement about the importance of resection completeness for meningiomas, since the residual meningiomas may continue to grow.[19] Complete resection surgery was an ideal choice for patients with WHO grade III meningiomas. Compared with benign cases, a Simpson grade I resection was much more difficult to achieve, especially venous sinus wall or skull-base was involved. In our study, only 9 patients (26.5%) achieved a Simpson grade I resection. The extent of resection is the most powerful prognostic factor for recurrence for all grades of meningiomas. Our study showed patients with tumor resected completely had a superior outcome than those with incomplete resection, which was in accordance with some studies.[4,6,7,20] Choi et al[20] reported a cohort with 37 patients diagnosed with WHO grade III meningiomas, and extent of surgical resection was identified as prognostic for local control and OS, but the patients were analyzed together with WHO grade II patients. Sughrue et al found that, compared with patients received gross-total resection, patients treated with near-total resection at initial or repeat surgery had extended OS. Meanwhile, the author also noticed the risks of aggressive gross-total resection.[7] Champeaux et al[4] presented a series of 62 patients, and highlighted that complete or subtotal resection improved OS.
4.2. Radiotherapy
Radiotherapy was a choice for management of patients with WHO grade III meningioma. Owing to the high recurrence rate and poor outcome of WHO grade III meningiomas, it appears that the majority of neurosurgeons would refer patients with partially resected WHO grade III meningiomas to radiotherapy.[10,21] In our study, 21 patients received adjuvant radiotherapy after surgical resection, but we could not confirm the efficiency of radiotherapy, which was consistent with Champeaux et al.[4] On the contrary, several studies addressed the usefulness of radiotherapy in the management of WHO grade III meningiomas. Balasubramanian et al reported that radiotherapy was associated with increased OS, and Zhao et al reported that radiotherapy was associated with increased PFS and OS in WHO grade III meningiomas.[5,6]
4.3. Ki-67
The Ki-67 index is a useful predictor of risk of tumor recurrence.[22] Perry et al summarized different studies of mean Ki-67 and reported a range of 11% to 16.3% in WHO grade III meningiomas, and Ki-67 beyond 4% indicated an increased recurrence rate.[23] In our study, the mean Ki-67 index was 19.9% (range 5%–85%). Bruna et al identified Ki-67 as the prognostic factor related to tumor recurrence and OS.[24] In our series, we failed to identify Ki-67 as the independent factor associated with tumor recurrence and OS either for all patients or newly diagnosed patients.
4.4. Limitations
This is one of the largest retrospective study to evaluate the outcome and prognostic factors associated with tumor recurrence and OS of patients with WHO grade III meningiomas to the best of our knowledge. However, this study had several limitations. One weakness is this study's retrospective nature. Moreover, the decision to employ radiotherapy for patients was not randomized, and for the analysis, we considered both gamma knife and conventional radiotherapy equally, thus biases were introduced, objectively. As the rarity of WHO grade III meningiomas, small size of the sample was also a weakness of this study. Larger retrospective, prospective, randomized, or multicenter clinical trials are needed to evaluate the prognostic factors in the patients with WHO grade III meningiomas.
5. Conclusions
In conclusion, WHO grade III meningioma is rare, and difficult to manage with a high rate of recurrence and poor overall survival. Extent of resection is an independent prognostic factor related to tumor recurrence and OS. We could not confirm the usefulness of Ki-67. We suggest that more aggressive treatment, such as safety maximizing cytoreduction by surgery, would improve treatment outcomes.
Acknowledgments
The authors thank the reviewers for their constructive comments.
Footnotes
Abbreviations: HR = hazard ratio, IQR = interquartile range, MRI = magnetic resonance image, OS = overall survival, RFS = recurrence-free survival, WHO = World Health Organization.
BS, JZ, and YS are equal contributors.
The authors report no conflicts of interest.
References
- [1].Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neurooncol 2010;99:307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dolecek TA, Dressler EV, Thakkar JP, et al. Epidemiology of meningiomas post-Public Law 107-206: The Benign Brain Tumor Cancer Registries Amendment Act. Cancer 2015;121:2400–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hanft S, Canoll P, Bruce JN. A review of malignant meningiomas: diagnosis, characteristics, and treatment. J Neurooncol 2010;99:433–43. [DOI] [PubMed] [Google Scholar]
- [4].Champeaux C, Wilson E, Brandner S, et al. World Health Organization grade III meningiomas. A retrospective study for outcome and prognostic factors assessment. Br J Neurosurg 2015;29:693–8. [DOI] [PubMed] [Google Scholar]
- [5].Balasubramanian SK, Sharma M, Silva D, et al. Longitudinal experience with WHO Grade III (anaplastic) meningiomas at a single institution. J Neurooncol 2017;131:555–63. [DOI] [PubMed] [Google Scholar]
- [6].Zhao P, Hu M, Zhao M, et al. Prognostic factors for patients with atypical or malignant meningiomas treated at a single center. Neurosurg Rev 2015;38:101–7. [DOI] [PubMed] [Google Scholar]
- [7].Sughrue ME, Sanai N, Shangari G, et al. Outcome and survival following primary and repeat surgery for World Health Organization Grade III meningiomas. J Neurosurg 2010;113:202–9. [DOI] [PubMed] [Google Scholar]
- [8].Adeberg S, Hartmann C, Welzel T, et al. Long-term outcome after radiotherapy in patients with atypical and malignant meningiomas--clinical results in 85 patients treated in a single institution leading to optimized guidelines for early radiation therapy. Int J Radiat Oncol Biol Phys 2012;83:859–64. [DOI] [PubMed] [Google Scholar]
- [9].Ye J, Lv G, Qian J, et al. Clinical features and prognostic factors of WHO II and III adult spinal meningiomas: analysis of 25 cases in a single center. J Neurooncol 2016;128:349–56. [DOI] [PubMed] [Google Scholar]
- [10].Sun SQ, Hawasli AH, Huang J, et al. An evidence-based treatment algorithm for the management of WHO Grade II and III meningiomas. Neurosurg Focus 2015;38:E3. [DOI] [PubMed] [Google Scholar]
- [11].Zhang M, Ho AL, D’Astous M, et al. CyberKnife stereotactic radiosurgery for atypical and malignant meningiomas. World Neurosurg 2016;91:574–81. e571. [DOI] [PubMed] [Google Scholar]
- [12].Wang WH, Lee CC, Yang HC, et al. Gamma knife radiosurgery for atypical and anaplastic meningiomas. World Neurosurg 2016;87:557–64. [DOI] [PubMed] [Google Scholar]
- [13].Piscevic I, Villa A, Milicevic M, et al. The influence of adjuvant radiotherapy in atypical and anaplastic meningiomas: a series of 88 patients in a single institution. World Neurosurg 2015;83:987–95. [DOI] [PubMed] [Google Scholar]
- [14].Walcott BP, Nahed BV, Brastianos PK, et al. Radiation treatment for WHO grade II and III meningiomas. Front Oncol 2013;3:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Engenhart-Cabillic R, Farhoud A, Sure U, et al. Clinicopathologic features of aggressive meningioma emphasizing the role of radiotherapy in treatment. Strahlenther Onkol 2006;182:641–6. [DOI] [PubMed] [Google Scholar]
- [16].Ferraro DJ, Funk RK, Blackett JW, et al. A retrospective analysis of survival and prognostic factors after stereotactic radiosurgery for aggressive meningiomas. Radiation Oncol (London, England) 2014;9:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nayak L, Iwamoto FM, Rudnick JD, et al. Atypical and anaplastic meningiomas treated with bevacizumab. J Neurooncol 2012;109:187–93. [DOI] [PubMed] [Google Scholar]
- [18].Rammo R, Rock A, Transou A, et al. Anaplastic meningioma: octreotide therapy for a case of recurrent and progressive intracranial disease. J Neurosurg 2016;124:496–500. [DOI] [PubMed] [Google Scholar]
- [19].Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry 1957;20:22–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Choi Y, Lim DH, Jo K, et al. Efficacy of postoperative radiotherapy for high grade meningiomas. J Neurooncol 2014;119:405–12. [DOI] [PubMed] [Google Scholar]
- [21].Paldor I, Awad M, Sufaro YZ, et al. Review of controversies in management of non-benign meningioma. J Clin Neurosci 2016;31:37–46. [DOI] [PubMed] [Google Scholar]
- [22].Abry E, Thomassen IO, Salvesen OO, et al. The significance of Ki-67/MIB-1 labeling index in human meningiomas: a literature study. Pathol Res Pract 2010;206:810–5. [DOI] [PubMed] [Google Scholar]
- [23].Perry A, Stafford SL, Scheithauer BW, et al. Meningioma grading: an analysis of histologic parameters. Am J Surg Pathol 1997;21:1455–65. [DOI] [PubMed] [Google Scholar]
- [24].Bruna J, Brell M, Ferrer I, et al. Ki-67 proliferative index predicts clinical outcome in patients with atypical or anaplastic meningioma. Neuropathology 2007;27:114–20. [DOI] [PubMed] [Google Scholar]


