Abstract
Rationale:
Tick infestation of the external auditory canal (EAC) constitutes <1% of all patients presenting with ear complaints. Consequently, parameters for the diagnosis and management of ticks in the EAC have not been established.
Patient concerns:
We report 2 cases of EAC infestation by Amblyomma testudinarium in 2 female patients, aged 12- and 72 years old.
Diagnoses interventions and outcomes:
In both patients, otoscopic examination revealed engorged ticks attached to the ear canal. The 12-year-old girl had a narrow EAC, necessitating slight dislodging of the tick to determine if its mouth parts were embedded in the EAC skin. The tick's mouth parts were confirmed to be free, enabling complete removal of the tick. The 72-year-old woman had a wide EAC, enabling tick removal using Hartman's forceps because the tick's mouth parts were confirmed to be free of the skin. Prophylactic tetracycline (200 mg/day) was administered to each patient for 7 days; neither showed any signs of fever or rash.
Lessons:
This report describes 2 patients with A testudinarium infestation of the EAC, and proposes a simple, noninvasive protocol for determining if the tick is anchored to the ear canal.
Keywords: Amblyomma testudinarium, external auditory canal, human otoacariasis, ticks
1. Introduction
Tick infestations of the external auditory canal (EAC) are responsible for <1% of all patients presenting with ear complaints.[1] However, this is a particularly painful and distressing condition associated with severe complications, including labyrinthitis-induced tinnitus, hearing loss, vertigo, or facial paralysis (palsy).[2] Labyrinthitis is caused by bacteria or viruses transmitted via tick saliva. Amblyomma testudinarium is a known carrier of Rickettsia tamurae,[3] recently found to be responsible for skin lesions, erythema, and pain.[4] In this report, we present 2 cases of A testudinarium infestation of the EAC. The removal of these parasites remains challenging, even for specialized surgeons, because ticks are highly resistant to most insecticidal agents.[1] As a result of our experience, we propose guidelines for the successful and minimally invasive management of these patients.
2. Case reports
2.1. Case 1
A 12-year-old girl developed pain, without tinnitus, in her right ear that persisted for 2 days. She did not have a history of overseas travel, but her family owned a small dog that might have carried ticks. Her body weight was 50 kg, and pulse rate was 72 beats/min. She was afebrile and lacked any other systemic symptoms.
Otoscopy revealed an engorged tick blocking the bony part of the girl's narrow EAC. Further examination revealed the moving legs of the tick (Fig. 1A), but the mouth parts were not visible. Incomplete removal of the tick may result in the development of a foreign body granuloma, therefore, the tick was slightly dislodged under surgical microscopy, to determine whether or not the mouth parts were embedded in the patient's skin. Since they were not, the tick was gently removed using Hartman's forceps without the need for anesthesia or agents to facilitate the detachment of the arachnid. In this case, although the EAC was slightly reddened, a bite site was not apparent, suggesting that the tick had detached following feeding. Tetracycline (200 mg/day) was administered for 7 days. Over the 1 week follow-up period, the patient did not lose weight and her vital signs remained normal. The patient did not demonstrate any signs of fever or rashes. The extracted specimen (Fig. 1B–D) was identified as a female A testudinarium tick, based on its morphological characteristics (nymph stage, 6.3 × 5.3 mm).
Figure 1.
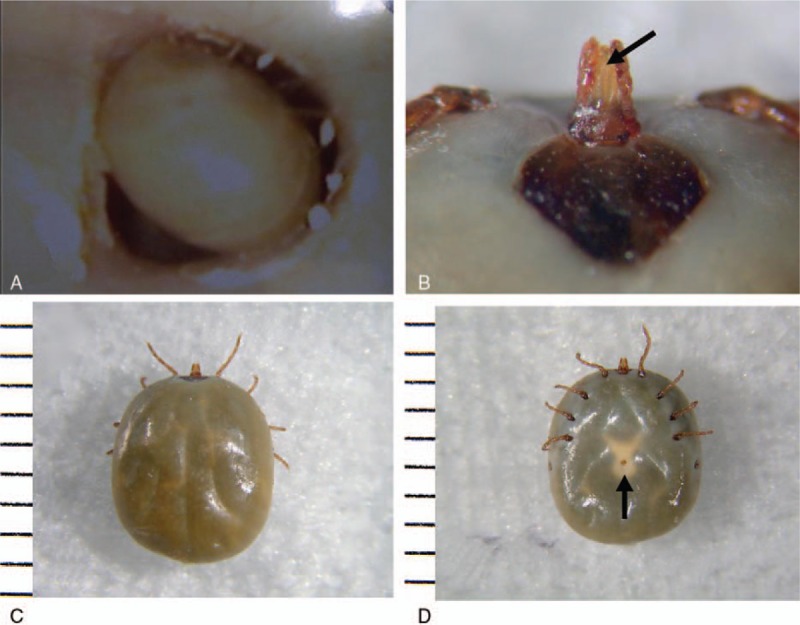
Otoscopic view of the right external auditory canal in a 12-year-old girl. (A) Although tick leg movement was confirmed, we could not see the parasite's hypostome. The extracted specimen was identified as a female A testudinarium tick (nymph stage, 6.3 × 5.3 mm); tick's (B) hypostome; (C) back; and (D) abdomen. Note the hypostome (B, arrow) and genital pore (D, arrow).
2.2. Case 2
A 72-year-old woman presented with a 2-week history of intermittent tinnitus and itching of her right ear. There was no family or medical history of ear complaints. She did not have a history of overseas travel and did not own a pet. However, she resided in a rural area and often went for walks, which might have facilitated her exposure to ticks. People working in forests and agricultural setups are vulnerable to diseases associated with tick bites.[5] Her body weight was 48 kg, with a pulse rate of 60 beats/min. She was afebrile and did not have any apparent systemic symptoms.
Similar to patient 1, otoscopy revealed a dark green mass blocking the bony part of the EAC. Further observation revealed an engorged tick moving within the EAC. Because the patient had a wide EAC, we could see the legs, abdomen, and mouth parts of the tick and that they were not attached to the skin of the EAC (Fig. 2A). After re-confirming that the mouth parts of the tick were not embedded in the patient's skin, the tick was gently removed using Hartman's forceps. The EAC was slightly reddened after removal of the tick. Similar to the first case, this patient's tick had also detached from the EAC after feeding, and there was no evidence of tympanic membrane perforation. Tetracycline (200 mg/day) was also administered to this patient for 7 days. Over this time, the patient did not lose weight, had normal vital signs, and did not demonstrate any signs of fever or rashes. The extracted arachnid was identified as a female A testudinarium tick (nymph stage, 5 × 4.5 mm) (Fig. 2B and C). The study protocol was approved by the ethics review committee of our hospital (approval number: 2013, 4-1). Written informed consent was obtained from the patients.
Figure 2.

Otoscopic view of the right external auditory canal in a 72-year-old woman. Because the patient had a wide external auditory canal, we could observe the legs, abdomen, and mouth parts of the tick, and that there was no attachment to the skin of the EAC. Tick's (A) mouth parts (arrow), abdomen (asterisk), and 8 legs. The extracted specimen was a female Amblyomma testudinarium tick (nymph stage, 5 × 4.5 mm); (B) back; and (C) abdomen. EAC = external auditory canal.
3. Discussion
In Japan, ticks are rarely found in human EACs. Since 1990, only 16 reports, involving Haemaphysalis longicornis,[6–10], Dermacentor sp,[11,12]Ixodes persulcatus,[13,14] and Ixodes ovatus,[15] have described such occurrences. These ticks usually infest moist areas of the body, such as interdigital, anal, or other hidden areas,[16] and rarely enter the EAC. However, in some countries such as India, South Africa, Nepal, Chile, Malaysia, and Sri Lanka, ticks are sometimes found in human EACs.[1,17] The likelihood of tick attachment differs between rural and urban areas.[17,18] To our knowledge, this is the first report of an A testudinarium tick found in the EAC.
The most common complaint associated with a tick infestation in the EAC is acute ear pain,[1] caused by an enzyme in tick saliva that produces intense, local inflammation, and pain. An aural sensation of pressure may develop when ticks become so blood-engorged that they fully block the EAC.[6,7,10,15] Further, tinnitus may develop if the tick attaches to the eardrum. Indudharan et al[19] described a rare tick infestation, with tympanic membrane perforation, that resulted in paralysis of the facial nerve. They speculated that the perforation of the tympanic membrane enabled tick saliva to enter the middle ear and reach the facial nerve through the natural dehiscence of the facial canal. They also suggested that tick saliva may include neurotoxins. Therefore, strict follow-up is required if the tympanic membrane is perforated.
Determination of whether or not a tick's mouth parts are attached to the skin is essential before attempting its removal from the EAC because any remaining tick parts may trigger the development of a foreign body granuloma, damaging the ear canal.[20] Accordingly, the cases presented in this report provide excellent examples of the 2 scenarios a clinician may encounter during the diagnosis of an unattached tick in the EAC of a patient.
Patient 1 was particularly problematic because the tick filled the narrow EAC, and its mouth parts were not visible by otoscopy. In such situations, Hartman's forceps may be used to gently move the body around to determine if the mouth parts are attached to the skin. If they are not, tick may be safely removed in its entirety. In the literature, previous reports of tick removal from the EAC have recommended resection of the skin around the parasite's mouth parts, if attachment to the skin cannot be confirmed.[8,9,14,15] However, the procedure described in the present report is a superior, minimally invasive approach that does not involve skin excision when the tick's mouth parts are only suspected of being embedded in the skin. Our new maneuver allows for the determination of tick attachment and should be utilized to minimize any EAC morbidity.
The case of the 72-year-old patient was intriguing because the tick had obviously been feeding on the host, resulting in its engorged state. However, the mouth part was not attached to the skin, as clearly indicated by the otoscopic examination. This discrepancy may be explained by the life cycle of the tick. In this case, the patient had suffered from intermittent tinnitus and aural pruritis for 2 weeks, suggesting that the tick had been feeding for that period of time. During the life-cycle, the female A testudinarium tick feeds on the host over a period of 7 to 12 days.
Hard ticks are important vectors of diseases in humans, for example, Rickettsia japonica (Japanese spotted fever), Francisella tularensis (rabbit fever), and Borrelia burgdorferi (Lyme disease). However, not all tick bites cause disease because not all ticks are infected. This fact has led to some controversy regarding which tick bites should be treated with antibiotics. Hashimoto et al[21] reported that only 56 (8%) of 700 patients with tick bites developed Lyme disease between 1995 and 2000 in Hokkaido prefecture, Japan. Nonetheless, Shimizu[22] recommended that standard dose antibiotics (minocycline) should be prophylactically administered for 1 week to prevent Lyme disease after the removal of a tick. Therefore, we administered prophylactic antibiotics to our patients.
4. Conclusion
This case series presents 2 cases of A testudinarium infestation in the EAC, and proposes a simple protocol for determining if the tick is anchored to the ear canal, even when the diagnosis cannot be confirmed by a routine otoscopic examination. The utilization of this technique minimizes the EAC morbidity associated with earlier, more invasive recommendations.
Acknowledgment
The authors are grateful to Dr Teruki Kadosaka (Department of Parasitology, Aichi Medical University) for identification of the tick species in these 2 patients.
Footnotes
Abbreviation: EAC = external auditory canal.
The authors have no conflicts of interest to disclose.
References
- [1].Somayaji KSG, Rajeshwari A. Human otoacariasis. Indian J Otolaryngol Head Neck Surg 2007;59:237–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Shibghatullah AH, Abdullah MK, Pein CJ, et al. Acute labyrinthitis secondary to aural tick infestation. Southeast Asian J Trop Med Public Health 2012;43:857–9. [PubMed] [Google Scholar]
- [3].Fujita H, Watanabe Y, Ishikura M, et al. List of all isolates of spotted fever group rickettsiae from ticks in Japan 1993–1998. Ann Rep Ohara General Hosp 1999;42:45–50. [Google Scholar]
- [4].Imaoka K, Kaneko S, Tabara K, et al. The first human case of rickettsia tamurae infection in Japan. Case Rep Dermatol 2011;3:68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dutkiewicz J, Cisak E, Sroka J, et al. Biological agents as occupational hazards—selected issues. Ann Agric Environ Med 2011;18:286–93. [PubMed] [Google Scholar]
- [6].Yoneda Y, Fukma T, Itoh T, et al. Eleven human cases infested with ixodid ticks. Med Entomol Zool 1992;43:263–6. [Google Scholar]
- [7].Ohi K, Onodera R, Kawada T, et al. Two cases of biotic foreign bodies in the external auditory canal (Haemaphysalis Longicornis). Otolaryngol Head Neck Surg (Tokyo) 1992;64:227–31. [Google Scholar]
- [8].Saiki T, Shinohara T, Aritomo H, et al. Two cases of ixodid tick in the external auditory canal. Otolaryngol Head Neck Surg (Tokyo) 1994;66:617–20. [Google Scholar]
- [9].Morita T, Tabuchi K, Kitajiri S. Tick bite in the external auditory canal: a human case report. Pract Otol (Kyoto) 1998;91:1103–6. [Google Scholar]
- [10].Nakayama T, Kawaguchi H, Saito K, et al. A case of tick bite in the external auditory canal. Otolaryngol Head Neck Surg (Tokyo) 2003;75:809–11. [Google Scholar]
- [11].Nishiyama A, Nakajima Y, Masuzama T, et al. A tick-bite in the left ear: a case report. Ann Rep Ohara Hosp 1995;38:51. [Google Scholar]
- [12].Masaki T, Okamoto M, Hoshino I. Biotic foreign body in the external auditory canal (Dermacentor sp); case report. Pract Otol (Kyoto) 2004;97:21–4. [Google Scholar]
- [13].Miyamoto K, Nakao M. Frequent occurrence of human tick bites and monthly fluctuation of ixodid ticks in Hokkaido, Japan. Jpn J Saint Zool 1991;42:267–9. [Google Scholar]
- [14].Kogashiwa Y, Moro Y, Kono N, et al. A case of tick bite (Ixodes persulcatus) in the external auditory canal. Otolaryngol Head Neck Surg (Tokyo) 2009;81:670–2. [Google Scholar]
- [15].Iwasaki S, Takebayashi S, Watanabe T. Tick bites in the external auditory canal. Auris Nasus Larynx 2007;34:375–7. [DOI] [PubMed] [Google Scholar]
- [16].Yamaguchi N. Human tick bite: variety of tick species and increase of cases. Saishin-Igaku 1989;44:903–8. (in Japanese). [Google Scholar]
- [17].Dilrukshi PR, Yasawardene AD, Amerasinghe PH, et al. Human otoacariasis: a retrospective study from an area of Sri Lanka. Trans R Soc Trop Med Hyg 2004;98:489–95. [DOI] [PubMed] [Google Scholar]
- [18].Gökdoğan O, Çakabay T, Baran H, et al. Otoacariasis: demographic and clinical outcomes of patients with ticks in the ear canal. Braz J Otorhinolaryngol 2016;82:416–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Indudharan A, Dharap AS, Ho TM. Intra-aural tick causing facial palsy. Lancet 1996;348:613. [DOI] [PubMed] [Google Scholar]
- [20].Castelli E, Caputo V, Morello V, et al. Local reactions to tick bites. Am J Dermatopathol 2008;30:241–8. [DOI] [PubMed] [Google Scholar]
- [21].Hashimoto Y, Kinouchi M, Takahashi H, et al. An epidemic study of 700 cases with tick bites in Hokkaido prefecture during the past 6 years: relationship to Lyme disease. Jpn J Dermatol 2002;112:1467–73. [Google Scholar]
- [22].Shimizu H. Shimizu H. Text of Modern Dermatology. 1st edn.Tokyo: Nakayama Shoten Co.; 2005. 500–1. [Google Scholar]


