Abstract
Fibrocytes, ahematopoietic stem cell source of fibroblasts/myofibroblasts, were previously implicated to infiltrate into the intestinal and enhance inflammation.
The aims of the present study were to elucidate the role of fibrocytes in necrotizing enterocolitis (NEC) pathogenesis and to explore the mechanisms by which fibrocytes contributed to the inflammatory responses.
We investigated circulating and intestinal local fibrocytes from 32 patients with NEC, 8 patients with noninflammatory conditions of the gastrointestinal tract and 12 normal subjects.
Significantly higher numbers of circulating fibrocytes were found in the peripheral blood from NEC patients than the controls (P < .01). Numerous fibrocytes were found infiltrating the NEC intestinal mucous membranes. The percentage of fibrocytes to total leukocytes in the NEC inflammatory lesions was significantly increased compared with the percentage in the noninflammatory gastrointestinal tract. The fibrocyte attractant chemokine C-X-C motif chemokine ligand 12 (CXCL12) was significantly increased in the plasma and was detectable in 80% of the peritoneal lavage fluid from NEC patients but not the controls. Furthermore, chemokine expression was increased in fibrocytes infiltrating and trafficking to leukocyte sites. In culture, lipopolysaccharide (LPS) induced a significant increase in the expression of the Toll-like receptor (TLR4) signal, with the upregulation of p38 in both the isolated fibrocytes and macrophages. Similarly, interleukin (IL)-1β induced increased the upregulation of the IL-6, tumor necrosis factor (TNF)-α, and intercellular cell adhesion molecule-1 mRNAs but downregulated ColI in fibrocytes isolated from NEC patients compared with the controls.
These findings indicate that circulating fibrocytes are increased in NEC patients and may be recruited to the inflammatory intestinal track, most likely through the CXCR4/CXCL12 axis. These cells may contribute to intestinal inflammation through TLR4 signaling by producing the TNF-α and IL-6 cytokines.
Keywords: circulating fibrocytes, necrotizing enterocolitis (NEC), SDF-1/CXCL12, TLR4
1. Introduction
Necrotizing enterocolitis (NEC) is the most common gastrointestinal disease in infants. Its incidence has become more prevalent in recent years due to the increasing survival of low-birth weight neonates.[1,2] The prominent pathophysiology of NEC is characterized by persistent active gastrointestinal hemorrhagic inflammatory necrosis that affects the intestinal tracts of premature neonates. Although any segments of the gastrointestinal tract can be involved, the terminal ileum and proximal colon are the most commonly involved positions.[3,4] Over the previous 10 years, accumulating studies have penetrated all aspects of the mechanisms mediating NEC, including gut barrier functions, vascular dysfunction, infectious complications, and intensive proinflammatory cytokine production.[5,6] Interestingly, the anti-inflammatory cytokine interleukin-10 (IL-10) was reported to dampen the inflammatory responses and protect against NEC.[7] Although a variety of components have been implicated, the exact cellular and molecular mechanisms involved in NEC pathogenesis are unknown.
Circulating fibrocytes are round-shaped mesenchymal cells that arise from monocyte precursors. Although these cells comprise only approximately 0.1% to 0.5% of peripherally circulating cells in normal humans, they are present in injured organs and participate in many biological processes.[8] In response to injurious and inflammatory stimuli, fibrocytes are believed to undergo tissue recruitment and activation from circulation. Fibrocytes are involved in the pathologies of inflammation and fibroblast activation, which is a key regulatory step in the inflammatory response.[9] In response to acute injury stress, fibrocytes can express a macrophage-like inflammatory gene program that encodes proinflammatory cytokines and chemokine receptors involved in antigen presentation and leukocyte trafficking. Fibrocytes can also function as antigen-presenting cells for CD8+ T cell activation by expressing main histologic complex class I and II molecules and the costimulatory proteins CD80 and CD86.[10] Clinically, fibrocytes are involved in several inflammatory and fibrotic conditions, such as inflammatory bowel disease[11] and idiopathic pulmonary fibrosis.[12] However, the role of fibrocytes in NEC pathogenesis is unknown.
The purpose of this study was to test whether fibrocytes were involved in the pathogenesis of human NEC. Therefore, we evaluated the trafficked number of fibrocytes in inflamed tissues from NEC patients. We also investigated the response of tissue fibrocytes in the intestinal mucosa following exposure to bacterial components.
2. Methods
2.1. Patient population and sample collection
Patients with detailed clinical data were consecutively recruited from January 2013 to July 2015 at the Affiliated Hospital of Chongqing Medical University, Chongqing, People's Republic of China. The study protocols for consent, data collection, and privacy protection were approved by the Institutional Review Board of the Chongqing Medical University, and written informed consent was obtained from the patients’ guardians. The NEC diagnosis was based on the criteria originally proposed by Bell et al and subsequently modified by Walsh and Kliegman.[13] The criteria for enrollment included a gestational age of more than 34 weeks and stage III NEC requiring bowel surgery. The exclusion criteria were patients with the presence of an acute pulmonary bacterial infection, intestinal malformation (aproctia, intestinal atresia, or Hirschsprung disease), and severe cardiac dysfunction. In addition, to minimize severity differences in the study population, patients managed in the intensive care unit (ICU) for more than 30 days were excluded. Once the NEC case was included in the present study, detailed clinical data from the medical records, including all physicians’ and nurses’ notes, daily flow sheets, laboratory and radiographic reports, underlying illnesses, surgical records, and medications, were reviewed by one of the investigators. Tissue was processed for histological evaluation by light microscopy to confirm the diagnosis of NEC. Patients with NEC underwent serial blood sampling preoperatively and at 1 week after laparotomy. Resected intestine remnants and 10-mL peritoneal lavage samples were obtained during laparotomy from all cases. Control groups with comparable age to patients with NEC included patients with noninflammatory conditions intestine tissue (n = 8, intussusception) and the volunteers matched by age and sex were studied as control subjects for determination of the circulating fibrocytes and serum SDF-1/CXCL12.
2.2. Isolation of fibrocytes and treatment
The peripheral blood of patients with NEC and healthy donors were subjected to peripheral blood mononuclear cells (PBMCs) isolation using a gradient with Ficoll-Paque (Amersham Biosciences, Piscataway, NJ). The PBMCs were further undertaken positive selection for CD14+ monocytes with anti-CD14 Dynabeads (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. Cells were then cultured for 48 hours in fibronectin-coated dishes (BD Biosciences, San Jose, CA) in Dulbecco's modified Eagle's medium (Life Technologies, Gaithersburg, MD) supplemented with 20% fetal bovine serum (FBS; Invitrogen). Nonadherent cells were then removed by gentle aspiration, and they were cultured for another 14 days. Some of sample was further subjected to flow cytometry (FCM) analysis to determine the fibrocytes using specific fibrocyte markers. Isolated cultured fibrocytes were treated with 10 lg/mL of lipopolysaccharide (LPS, InvivoGen, San Diego) for 12 hours.
2.3. Immunohistochemistry of surgical specimens
Details of the immunohistological staining protocols were published elsewhere.[14] Briefly, deparaffinized 4-μm thick sections were incubated with the primary SDF-1/CXCL12 (1:200; R&D Systems, Minneapolis, MN) antibody for 2 hours at room temperature, followed by incubation for 1 hour with the appropriate secondary antibody. The sections were incubated with the AB enzyme reagent for 30 minutes. The peroxidase substrate was added for stain development. The sections were dehydrated, permanently mounted with mounting medium, and observed by light microscopy.
2.4. Dual immunofluorescence
The deparaffinized sections were incubated with the anti-CD45 (mouse anti-human CD45; Santa Cruz Biotechnology, Santa Cruz, CA) and anti-collagen type I (rabbit anti-human Col I; Santa Cruz Biotechnology, Santa Cruz, CA) antibodies in the previously described blocking solution.[14] This process was followed by a second incubation for 1 hour with the donkey anti-mouse Alexa Fluor 488 (isotype IgG, 1:200, ab21202; Invitrogen) and donkey anti-rabbit Alexa Fluor 594 (isotype IgG; 1:200, ab21207; Invitrogen) secondary antibodies. The tissue sections were counterstained with 4’,6-diamidino-2-phenylindole (DAPI; 1:1000; Sigma-Aldrich, Ann Arbor, MI) and mounted with glycerol. Negative control sections were incubated with either normal rabbit serum instead of the anti-collagen type I antibody or normal mouse serum instead of the anti-CD45 antibody during all of the staining procedures, and no cross-reactivity was observed. The samples were analyzed using a Zeiss Axioplan 2 fluorescence microscope (Carl Zeiss, Inc., Thornwood, NY), equipped with FITC (green), PE (red), and DAPI (blue) filter sets. All digital images were processed and merged using the Photoshop software, version 6.0 (Adobe Systems Inc., San Jose, CA). For quantitative measurement of fibrocytes, double-stained cells were counted in a field with 6 randomly selected areas (×1000 magnification). For quantitative measurement of tissue fibrocytes, stained cells were acquired in 3 nonoverlapping random fields and counted as the number of fibrocytes per mm2.
2.5. Flow cytometric analysis
Three milliliters (3 mL) of peripheral venous blood was collected in a sterile test tube and frozen at -80°C before processing for FCM and the enzyme-linked immunosorbent assay (ELISA). Freshly isolated intestinal tissues were gently minced, passed through a 40-μm cell strainer, and centrifuged at 300g. Mononuclear cells were resuspended in RPMI 1640 medium (Invitrogen, Carlsbad, CA) and stained with the PE-conjugated surface antigen anti-CD45 and PerCP-Cy5-conjugated surface antigen anti-CXCR4 (CD45-PE, 368509; CXCR4-PerCP-Cy5.5, 306515; Biolegend, San Diego, CA) or selectively PE-conjugated anti-intercellular cell adhesion molecule-1 (ICAM1) (BD Biosciences, San Jose, CA). Then, the cells were permeabilized with a Cytofix/Cytoperm kit (BD Biosciences) for intracellular antigen detection before intracellular staining of collagen-1. Next, the cell pellet was incubated with a FITC-conjugated antihuman collagen-1 antibody (Rockland Immunochemicals, Gilbertsville, PA) or the IgG isotype control for 30 minutes. The cell suspension was analyzed with a BD FACScan flow cytometer (BD Biosciences, San Jose, CA) using the CellQuest 3.2.1f1 software. All data were analyzed with the FlowJo software (Tree Star, Inc., Ashland, OR). Baseline leukocyte counts were expressed as a percentage of the total leukocyte counts.
2.6. ELISA measurement of SDF-1/CXCL12
The concentration of SDF-1/CXCL12 in the plasma and peritoneal lavage samples was determined by ELISA using commercially available ELISAs according to the manufacturer's instructions (Quantikine; R&D Systems, Minneapolis, MN). The optical densities were determined using a microtiter plate reader (Multiscan RC Type 351; Labsystems, Helsinki, Finland) at 405 nm, with a correction wavelength set at 540 or 570 nm. The blank was subtracted from duplicate readings for each standard and sample without any knowledge of survival or other clinical data. All of the analyses and calibrations were performed at least in duplicate. The mean values were used for the statistical analysis.
2.7. Quantitative real-time PCR
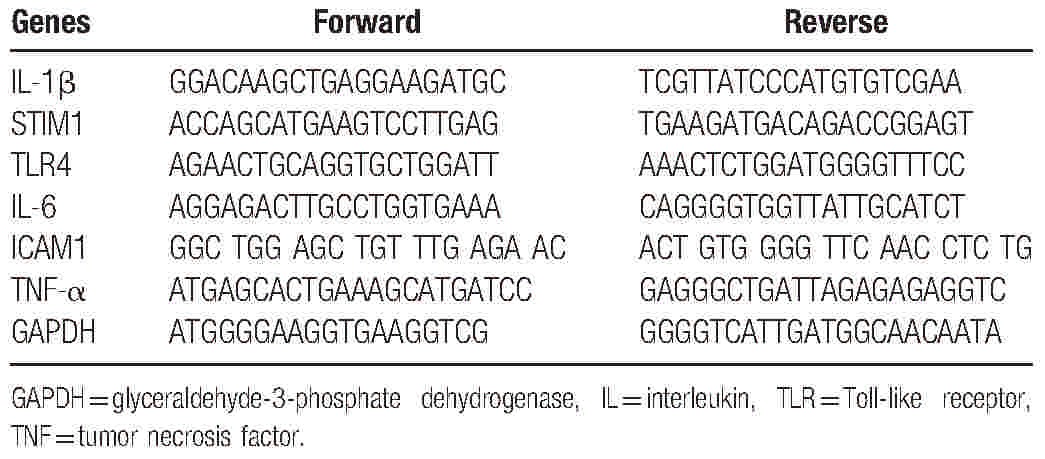
PBMCs were separated from whole blood by Ficoll–Hypaque density gradient centrifugation. After isolating the PBMCs, positive selection for CD14+ monocytes was performed with anti-CD14 Dynabeads (Invitrogen) according to the manufacturer's protocol. Nonadherent cells were removed, and the CD14+ monocytes were cultured for 14 days. The cultured fibrocytes were treated with 10 μg/mL of LPS (a TLR4 agonist; InvivoGen, San Diego, CA) and IL-1β (10 ng/mL) (401-ML-025; R&D Systems) separately. At the scheduled time points, the cultured cells were subjected to RNA extraction using TRIzol (Invitrogen, Carlsbad, CA). Quantitative real-time PCR was performed using proprietary primers and probes (Taqman Gene Expression Assays; Applied Biosystems, Foster City, CA) to measure mRNA expression with the primers summarized in Table 1 (synthesized by GenePharma, Inc., Shanghai, China). glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. The relative quantification method[14] was used to determine the Ct values for the PCR products of the target genes and GAPDH. The relative quantification formula was: 2−ΔCt × 100%, where ΔCt = Ct(target gene) − Ct(GAPDH).
Table 1.
Primer sequences used for the qRT-PCR analysis.
2.8. Statistical analysis
Statistical comparisons were conducted with GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA) and SPSS 13.0 (SPSS Inc., Chicago, IL). Continuous variables are expressed in the figures as the mean ± SEM. Student t test and the Mann–Whitney U test were used to compare distributed continuous variables. The 1-way analysis of variance (ANOVA) multiple comparison test was used to identify differences in mRNA expression and quantitative histological measurements. Statistical significance was accepted for 2-sided P values less than .05.
3. Results
3.1. Patient characteristics
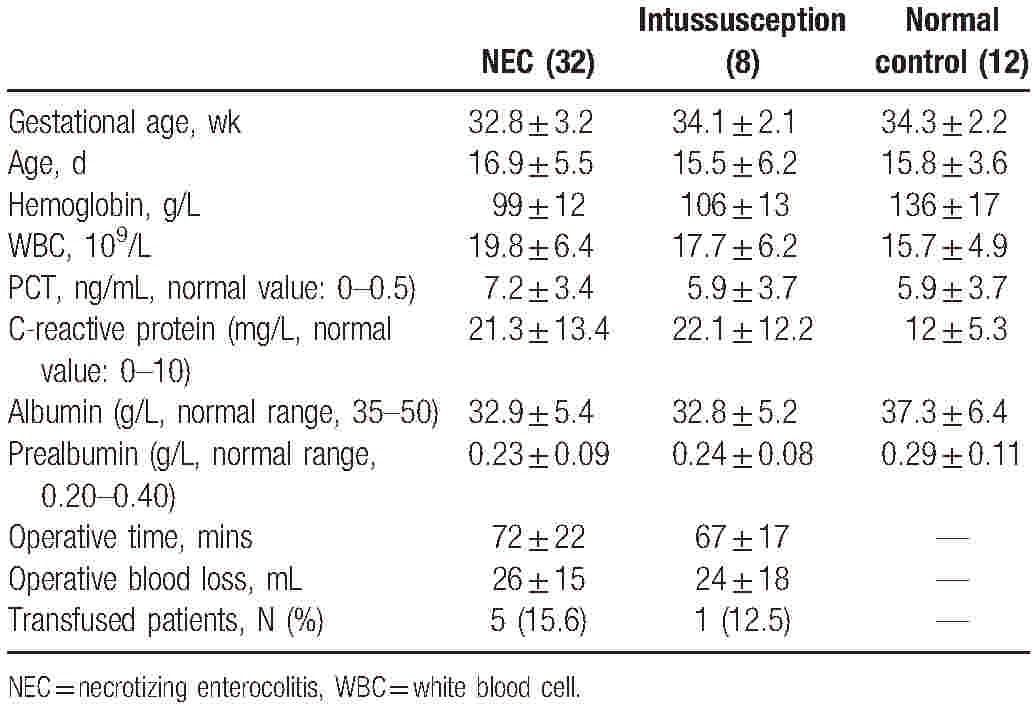
The baseline features of the infants are summarized in Table 1. Thirty-two patients met the inclusion criteria for admission, with 20 cases for PBMCs measurement and 12 cases for surgical sample collection. Serial blood sampling was performed preoperatively and at 1 week after emergency gastrointestinal surgery during routine clinic visits. Control groups with comparable ages to the NEC patients included patients who required gastrointestinal tract (e.g., intussusception) resection with noninflammatory conditions (n = 8) and normal control for PBMC collection (n = 12)There were no significant differences in the demographic features of the patients between the 2 surgical groups, including the mean age, gender distribution, and initial mean body weight. Nutritional characteristics were also similar between the 2 groups as assessed by the mean serum prealbumin and albumin concentrations. In addition, there were no significant differences in the surgical approach between the patients with NEC and intussusception (Table 2). The operative magnitude was evaluated by measurement of the operative time, estimated blood loss, and total units of blood transfused within the 24-hour perioperative period. View it in a separate window
Table 2.
Comparison of demographic and clinical characteristics between infants with and without NEC.
3.2. Necrotizing enterocolitis is characterized by fibrocyte infiltration
The tissue specimen sections from the NEC and noninflammatory gastrointestinal tracts were evaluated with hematoxylin and eosin staining. A prominent inflammatory infiltrate was indicated in NEC patients compared with the noninflammatory gastrointestinal tract samples (Fig. 1A). To characterize fibrocyte infiltration in NEC, the surgical specimens were subjected to immunofluorescence with CD45 and collagen-1 (ColI) to identify fibrocytes in the intestinal mucosa. The CD45+ColI+ fibrocytes were primarily found in varying numbers and locations in the mucosal layers and the submucosal layers. In the inflammatory intestines of NEC patients, CD45+ColI+ fibrocytes preferentially infiltrated into the inflammatory mucosa, whereas fibrocytes were scarcely found in the intestines without inflammation (Fig. 1B). Next, we determined the number of fibrocytes in NEC patients. Significantly higher numbers of fibrocytes were found in the NEC patients than in the patients with noninflammatory intestinal tracts (P < .01). The CD45+ColI+ fibrocyte counts in the randomly selected areas were remarkably increased in the inflammatory lesions compared with the intestines without inflammation (P < .01) (Fig. 1C), indicating that fibrocytes might exacerbate inflammation in NEC.
Figure 1.
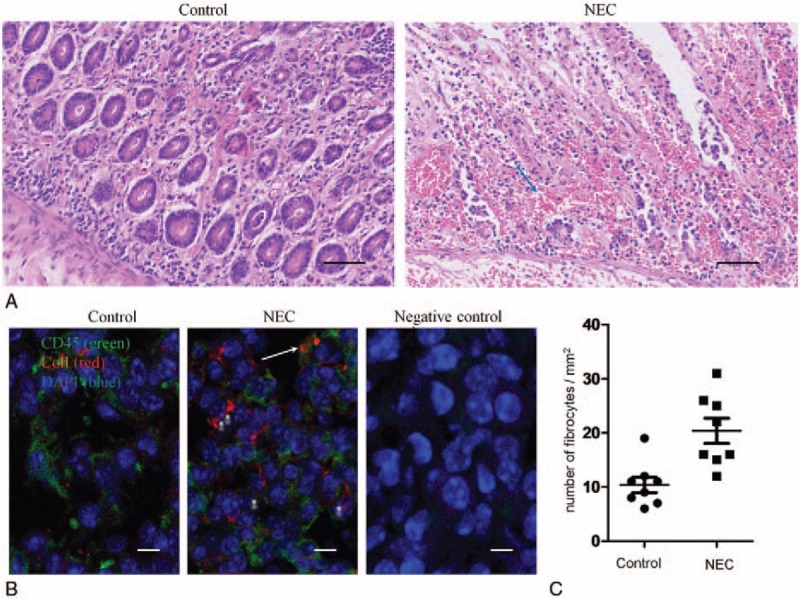
Necrotizing enterocolitis (NEC) is characterized by a fibrocyte-rich inflammatory infiltrate. (A) Histologic H&E staining of intestinal tissue obtained from patients with NEC and noninflammatory gastrointestinal tracts as previously described. Left panel: Normal intestine (jejunum) from a 21-day-old neonate who underwent emergency surgery and was diagnosed with intestinal atresia showing normal cellularity and crypt villus histoarchitecture. Right panel: NEC in a 23-day-old neonate showing epithelial necrosis and a prominent infiltrating cell infiltrate (hematoxylin & eosin, Bar = 50 μm). Blue arrow, a few polymorphonuclear leukocytes (PMNs). (B) Dual immunofluorescence of intestinal tissues obtained from patients with NEC and noninflammatory gastrointestinal tracts with CD45 (green), ColI (red), and 40,6-diamidino-2-phenylindole (DAPI) (blue) staining; the fluorescence microscopy images were merged and evaluated. Scale bar 10 μm. (C) The number of CD45+ColI+ cells per mm2 in surgical specimens from patients with NEC and noninflammatory gastrointestinal tracts. Horizontal lines represent the mean ± standard error of the mean (SEM). ∗P < .01. Arrows point to colocalization by both CD45 and ColI-positive cells and ∗ represents various fibrocyte morphologies. Bar = 10 μm.
3.3. Fibrocytes are increased in the plasma and local intestinal tissues of NEC patients
We determined the overall circulating fibrocyte counts in the peripheral blood and intestinal tissues using quantitative FCM analysis. As shown in Figure 2A and B, CD45+ColI+ fibrocytes were significantly elevated in the peripheral blood and intestinal tissues of the NEC patients (n = 20) compared with the patients without intestinal inflammation (n = 8) and the age- and gender-matched healthy controls (n = 12) (P = .002). The percentage of CD45+ColI+ fibrocytes/total CD45+ cells in the peripheral blood was equivalent to the percentage in the intestinal tissue.
Figure 2.
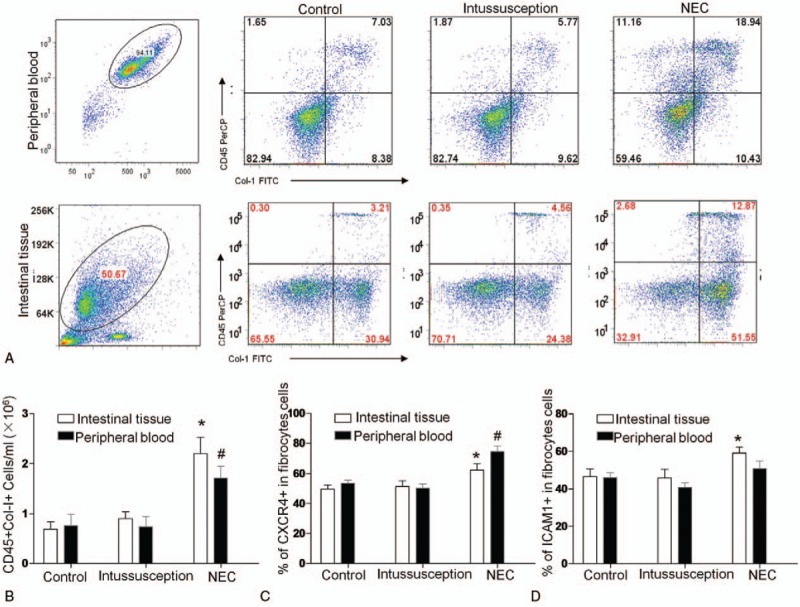
Flow cytometric analysis of circulating fibrocytes and their subpopulations in NEC patients, patients who required resection for noninflammatory conditions of the gastrointestinal tract and normal subjects. (A) Representative flow cytometry analysis of CD45+Col-I+fibrocytes. The histogram indicates the average number of (B) CD45+Col-I+, (C) CD34+CD45+Col-I+, (D) Col-I+CD45+CXCR4+, and (E) Col-I+CD45+ αSMA cells that are present (n = 20). The data are expressed as the mean ± SEM. ∗P < .05, †P < .05, vs Control, 1-way ANOVA.
Among the numerous microenvironmental cytokines, the CXCR4/CXCL12 chemokine axis plays a key role in the homing of fibrocytes to injured tissues. Next, we characterized circulating fibrocytes by evaluating CXCR4 and ICAM1 expression because CXCR4 facilitates fibrocyte recruitment and ICAM1 may influence leukocyte trafficking. We found that the majority of the CD45+ColI+ cells expressed CXCR4 and ICAM1 (Fig. 2B, C). In addition, CXCR4+ fibrocytes were significantly increased in the peripheral blood and the intestinal tissues from the NEC patients compared with the patients without intestinal inflammation and the healthy controls, respectively, demonstrating their enhanced ability for recruitment to wound sites. At the same time, the resident ICAM1+ fibrocytes were significantly increased in the NEC patients, as shown in Figure 2C, indicating their enhanced leukocyte trafficking ability.
3.4. SDF-1/CXCL12 concentration in NEC patients
We examined the concentration of the CXCR4 ligand SDF-1/CXCL12 in the plasma and peritoneal lavage samples from NEC patients. As illustrated in Figure 3A, the plasma concentration of this chemokine was significantly higher in the NEC patients than in the patients (n = 20) without intestinal inflammation (n = 8) and the normal subjects (n = 12). Similarly, SDF-1/CXCL12 was detected in most of the NEC peritoneal lavage samples but was undetectable in the control peritoneal lavage samples (Fig. 3B).
Figure 3.
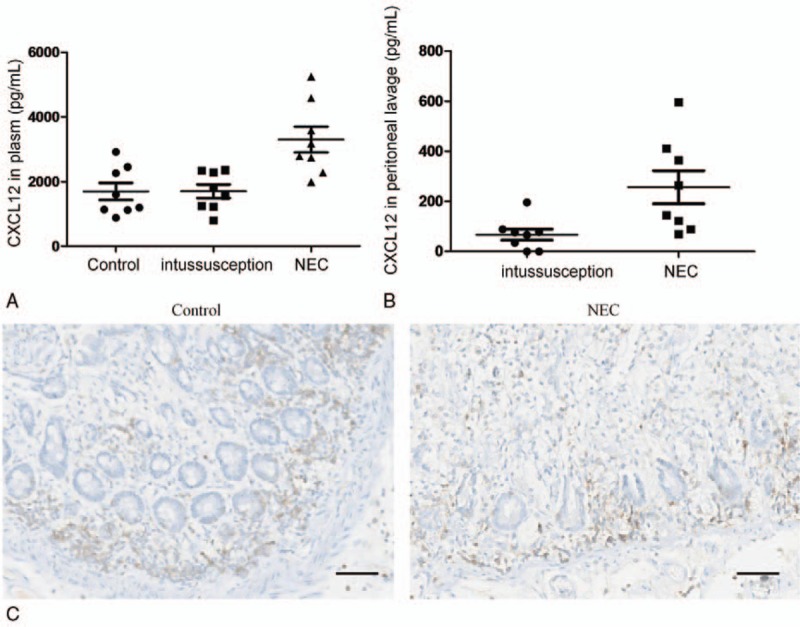
Concentration and localization of SDF-1/CXCL12 in NEC patients. The SDF-1/CXCL12 concentration in the plasma (A) and peritoneal lavage (B) as indicated within the figures in NEC patients, patients who required resection for noninflammatory conditions of the gastrointestinal tract and normal subjects. (C) Immunolocalization of SDF-1/CXCL12 in patients with NEC and noninflammatory gastrointestinal tracts. Representative photomicrographs of immunohistochemical staining performed with the CXCL12 antibody in tissue sections. Bar = 50 μm. A strong immunoreactive protein was observed in NEC and was found primarily at the apex of the enterocytes, whereas no or weak staining was detected in the normal intestinal tissues, as exemplified in (C). All sections were counterstained with hematoxylin. Original magnification 400x. CXCL12 = C-X-C motif chemokine ligand 12.
Next, we examined the expression and localization of CXCL12 in intestinal sections from NEC patients in which fibrocytes were found using immunohistochemistry. As shown in Figure 3C, the immunoreactive protein was strongly expressed throughout the crypts and villi of the intestinal epithelium, whereas no or weak SDF-1/CXCL12 expression was found in the samples from patients without intestinal inflammation. In addition, increased SDF-1/CXCL12 expression was noted in the submucosa and muscularis layers, which may represent SDF-1/CXCL12 induction within the inflammatory cells in these layers.
3.5. Continuous stimulation by LPS leads to TLR4 signaling activation in fibrocytes
We investigated whether fibrocytes exerted their functions through TLR signaling because TLR4 was reported to be pivotal for NEC pathogenesis. We cultured fibrocytes from peripheral blood obtained during emergency gastrointestinal surgery from NEC patients (n = 12) and patients without intestinal inflammation (n = 8) for 14 days as described in the “ Methods.” As shown in Figure 4A, circulating fibrocytes from NEC expressed TLR4, and this expression was elevated by LPS exposure. The important TLR4 signaling downstream molecule (release of phosphorylated p38) was also elevated upon LPS treatment (Fig. 4B). In response to IL-1β, TNF-α mRNA expression was remarkably upregulated, which represented a proinflammatory ability in fibrocytes from NEC patients. In addition, the ICAM1 mRNA was remarkably upregulated, which would promote leukocyte trafficking. Conversely, LPS treatment significantly downregulated ColI mRNA expression (Fig. 4C).
Figure 4.
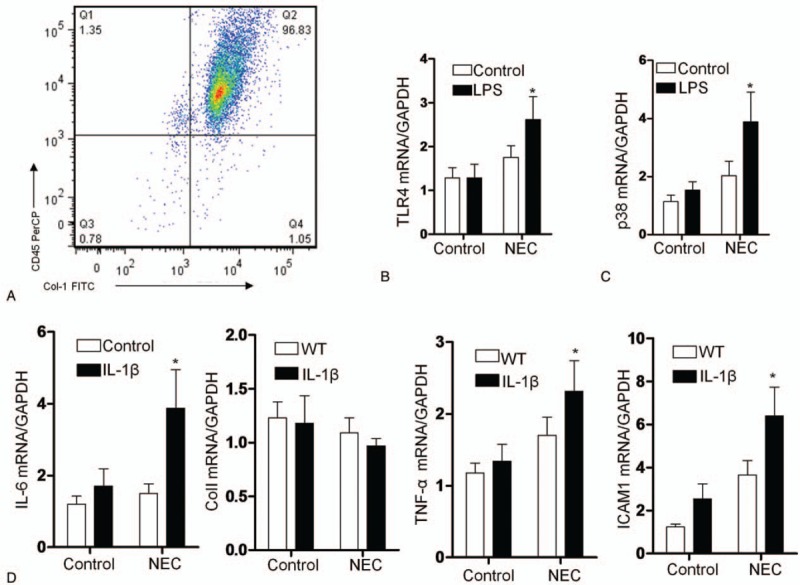
Gene expression in cultured human fibrocytes. (A) The histogram indicates the flow cytometric analysis of cultured fibrocytes with CD45+Col-I+ cells. Comparison of the TLR4 (B) and p38 mRNA (C) expression levels in cultured fibrocytes from patients treated with LPS (10 μg/mL) for 24 hours. (D) Comparison of the TNF-α, IL-6, ColI, and ICAM1 mRNA expression levels in cultured fibrocytes treated with IL-1β (10 ng/mL) for 24 hours. The expression levels were calculated as the mean level of induction compared to the housekeeping gene β-actin. Columns: average values of a minimum of three independent experiments; bars: ±SEM. ∗P < .01 compared with the corresponding air-exposed control (1-way ANOVA). ICAM1 = intercellular cell adhesion molecule-1.
4. Discussion
This study showed a significant increase in fibrocytes (CD45+ColI+) in the intestinal mucosa of NEC patients. Similar differences were detected for other circulating fibrocyte subpopulations, including CD45+ColI+CDCR4+ cells and CD45+ColI+ICAM1+ cells. Furthermore, the plasma and peritoneal fluid SDF-1/CXCL12 levels were elevated in NEC patients, implying the recruitment capability of these extraintestinal cells. We also quantified elevated levels of the pro-inflammation cytokines IL-6 and TNF-β and recruiting leukocytes in the NEC patients, suggesting their involvement in the pathogenesis of this disease. Cultured fibrocytes from the patients activated inflammatory TLR4 signals in response to LPS, suggesting that these cells may contribute to the accelerated inflammation by producing several proinflammatory cytokines.
Fibrocytes were revealed to be effector cells in chronic inflammation and were implicated in the pathogenesis of the chronic inflammatory state.[15,16] Consistent with this finding, we demonstrated for the first time a significant increase of fibrocytes in the intestine in NEC patients compared with resected intestines from patients with noninflammatory gastrointestinal conditions. This increase was consistently noted in all 12 NEC specimens with a variable spectrum of intestinal injury, although we could not correlate the abundance of fibrocytes with the severity of the intestinal injury. The results of the present study were in line with studies showing that fibrocytes emerged in inflammatory tissues, including colitis, asthma, Crohn disease, scleroderma, Graves’ disease, and rheumatoid arthritis.[17–20] To the best of our knowledge, no other studies have demonstrated the presence of fibrocytes in NEC patients. Our previous data demonstrated that the appearance of spindle-shaped mature fibrocytes and a-SMA myofibroblasts occurred during the healing phase of bronchopulmonary dysplasia, suggesting that these cells were recruited to repair the damage and not simply to amplify inflammation. Another possibility is that fibrocytes in the inflamed intestine may have a protective mechanism as suggested in other diseases, such as idiopathic pulmonary fibrosis. Another study showed that the number of fibrocytes was decreased in the fibrotic tissues of patients with Crohn disease,[18,21] which seemed to be paradoxical according to the hypothesis that fibrocytes were directly involved in the inflammatory reaction of NEC. Although it is beyond the scope of this study to speculate that fibrocytes may play a role in the repair of the mucosa, the induction of fibrocytes suggests a role in the inflammatory response of NEC. The comprehensive function of fibrocytes in NEC pathogenesis should be investigated in long-term processes in a future study.
The chemokine receptors CXCR4 and CCR7 are reported to be pivotal for the recruitment of fibrocytes to injured sites.[22,23] Here, we confirmed that a major proportion of human fibrocytes (nearly 80%) in the peripheral blood of NEC patients expressed CXCR4. At the same time, the CXCR4 receptors were present at a high level in the serum samples from NEC patients, which was similar to observations in patients with fibrotic lung disease.[24,25] Moreover, neutralization of CXCL12 using antibodies resulted in reduced recruitment of circulating fibrocytes to the injured lung and attenuated fibrosis.[26] The presence of these chemokine receptors further implied that the fibrocytes of patients isolated with CD45 and Col-I from peripheral blood leukocytes had a high ability to infiltrate to the injured sites, although no similar blocking experiment was performed to investigate their mechanism in gastroenterological disease. Some studies have proposed that fibrocytes are putative effector cells in the progression of inflammation in Crohn disease, where the accumulation of fibrocytes was found in the inflamed intestinal mucosa. Our results revealed that tissues fibrocytes were preferentially found in areas of inflammation close to inflammatory cells, such as macrophages and T lymphocytes, which potentially represents a process of migration and homing of these cells to these intestinal cell populations. During inflammation episodes, ICAM1 is primarily responsible for the migration and recruitment of neutrophils, monocytes, and macrophages into tissues in response to injury.[27] Importantly, we showed a significantly higher population of ICAM1-positive circulating fibrocytes in NEC patients than the controls, suggesting a high ability for leukocyte trafficking in NEC pathogenesis.
At present, it is unclear by which molecular mechanisms fibrocytes amplify the inflammatory and immune responses. The TLR ligand LPS is regarded as a predominant signal transduction pathway involved in exacerbating a wide variety of inflammatory genes in the intestinal mucosa during NEC pathogenesis.[28,29] We specifically examined TLR4 activation in isolated fibrocytes by LPS treatment and demonstrated for the first time that cultured human fibrocytes derived from the peripheral blood of patients with NEC expressed the TLR4 mRNA. In addition, we demonstrated that various cytokines (e.g., inflammatory cytokines, such as TNF-α and IL-6, and tissue remodeling factors, such as ColI) were increased in infants with NEC and might be responsible for the induction of TLR4 activation in NEC. Recent studies have shown that fibrocytes stimulated by TLR ligands produce a higher amount of IL-6 than the plasmacytoid dendritic cells.[30] LPS treatment specifically and remarkably upregulated the TNF-α and ColI mRNA levels in fibrocytes. Consistent with this finding, our investigation of signaling pathways in fibrocytes suggested that TLR signaling affected the function of the fibrocytes, thereby supporting the hypothesis that fibrocytes could infiltrate into the bacterial component rich environment and respond to TLR ligands in the inflammatory intestinal tissue of NEC patients. We postulate that fibrocytes may potentially be an even more attractive target for the treatment of inflammatory intestinal conditions because they are the sources of IL-6 and other proinflammatory cytokines.
Certain weakness should be considered in this study. Due to the purely cross-sectional design of the present study and the small sample size, we did not draw a line between the circulating fibrocytes infiltrated into the inflammatory lesions and NEC, although these cells were present in the intestinal mucosa of the NEC patients. Although these questions remain open, the current study has described circulating fibrocytes as a unique inflammatory infiltration profile observed in NEC, which supports the proinflammatory role of circulating fibrocytes involved in NEC pathogenesis. Further elucidation of the nature and the contribution of fibrocyte infiltration will be essential.
5. Conclusion
For the first time, we found increased fibrocyte counts in NEC patients that were possibly involved in the pathogenesis of this disease. The fibrocytes might enhance inflammation by TLR4 signaling to promote cytokine activation and leukocyte recruitment. However, these findings should be confirmed or refuted by larger studies. To understand the contribution of fibrocytes and to identify a potential therapeutic target for the effective treatment of this disorder, complementary studies should investigate the additive effects of fibrocytes in detail.
Acknowledgments
We thank Prof. Xianqing Jin for providing technical assistance and insightful discussions during the preparation of the manuscript. We thank Dr. Xiaoyong Zhang of the Wistar Institute (USA) for providing medical writing services.
Footnotes
Abbreviations: IL = interleukin, LPS = lipopolysaccharide, NEC = Necrotizing enterocolitis, PBMCs = peripheral blood mononuclear cells, TLR= Toll-like receptor.
Authorship: YL collected the clinical samples and carried out the images analysis and the quantitative PCR assays. QS and ZG drafted the manuscript. CG participated in the design of the study and performed the statistical analysis. CG conceived the study and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Funding/support: This research was supported by the National Natural Science Foundation of China (No. 81270058 and 30770950) and by the Chongqing Natural Science Foundation (CSTC, 2009BB6072).
The authors declare that they have no competing interests.
References
- [1].Patel RM, Knezevic A, Shenvi N, et al. Association of red blood cell transfusion, anemia, and necrotizing enterocolitis in very low-birth-weight infants. JAMA 2016;315:889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kantorowska A, Wei JC, Cohen RS, et al. Impact of donor milk availability on breast milk use and necrotizing enterocolitis rates. Pediatrics 2016;137:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cherrington NJ, Estrada TE, Frisk HA, et al. The hepatic bile acid transporters Ntcp and Mrp2 are downregulated in experimental necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 2013;304:G48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Halpern MD, Khailova L, Molla-Hosseini D, et al. Decreased development of necrotizing enterocolitis in IL-18-deficient mice. Am J Physiol Gastrointest Liver Physiol 2008;294:G20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yazji I, Sodhi CP, Lee EK, et al. Endothelial TLR4 activation impairs intestinal microcirculatory perfusion in necrotizing enterocolitis via eNOS-NO-nitrite signaling. Proc Natl Acad Sci U S A 2013;110:9451–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sodhi CP, Shi XH, Richardson WM, et al. Toll-like receptor-4 inhibits enterocyte proliferation via impaired beta-catenin signaling in necrotizing enterocolitis. Gastroenterology 2010;138:185–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Emami CN, Chokshi N, Wang J, et al. Role of interleukin-10 in the pathogenesis of necrotizing enterocolitis. Am J Surg 2012;203:428–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Reilkoff RA, Bucala R, Herzog EL. Fibrocytes: emerging effector cells in chronic inflammation. Nat Rev Immunol 2011;11:427–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Galligan CL, Fish EN. The role of circulating fibrocytes in inflammation and autoimmunity. J Leukoc Biol 2013;93:45–50. [DOI] [PubMed] [Google Scholar]
- [10].Chesney J, Bacher M, Bender A, et al. The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc Natl Acad Sci U S A 1997;94:6307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sazuka S, Katsuno T, Nakagawa T, et al. Fibrocytes are involved in inflammation as well as fibrosis in the pathogenesis of Crohn's disease. Dig Dis Sci 2014;59:760–8. [DOI] [PubMed] [Google Scholar]
- [12].Moeller A1, Gilpin SE, Ask K, et al. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2009;179:588–94. [DOI] [PubMed] [Google Scholar]
- [13].Sharma R, Hudak ML. A clinical perspective of necrotizing enterocolitis: past, present, and future. Clin Perinatol 2013;40:27–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Han W, Guo C, Liu Q, et al. Aberrant elastin remodeling in the lungs of O2-exposed newborn mice; primarily results from perturbed interaction between integrins and elastin. Cell Tissue Res 2015;359:589–603. [DOI] [PubMed] [Google Scholar]
- [15].Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol 2011;11:762–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Abe R, Donnelly SC, Peng T, et al. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol 2001;166:7556–62. [DOI] [PubMed] [Google Scholar]
- [17].Peng H, Herzog EL. Fibrocytes: emerging effector cells in chronic inflammation. Curr Opin Pharmacol 2012;12:491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rieder F, Fiocchi C. Intestinal fibrosis in inflammatory bowel disease: progress in basic and clinical science. Curr Opin Gastroenterol 2008;24:462–8. [DOI] [PubMed] [Google Scholar]
- [19].Metz CN. Fibrocytes: a unique cell population implicated in wound healing. Cell Mol Life Sci 2003;60:1342–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lei PP, Qu YQ, Shuai Q, et al. Fibrocytes are associated with the fibrosis of coronary heart disease. Pathol Res Pract 2013;209:36–43. [DOI] [PubMed] [Google Scholar]
- [21].Sorrentino D. Fibrocytes, inflammation, and fibrosis in Crohn's disease: another piece of the puzzle. Dig Dis Sci 2014;59:699–701. [DOI] [PubMed] [Google Scholar]
- [22].Wang CH, Punde TH, Huang CD, et al. Fibrocyte trafficking in patients with chronic obstructive asthma and during an acute asthma exacerbation. J Allergy Clin Immunol 2015;135:1154–62.e1–5. [DOI] [PubMed] [Google Scholar]
- [23].Dupin I, Allard B, Ozier A, et al. Blood fibrocytes are recruited during acute exacerbations of chronic obstructive pulmonary disease through a CXCR4-dependent pathway. J Allergy Clin Immunol 2016;137:1036–42.e7. [DOI] [PubMed] [Google Scholar]
- [24].Wang CH, Huang CD, Lin HC, et al. Increased circulating fibrocytes in asthma with chronic airflow obstruction. Am J Respir Crit Care Med 2008;178:583–91. [DOI] [PubMed] [Google Scholar]
- [25].Gomperts BN, Strieter RM. Fibrocytes in lung disease. J Leukoc Biol 2007;82:449–56. [DOI] [PubMed] [Google Scholar]
- [26].Phillips RJ, Burdick MD, Hong K, et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest 2004;114:438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Guo Y, Mishra A, Howland E, et al. Platelet-derived Wnt antagonist Dickkopf-1 is implicated in ICAM-1/VCAM-1-mediated neutrophilic acute lung inflammation. Blood 2015;126:2220–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shang Q, Bao L, Guo H, et al. Contribution of glutaredoxin-1 to S-glutathionylation of endothelial nitric oxide synthase for mesenteric nitric oxide generation in experimental necrotizing enterocolitis. Transl Res 2016;[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [29].Meng D, Zhu W, Shi HN, et al. Toll-like receptor-4 in human and mouse colonic epithelium is developmentally regulated: a possible role in necrotizing enterocolitis. Pediatr Res 2015;77:416–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Balmelli C, Alves MP, Steiner E, et al. Responsiveness of fibrocytes to toll-like receptor danger signals. Immunobiology 2007;212:693–9. [DOI] [PubMed] [Google Scholar]


