Abstract
Background:
Microcurrent electrical neuromuscular stimulation (MENS) has been suggested to improve muscle function and restore damaged muscle. However, current evidence is insufficient to determine the effectiveness of this therapy in age-dependent muscle weakness. Therefore, a prospective, randomized, double-blinded, sham-controlled clinical trial was designed to evaluate the effects of short-term MENS on muscle function in the elderly.
Methods:
A total of 38 healthy elderly participants aged 65 years and above were enrolled and randomly divided into 2 stimulation groups: real or sham MENS group. Real or sham MENS were applied to the 8 anatomical points of the dominant arm and leg during the course of 40 minutes. We performed muscle function tests at baseline and after the intervention: the handgrip strength tests (HGS, kg), the root mean square values (RMS, μV), and the single leg heel-rise tests (HRT) to determine changes in the strength, activity, and endurance of the elderly muscle, respectively.
Results:
In the real MENS group, the mean values of the HGS and the number of plantar flexions were significantly increased, but the RMS value of the electromyography signal was significantly decreased after the real intervention (P < .05). However, the sham MENS group showed a significant decrease in the number of plantar flexions and the total time for HRT after the sham stimulation (P < .05). The mean difference in the RMS value was significantly lower, but the number of plantar flexions and the total time for HRT was significantly higher in the real MENS group than in the sham MENS group (P < .05).
Conclusion:
The findings suggest that short-term application of MENS may play a partial role in enhancing physical activities of the elderly, as it can improve some muscle function.
Keywords: aged, elecrical stimulation, endurance, MENS, microcurrent, muscle, strength
1. Introduction
The loss of muscle force in aging process might worsen physical function that in turn causes adverse health outcomes, such as falling, disability, poor quality of life, and even mortality.[1–4] The skeletal muscle mass gradually decreases along with age-related alteration of the endocrinal and metabolic system.[5,6] Previous studies have explained that the age-dependent muscle weakness can be induced by impaired release of sarcoplasmic reticulum (SR) Ca2+ in underlying rise in cytoplasmic (Ca2+)cyt.[7–9] MENS is a physical modality delivering subsensory current to the tissues within the microampere (μA) range and thus mimics the electrical intensity found in living tissue. If microcurrents of a physiological amperage were delivered into damaged muscle tissues, it could control the altered membrane function by various mechanisms, such as the maintenance of intracellular Ca2+ homeostasis and the upregulation of ATP production.[10–12] Previous studies revealed that treatment of muscle damage with microcurrent therapy with low amperage <500 μA can reduce the severity of muscle symptoms.[11,13] However, the mechanism of MENS has not yet been clarified and it is presumed that MENS has various therapeutic effects owing to differences in microcurrent parameters such as ampere and frequency. The hypothesis of this journal is that MENS with tailored parameters could improve muscle function in the elderly by preventing muscle damage and promoting recovery. As far as we are aware, there have been no randomized clinical trials that have used microcurrent therapy for the rapid improvement of muscle function. The aim of this study was to evaluate the effects of short-term MENS on the strength, endurance, and activity of muscle in the elderly.
2. Materials and methods
2.1. Study design
This study used a prospective, randomized, double-blinded, sham-controlled design and was performed at Soonchunhyang University Hospital (SCHUH), Seoul, South Korea, from February 2016 to December 2016. The study was approved by the Institutional Review Board (SCHUH 2016–01–005–001) of SCHUH. Written informed consent was obtained from each participant. The trial was registered in the South Korea Clinical Trials Registry at https://cris.nih.go.kr (KCT0001976). Thirty-eight eligible participants were randomly divided into 2 groups (real and sham MENS groups) at a 1:1 allocation ratio. The participants, outcome assessors, and data analysts were blinded to group allocation.
2.2. Participants
Healthy volunteers who were 65 years of age and older were enrolled in this study (Fig. 1). Participants were excluded if any of the following criteria were present: neurologic deficits related with central nervous system disorders, peripheral neuropathies, neuromuscular junction disorders, or myopathy; history of brain or spine surgery or peripheral nerve entrapment surgery; severe bony deformities or articular contracture; hypersensitive reaction to surface electrode; senile dementia, impaired cognitive function, and severe psychological disorders; alcohol or drug abuse; ineligibility as confirmed by a physiatrist; refusal to participate in this study; or an inability to communicate in the Korean language.
Figure 1.
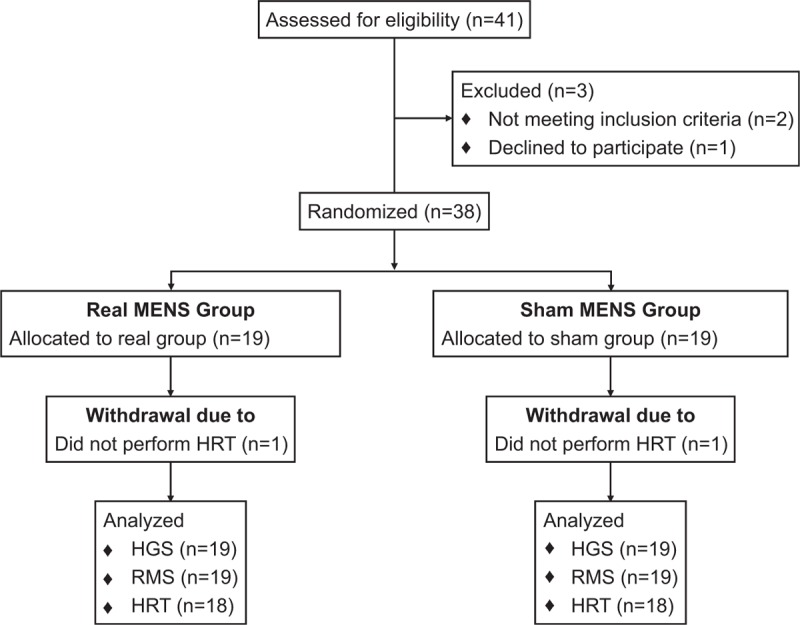
CONSORT flowchart of participants through the trial.
2.3. Randomization
Following the Consolidated Standards of Reporting Trials (CONSORT) statement, participants were randomly selected using a computerized random number generator by a professional statistician who did not know their assignment. Block randomization was conducted for eligible participants, and the written informed consents were obtained. Participants were randomly assigned to the real MENS group or the sham MENS group. The participants, outcome assessors, and data analyst were masked to the allocation.
2.4. Interventions
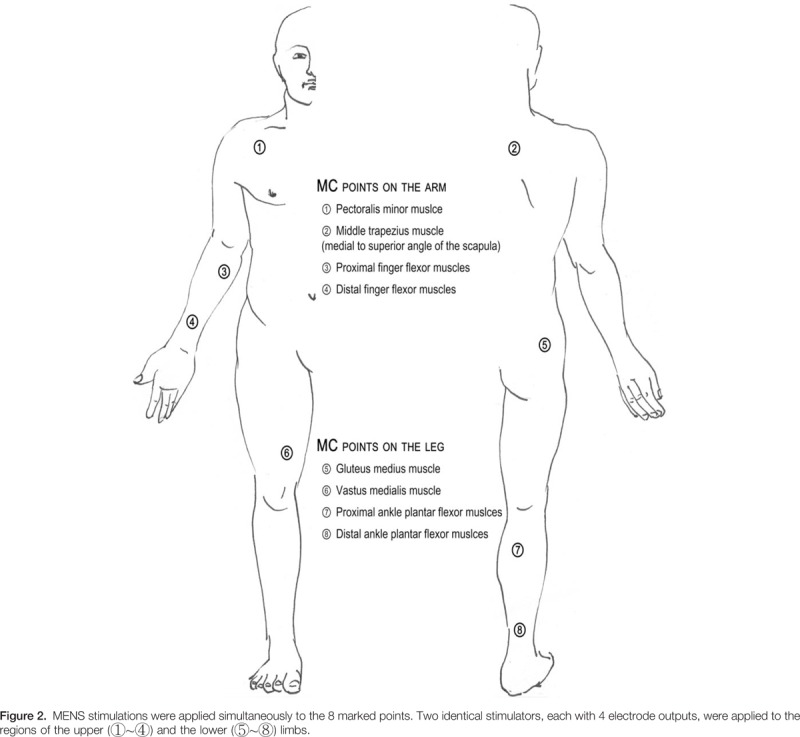
We applied real or sham MENS for 40 minutes on the 8 anatomical points of the dominant arm and leg, as illustrated in Figure 2. The application points were established based on previous reports,[14,15] which found that providing stability to the proximal muscle group may contribute to efficient power generation of the distal muscle group. The microcurrent generator (EMI; Cosmic Co, Seoul, Korea) was a portable handheld device that provides 4 electrode outputs. We applied 2 identical microcurrent devices to each the upper and lower limbs. It was programmed to provide an alternating current characterized by a monophasic rectangular pulse format, with polarity reversal every 3 seconds. The frequency was 8 Hz and the intensity was 25 μA. This current intensity was significantly lower than the human sensory threshold. The appearance of the sham microcurrent stimulator was identical to the real stimulator, but there was no electrical current, even when it was operational. During the 40 minutes of application, participants were allowed to perform gentle movements so that the equipment installed on the body was not hindered.
2.5. Outcome measurements
All outcomes were measured at baseline and after the application. Body composition was measured at baseline and the details of adverse events were recorded throughout the trial. The study protocol is presented in Figure 3. The primary outcomes were parameters of the HGS and the HRT. The secondary outcome was the RMS values of the EMG signal.
Figure 3.
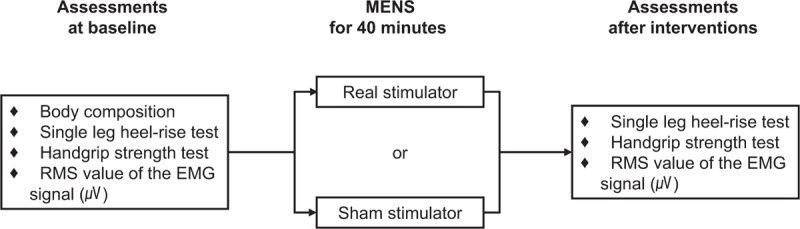
Study design for intervention and outcome assessments.
Single leg heel-rise test (HRT)[16,17] was designed to evaluate muscle endurance in elderly subjects. The parameters of HRT were: number of plantar flexions, time in seconds, and velocity (plantar flexions per second). The HRT was performed with the individual standing barefoot. Participants were supported by the dominant hand on the wall to maintain balance. An instrument was created so that the individual performed plantar flexion with a maximal range across all repetitions. To determine the full range of plantar flexion, participants initially performed the first plantar flexion as high as possible until they were supported by metatarsophalangeal joints. Then, the heights with their ankle in a full plantar flexion were measured using an elastic height-measuring instrument, and it was fixed at 1 cm below the level. To ensure the quality of the HRT, the subjects were instructed to touch the instrument with their head during every plantar flexion repetitions. Participants repeated plantar flexions as much as possible at full speed until they could no longer repeat owing to fatigue. Verbal instructions were given only at the beginning of the trial and no encouragements were given during the evaluation. The examiner registered the number of repetitions and the total time required for the trial.
Handgrip strength test (HGS, kg)[18] of the dominant arm was performed to evaluate handgrip muscle strength using a Jamar dynamometer fixed with the level II. Participants sat with an upright spine with their head in a neutral position and relaxed shoulders. The arm was adducted to the trunk with the elbow in a neutral position. The hip joints were flexed at 90 degree and slightly abducted with both feet supported on the ground. Only the test administrator was able to read the values and the participant did not know the values achieved. There was a break interval of approximately 45 seconds between each trial. The mean value (kilogram) of the 3 trials was calculated for statistical analysis. A total of 2 measurement sessions were obtained from each participant at baseline and after the intervention.
The root mean square values (RMS, μV)[19] of the EMG signal were measured during the maximal isometric contraction of the grasp muscles at the same time with HGS testing. The data of muscle spectrography were collected using a Medelec Synergy electromyographic device (Oxford Instruments Medical Inc, UK). The surface EMG signal was recorded with a pair of disc electrodes applied to the flexor digitorum profundus muscle belly and 4 cm apart using electroconducting gel with Ag/AgCl2 (Natus Neurology Inc). The EMG signal was sampled at 48 kHz and digitally processed with a filter “band pass” (10 Hz∼10 kHz) to reduce noise. The participant's skin surface was cleaned with alcohol and hair was removed before the electrodes were placed to reduce the chance of capturing interference. The precautions can reduce the surface impedance and improve the signal conductivity. The capture time of muscle activity was 50 ms during a period of maximal grasping. As with the HGS test, there was approximately a 45-second rest interval between each trial. The mean RMS value (μV) of the 3 trials was used for statistical analysis.
2.6. Statistical analysis
To estimate the sample size, we conducted a pilot trial as no previous studies could provide guidance regarding population size and power. In the trial, 9 volunteers underwent the HGS and the EMG test for evaluating RMS value before and after microcurrent stimulation. Among the outcomes, the RMS value was selected to estimate the number of subjects. To detect this difference in a randomized controlled trial using a paired t test assuming a 5% 2-tailed significant level (an alpha of 0.05), a power of 90%, and a 20% dropout rate, the suggested sample size was 18 subjects per group. With a slight increase in sample size, a total of 38 subjects (19 subjects in each group) participated in the present study. All demographic data are presented as the mean and standard deviation (SD), unless otherwise noted. Normality of distribution was assessed with the Shapiro-Wilk test. Student t test or Mann-Whitney U test was used for comparing the continuous variables and the χ2 test or Fisher exact test was utilized for comparing the categorical variables. Comparison between at baseline and after intervention data within each group was analyzed using a paired t test or Wilcoxon signed rank test. All variables with a P < .05 were considered statistically significant. Statistical Package for the Social Sciences (SPSS) software 14.0 (SPSS Inc, Chicago, IL) was used to conduct data analysis.
3. Results
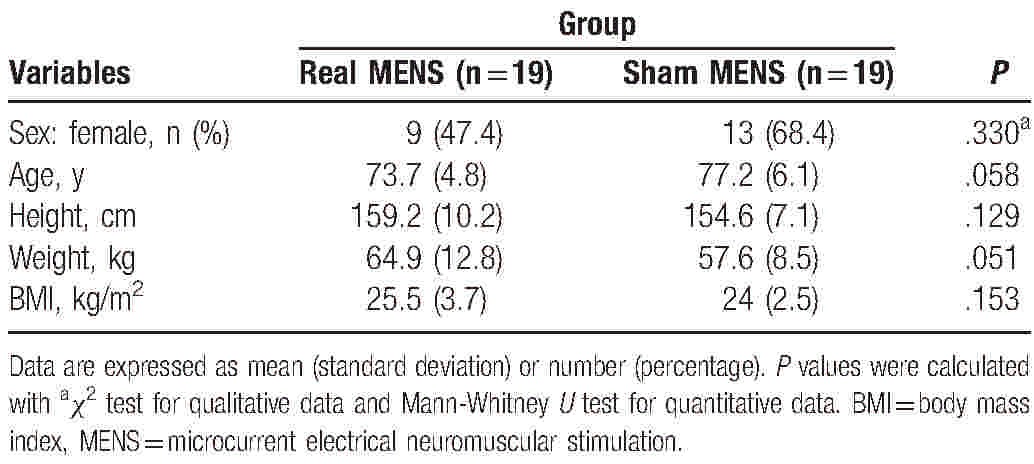
A total of 41 participants were initially screened and enrolled in the study (Fig. 1). Of these 41 subjects, 2 did not meet the study inclusion criteria and 1 declined to participate. Therefore, 38 eligible participants were randomized into the real and the sham MENS group. All 38 subjects were evaluated using the HGS and the EMG evaluation tests, but 2 subjects did not perform the HRT because of an acute muscle cramp. Participant characteristics are summarized in Table 1. The 2 groups did not differ significantly in all the characteristics at the baseline visit. At baseline, the mean (SD) age was 73.7 (4.8) years in the real MENS group and 77.2 (6.1) years in the sham MENS group. The body mass index (BMI) was 25.5 (3.7) in the real MENS group and 24.0 (2.5) in the sham MENS group.
Table 1.
Baseline characteristics of participants.
We examined the mean change from baseline by intervention and the difference (95% CI) between the real and sham MENS groups to evaluate the efficacy of real MENS in enhancing muscle function. Results from functional testing after 40 minutes of interventions are summarized in Tables 2 and 3. Regarding the changes in HGS and the RMS values from baseline (Table 2, Fig. 4), HGS (kg) was significantly increased (P = .01), while the RMS value of the EMG signal (microvolt) was significantly decreased after real microcurrent stimulation (P = .003). However, the sham MENS group displayed no significant differences in HGS (kilograms) and the RMS of the EMG signal (microvolt) after the sham microcurrent stimulation. The mean difference in the RMS following intervention was significantly lower in the real MENS group than in the sham MENS group (P = .002). Regarding the mean changes of the HRT variables form baseline (Table 3, Fig. 5), the real MENS group showed a significant increase in the number of plantar flexions (P = .006) and a tendency to increase the total time (seconds) of the HRT (P = .09) after real intervention. However, the sham MENS group showed a significant decrease in the number of plantar flexions (P = .02) and the total time (seconds) for HRT (P = .04) after the sham intervention. The mean difference in the number of plantar flexions (P < .001) and the total time for HRT (P = .005) was significantly higher in the real MENS group than in the sham MENS group. However, the velocity (number of plantar flexions per second) and the relative value of HRT was not different both in the mean change from baseline by intervention and in the mean difference between the 2 groups. There were no serious adverse events throughout the trial. However, 2 participants could not complete the HRT because of a calf muscle cramp that disappeared shortly after stopping the evaluation.
Table 2.
Comparison of handgrip strength tests within groups and between groups.
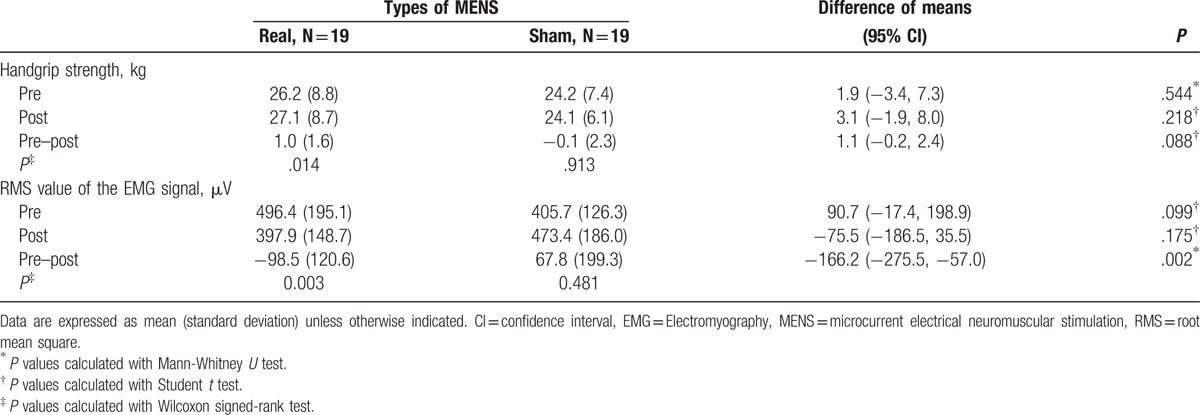
Table 3.
Comparison of single leg heel-rise test within groups and between groups.
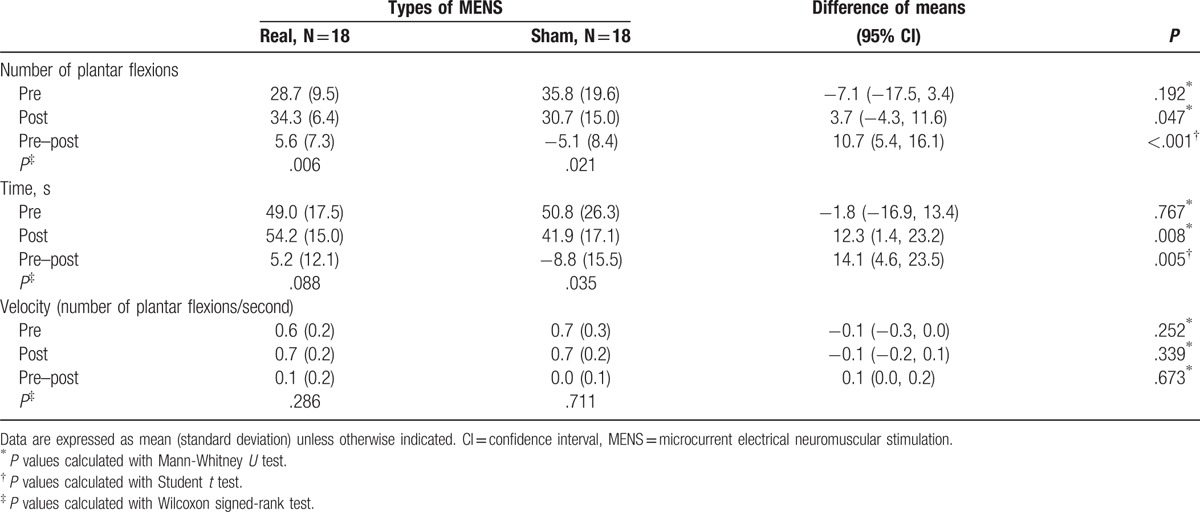
Figure 4.
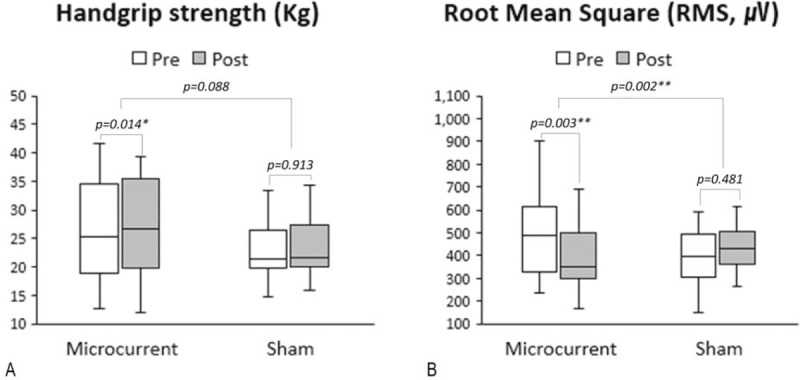
Box plots (median, IQR, minimal, and maximal values) illustrated absolute changes from baseline in the HGS (A) and the RMS of EMG signal (B) in each real and sham MENS group. Real MENS significantly increased HGS (kilogram), while it markedly reduced the RMS value of the EMG signal that represents the activity of muscle fibers. Wilcoxon signed-ranks test was used for comparison between at baseline and after intervention data within each group and Student's t-test or Mann-Whitney U test was used for comparing the median differences between group. ∗ = P < .05, ∗∗ = P < .01, EMG = electromyography, HGS = handgrip strength, IQR = interquartile range, MENS = microcurrent electrical neuromuscular stimulation, RMS = root mean square.
Figure 5.
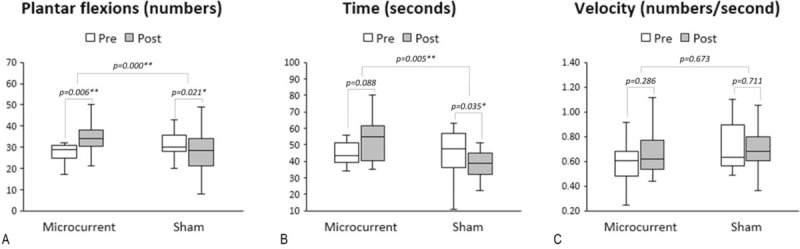
Box plots (median, IQR, minimal, and maximal values) illustrated absolute changes from baseline in the HRT variables in each real and sham MENS group. Real MENS significantly increased the number of plantar flexions. However, sham MENS significantly decreased the number of plantar flexions and the total time (seconds) for HRT. The median difference in the number of plantar flexions and the total time for HRT was significantly higher in the real MENS group than in the sham MENS group. Wilcoxon signed-ranks test was used for comparison between at baseline and after intervention data within each group and Student's t-test or Mann-Whitney U test was used for comparing the median differences between group. ∗ = P < .05, ∗∗ = P < .01, HRT = single leg heel rise test, IQR = interquartile range, MENS = microcurrent electrical neuromuscular stimulation.
4. Discussion
The results of this study met our hypothesis that real microcurrent stimulation was superior in enhancing muscle function in healthy elderly subjects after 40 minutes of short-term application compared with the sham microcurrent stimulation. Previous studies reported that tailored microcurrent stimulation can be helpful in restoring or regenerating damaged muscles. Studies on the efficacy of microcurrent stimulation for recovery from delayed onset muscle soreness (DOMS) have reported that 200 μA of microcurrent therapy or an electromembrane microcurrent patch of 20 μA provided significant protection and recovery from DOMS.[11,13] Moreover, recent studies have reported that the MENS could be effective in promoting the regeneration process of injured skeletal muscles or the activation of local stem cells in animal models.[20–22] However, there might be no conclusion as to the specific mechanisms of the results and whether these mechanisms can be also helpful for rapid improvements in muscle function. In this study, real microcurrent stimulation significantly improved the mean change in handgrip strength (kilograms), whereas it markedly reduced the RMS value of the EMG signal that represents the activity of muscle fibers. It is possible that participants in the real MENS group achieved more handgrip power efficiently through minimal recruitment of the muscle. Moreover, the real microcurrent stimulation also significantly improved the numbers of plantar flexions and time for HRT. However, the sham MENS group showed the opposite results, which is thought to be because muscle fatigue induced by baseline HRT was not sufficiently relieved during the 40 minutes of sham microcurrent stimulation. The way in which microcurrent therapy caused the discrepancy between the 2 groups is not known. Previous studies also reported that microcurrent therapy can prevent muscle damage compared to the sham treatment.[10,11,23] Excessive accumulation of intracellular Ca2+ can alter membrane integrity, which gradually induces morphological and functional changes in the contractile structure of the muscle.[9,10] The main mechanism of MENS is maintaining intracellular Ca2+ homeostasis in damaged muscle cells.[10,23] Muscle weakness during aging suggests that the Ca2+-dependent excitation-contraction coupling might be impaired.[7,8,10] During muscle contraction, membrane depolarization activates voltage sensing Ca2+ channels in transverse tubules (Cav1.1). The activated Ca2+ channels can then activate the SR Ca2+ release channel and ryanodine receptor 1 (RYR-1). The subsequent increase in cytoplasmic [Ca2+]cyt is essential for attenuating age-dependent muscle strength.[7,8,10] Our previous study (unpublished data) showed that calcium homeostasis was achieved after application of microcurrent (25 μA, 8 Hz) for 1 minute to increase intracellular calcium by tachypacing in mouse heart cells. However, the mechanism of impaired SR Ca2+ release in aging muscle has to be explained.
This study had several limitations. First, it is difficult to determine the definition of the healthy elderly because the spectrum of physical abilities might be wide, even though the same selection criteria were applied. Therefore, it is unclear whether the results are applicable to all elderly population, regardless of the degree of muscle dysfunction, or to other age groups. Second, the application time of 40 minutes can be too short to cause sufficient changes in muscle function, although the main purpose of this study was to observe improvements in muscle performance through short-term MENS. Finally, this study did not include a follow-up evaluation. Thus, the long-term effects of MENS on muscle function still need to be addressed in the future.
In conclusion, the results of this study found that short-term application of MENS could enhance the muscle functions in the elderly. Long-term randomized, controlled trials with follow-up assessments are needed to confirm our results.
Footnotes
Abbreviations: BMI = body mass index, CI = confidence interval, DOMS = delayed onset muscle soreness, EMG = Electromyography, HGS = handgrip strength test, HRT = single leg heel-rise test, IQR = interquartile range, MENS = Microcurrent electrical neuromuscular stimulation, RMS = root mean square, SD = standard deviation, SR = sarcoplasmic retinaculum.
This research was supported by the Soonchunhyang University Research Fund.
The authors report no conflicts of interest.
References
- [1].Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 2002;50:889–96. [DOI] [PubMed] [Google Scholar]
- [2].Kamel HK. Sarcopenia and aging. Nutr Rev 2003;61(5 pt 1):157–67. [DOI] [PubMed] [Google Scholar]
- [3].Nair KS. Aging muscle. Am J Clin Nutr 2005;81:953–63. [DOI] [PubMed] [Google Scholar]
- [4].Newman AB, Kupelian V, Visser M, et al. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc 2003;51:1602–9. [DOI] [PubMed] [Google Scholar]
- [5].Lee ES, Park HM. Prevalence of sarcopenia in healthy Korean elderly women. J Bone Metab 2015;22:191–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zamboni M, Mazzali G, Fantin F, et al. Sarcopenic obesity: a new category of obesity in the elderly. Nutr Metab Cardiovasc Dis 2008;18:388–95. [DOI] [PubMed] [Google Scholar]
- [7].Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 2008;88:287–332. [DOI] [PubMed] [Google Scholar]
- [8].Andersson DC, Marks AR. Fixing ryanodine receptor Ca leak—a novel therapeutic strategy for contractile failure in heart and skeletal muscle. Drug Discov Today Dis Mech 2010;7:e151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wessberg GA, Carroll WL, Dinham R, et al. Transcutaneous electrical stimulation as an adjunct in the management of myofascial pain-dysfunction syndrome. J Prosthet Dent 1981;45:307–14. [DOI] [PubMed] [Google Scholar]
- [10].Kwon DR, Park GY. Efficacy of microcurrent therapy in infants with congenital muscular torticollis involving the entire sternocleidomastoid muscle: a randomized placebo-controlled trial. Clin Rehabil 2014;28:983–91. [DOI] [PubMed] [Google Scholar]
- [11].Lambert MI, Marcus P, Burgess T, et al. Electro-membrane microcurrent therapy reduces signs and symptoms of muscle damage. Med Sci Sports Exerc 2002;34:602–7. [DOI] [PubMed] [Google Scholar]
- [12].Maenpaa H, Jaakkola R, Sandstrom M, et al. Does microcurrent stimulation increase the range of movement of ankle dorsiflexion in children with cerebral palsy? Disabil Rehabil 2004;26:669–77. [DOI] [PubMed] [Google Scholar]
- [13].Curtis D, Fallows S, Morris M, et al. The efficacy of frequency specific microcurrent therapy on delayed onset muscle soreness. J Bodyw Mov Ther 2010;14:272–9. [DOI] [PubMed] [Google Scholar]
- [14].Kobesova A, Dzvonik J, Kolar P, et al. Effects of shoulder girdle dynamic stabilization exercise on hand muscle strength. Isokinet Exerc Sci 2015;23:21–32. [Google Scholar]
- [15].Thomas EM, Sahlberg M, Svantesson U. The effect of resistance training on handgrip strength in young adults. Isokinet Exerc Sci 2008;16:125–31. [Google Scholar]
- [16].Lunsford BR, Perry J. The standing heel-rise test for ankle plantar flexion: criterion for normal. Phys Ther 1995;75:694–8. [DOI] [PubMed] [Google Scholar]
- [17].Monteiro DP, Britto RR, Lages AC, et al. Heel-rise test in the assessment of individuals with peripheral arterial occlusive disease. Vasc Health Risk Manag 2013;9:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Han SH, Nam KS, Ahn TK, et al. Analysis of Grip and Pinch Strength in Korean People. J Korean Orthop Assoc DE - 2009-04-01 2009;44:219–25. [Google Scholar]
- [19].Fukuda TY, Echeimberg JO, Pompeu JE, et al. Root mean square value of the electromyographic signal in the isometric torque of the quadriceps, hamstrings and brachial biceps muscles in female subjects. J Appl Res 2010;10:32–9. [Google Scholar]
- [20].Fujiya H, Ogura Y, Ohno Y, et al. Microcurrent electrical neuromuscular stimulation facilitates regeneration of injured skeletal muscle in mice. J Sports Sci Med 2015;14:297–303. [PMC free article] [PubMed] [Google Scholar]
- [21].Ohno Y, Fujiya H, Goto A, et al. Microcurrent electrical nerve stimulation facilitates regrowth of mouse soleus muscle. Int J Med Sci 2013;10:1286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zickri MB. Possible local stem cells activation by microcurrent application in experimentally injured soleus muscle. Int J Stem Cells 2014;7:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kim MY, Kwon DR, Lee HI. Therapeutic effect of microcurrent therapy in infants with congenital muscular torticollis. PM R 2009;1:736–9. [DOI] [PubMed] [Google Scholar]


