Abstract
The rapid and orderly folding of epithelial tissue during developmental processes such as gastrulation requires the precise coordination of changes in cell shape. Here, we report that the perforin-like protein Torso-like (Tsl), the key extracellular determinant for Drosophila embryonic terminal patterning, also functions to control epithelial morphogenesis. We find that tsl null mutants display a ventral cuticular hole phenotype that is independent of the loss of terminal structures, and arises as a consequence of mesoderm invagination defects. We show that the holes are caused by uncoordinated constriction of ventral cell apices, resulting in the formation of an incomplete ventral furrow. Consistent with these data, we find that loss of tsl is sensitive to gene dosage of RhoGEF2, a critical mediator of Rho1-dependent ventral cell shape changes during furrow formation, suggesting that Tsl may act in this pathway. In addition, loss of tsl strongly suppressed the effects of ectopic expression of Folded Gastrulation (Fog), a secreted protein that promotes apical constriction. Taken together, our data suggest that Tsl controls Rho1-mediated apical constriction via Fog. Therefore, we propose that Tsl regulates extracellular Fog activity to synchronize cell shape changes and coordinate ventral morphogenesis in Drosophila. Identifying the Tsl-mediated event that is common to both terminal patterning and morphogenesis will be valuable for our understanding of the extracellular control of developmental signaling by perforin-like proteins.
Keywords: morphogenesis, gastrulation, Torso-like, MACPF, Drosophila
MORPHOGENESIS is the fundamental biological process by which organisms acquire their form, and involves a complex orchestration of cell fate decisions and gross movements of cell populations. The cell movements that occur during morphogenesis are governed by concerted tissue-wide changes to cellular shape (Ip and Gridley 2002; Sawyer et al. 2010). One of the best studied examples of morphogenesis is the early stages of gastrulation in the Drosophila embryo, whereby cells in defined regions of the embryo are rapidly internalized (Leptin and Grunewald 1990; Knust and Muller 1998). The two major morphogenetic movements that occur during Drosophila gastrulation are the invaginations of the ventral furrow and the posterior midgut (Leptin and Grunewald 1990; Sweeton et al. 1991; Leptin et al. 1992). These events occur 3-hr postfertilization, immediately following the completion of cellularization, and serve to bring mesodermal and endodermal precursors to the interior of the embryo (Wieschaus and Nüsslein-Volhard 1986; Leptin 1995).
Underpinning these tissue invaginations is the ability of cells to constrict at their apical edges and adopt a wedge-like shape [for review see Lecuit and Lenne (2007)]. Intracellularly, this occurs via the remodelling of the actomyosin cytoskeleton, while cytoskeleton-linked connections between neighboring cells known as adherens junctions (AJs) provide tensile strength to allow the tissue to fold as a sheet (Martin et al. 2010). A remarkable aspect of Drosophila ventral morphogenesis is the rapid speed at which it occurs (Kam et al. 1991; Oda and Tsukita 2001). It is therefore critical that the apical constriction of individual ventral cells is precisely timed and synchronized across the entire ventral domain to permit a productive furrow that can complete invagination.
The key developmental signal that is required to initiate apical constriction is encoded by Folded Gastrulation (Fog) (Costa et al. 1994). Fog is a secreted protein that becomes expressed in subsets of cells fated for actomyosin-based shape changes, for example in the ventral mesoderm prior to ventral furrow formation (Costa et al. 1994). Fog is thought to signal to a local field of cells via the G-protein-coupled receptor Mesoderm-invagination signal transducer (Mist) Manning et al. 2013). Upon binding of Fog, localized activation of Mist induces apical constriction in cells of the presumptive mesoderm via G-protein signaling and activation of the highly conserved GTPase Rho1 by its guanine nucleotide exchange factor RhoGEF2 (Barrett et al. 1997; Morize et al. 1998; Nikolaidou and Barrett 2004; Dawes-Hoang et al. 2005; Manning et al. 2013). Rho1 activates Rho kinase, which phosphorylates the regulatory light chain of nonmuscle myosin II to induce contraction of the apical actomyosin network in the cells that receive the Fog signal (Dawes-Hoang et al. 2005).
As well as promoting apical constriction, the Fog/Mist pathway has been implicated as a central regulator of its coordination between cells. In addition to delayed initiation of morphogenesis, fog mutants display uncoordinated ventral cell apical constriction, a highly disorganized ventral furrow, and often fail to complete invagination (Costa et al. 1994; Oda and Tsukita 2001). However, unlike its role in initiating constriction, the mechanism by which the Fog pathway coordinates constriction between cells remains to be elucidated.
Here, we report that the maternal patterning protein Torso-like (Tsl), long known as the localized determinant of embryonic terminal patterning (Stevens et al. 1990; Savant-Bhonsale and Montell 1993; Martin et al. 1994), is also essential for the promotion and coordination of mesoderm invagination. Our data implicate Tsl as a new extracellular member of the Fog/Mist pathway required for ventral morphogenesis in Drosophila, and suggest that, while terminal patterning and ventral morphogenesis are distinct in many ways, these processes may share a common regulatory mechanism.
Materials and Methods
Drosophila stocks and maintenance
The following stocks were used: w1118 (BL5905), tslΔ (Johnson et al. 2013), torXR1 (Sprenger et al. 1989), HA-Tsl (Jimenez et al. 2002), tsl2, tsl3, tsl4, tsl5 (Savant-Bhonsale and Montell 1993), slbo-Gal4 (Rorth et al. 1998), Ecad-GFP (Oda and Tsukita 2001), fogS4 (BL2100), RhoGEF24·4 (BL9382; Barrett et al. 1997), and Gal4::VP16-nos.UTR (BL7253). All flies were maintained on standard media at 25°.
Cloning and transgenesis
To generate the upstream activating sequence (UAS)-Tsl-GFP construct, the open reading frame of tsl followed by a short linker encoding the peptide SAGSAS, three tandem myc epitopes, and the open reading frame for enhanced GFP (eGFP) was synthesized (Genscript) and subcloned in pUASTattB via BglII and XhoI sites. For UASP-fog, the full-length fog cDNA transcript was excised from an existing clone (SD02223; Rubin et al. 2000) and inserted into pUASP (Rorth 1998) via EcoRI/XhoI. Transgenic lines were made (BestGene) via ΦC31integrase-mediated transformation (Bischof et al. 2007) using the ZH-51CE attP-landing site for UAS-Tsl-GFP, and standard P-element transformation methods (Rubin and Spradling 1982) for genomic integration of UASP-fog into the w1118 background.
Cuticle preparations
Adults were allowed to lay on media containing apple juice supplemented with yeast paste for 24 hr before being removed. Embryos developed for a further 24 hr before dechorionation in 50% (v/v) bleach and mounting on slides in a 1:1 (v/v) mixture of Hoyer’s solution:lactic acid. Slides were incubated for several hours or overnight at 65° and imaged using dark-field optics (Leica). Cuticles from at least three separate overnight lays were scored and the means of each phenotypic category calculated. Significant differences between genotypes were determined by two-tailed unpaired t-tests.
Immunohistochemistry
For immunostaining, adults of the genotypes indicated were allowed to lay for 5 hr or overnight to isolate gastrulae and late-staged embryos, respectively. Embryos were collected, dechorionated, and either heat-fixed (for anti-β-cat) by pouring boiling salt solution (70 mM NaCl and 0.03% Triton X-100) over the embryos and cooling immediately on ice, or by chemical fixation (100 mM PIPES pH 6.9, 2 mM EGTA, 1 mM MgSO4, and 4% formaldehyde) with an equal volume of n-heptane for 25 min rocking. Embryos were devitellinized with n-heptane and methanol and rehydrated with phosphate buffered saline (PBS) with 0.1% Triton X-100 (PTx) before being blocked for 1 hr in PTx containing 5% normal goat serum (Sigma [Sigma Chemical], St. Louis, MO). Primary antibodies (anti-Nrt, 1:50; anti-β-cat, 1:20; and anti-Twi, 1:1000) were diluted in fresh block solution and incubated overnight with shaking at 4°. Secondary antibodies (anti-mouse and rabbit Alexa488 and 588 conjugated, 1:500, Molecular Probes, Eugene, OR) were applied after several washes in PTx for 1 hr, washed further, stained with DAPI (Sigma), and mounted in vectashield (Vector Laboratories, Burlingame, CA). Embryos were left whole, or hand-sliced using a 21-gauge needle and imaged using a spinning disk confocal microscope (Olympus CV1000).
RNA in situ hybridizations
RNA in situ hybridizations on whole-mount, 4-hr-old fixed (4% paraformaldehyde in phosphate buffered saline), and methanol devitellinized embryos were performed using a DIG-labeled antisense RNA probe transcribed from a pGEMT-Easy (Promega, Madison, WI) clone of sna (F-5′-CGCAGGATCTATCCCTGAAA-3′, R-5′-AGCGACATCCTGGAGAAAGA-3′) following standard protocols (Tomancak et al. 2002). Briefly, probes were hybridized to embryos overnight at 55° and washed in hybridization buffer (4 × saline sodium citrate buffer, 50% v/v formamide, 0.1% v/v Tween-20, and 50 mg/ml heparin) for 36 hr before incubation with alkaline phosphatase-conjugated anti-DIG and color development with 5-bromo-4-chloro-3-indolyl phosphate and nitro blue tetrazolium chloride. Imaging was performed under differential interference contrast optics on a Leica DM LB compound microscope.
Live imaging
Flies were allowed to lay for 4 hr and their embryos collected, dechorionated, and placed ventral side down in the wells of an eight-chambered slide and covered in PBS. Following the completion of cellularization, 10 optical slices, covering a 20 μm range starting at the ventral surface, were captured every 30 sec for each embryo using a 20 × objective (UPLSApo, 0.7NA) and CV1000 microscope. Movies were generated at 10 frames per second using images captured 9 μm below the embryo surface.
Data availability
Data and reagents are available upon request. Supplemental Material, File S1 contains one figure and the legends for File S2 and File S3.
Results
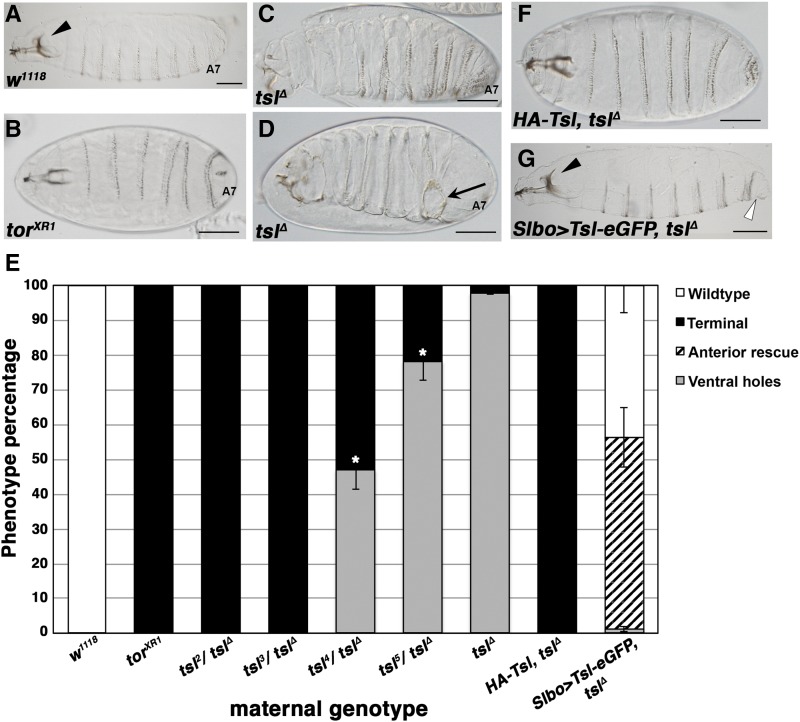
We previously generated a null mutant of tsl (tslΔ) via ends-out gene targeting (Johnson et al. 2013), as the available tsl alleles (e.g., tsl1-5 and tsl00617; Stevens et al. 1990; Savant-Bhonsale and Montell 1993) were point mutations or P-element insertions and potentially hypomorphic. As expected, and similar to observations for other maternal terminal class genes, embryos laid by tslΔ homozygous females lacked terminal structures (including abdominal segment 8, the telson, and filzkorper, Figure 1, B and C). Strikingly however, a large proportion (> 90%) of these embryos also displayed ventrally-located cuticular holes (Figure 1, D and E). These holes were variable in size and number and most often occurred in the posterior region of the embryo (Figure S1 in File S1).
Figure 1.
Loss of maternal tsl results in ventral cuticular holes that are independent of terminal patterning failure. (A) Wild-type larval cuticle with complete head skeleton (arrowhead) and abdominal segment 7 (A7) present. (B) Cuticle of embryos laid by torXR1 females showing the terminal class mutant phenotype. Note that the head skeleton is reduced and structures posterior to A7 are absent. (C and D) Cuticles of embryos laid by tslΔ mothers display the terminal phenotype (C) similar to torXR1; however, a large proportion also possess ventral cuticular holes (D, arrow). (E) Quantification of phenotypes observed in embryos laid by mothers of the genotypes shown. Hypomorphic alleles of tsl in trans with the null allele (tslΔ) form an allelic series with respect to the cuticular hole phenotype. Asterisks indicate significant differences from the tslΔ phenotype (t-test, P < 0.05). Means are plotted and error bars represent one SE calculated from at least three cuticle preparations (> 100 cuticles scored for each). (F) A genomic transgene containing HA-tagged tsl (HA-Tsl) completely rescues the cuticular hole phenotype of tslΔ; however, it does not restore terminal patterning. (G) Expression of an enhanced GFP (eGFP)-tagged tsl transgene in the endogenous ovarian pattern of tsl (Slbo-Gal4) restores the tslΔ ventral cuticle and partially restores terminal patterning, as assessed by rescue of the anterior head skeleton and presence of abdominal segment 8. The cuticle shown is a representative image where the addition of A8 tissue (open arrowhead) and a wild-type head skeleton (arrowhead) is observed. Anteriors are to the left. Bar, 100 μm.
To ensure that this phenotype was due to loss of tsl, we first performed complementation tests with other available tsl alleles. Placing the tslΔ allele in trans with chromosomes carrying tsl2 or tsl3 alleles produced embryos with only terminal defects and no ventral holes (Figure 1E). However, in transheterozygotes for tslΔ and the tsl4 and tsl5 alleles, both of which are known to be stronger with respect to the terminal class phenotype (Savant-Bhonsale and Montell 1993), cuticular holes were readily observed (Figure 1E). We also performed a rescue experiment using a genomic tsl construct in which Tsl is tagged at the N-terminus by a hemaglutinin (HA) epitope (HA-Tsl; Jimenez et al. 2002). We have previously shown that this transgene fails to rescue the terminal patterning defects in tslΔ embryos (Johnson et al. 2013), possibly due to its inability to accumulate at the embryonic plasma membrane (Mineo et al. 2015). Despite this, HA-Tsl fully rescues the ventral hole phenotype (Figure 1F).
In terminal patterning, maternal Tsl originates from ovarian follicle cells at the anterior and posterior poles of the developing oocyte (Stevens et al. 1990; Savant-Bhonsale and Montell 1993; Martin et al. 1994). Given the maternal nature of both the terminal and the cuticular hole phenotypes, we were interested to know whether the latter phenotype is also caused by loss of tsl from the follicle cells, or whether it reflects an unreported expression pattern of tsl. To this end, we expressed a functional UAS transgene encoding a carboxy-terminal eGFP-tagged form of Tsl (Tsl-eGFP) in the tsl null background specifically in the ovarian follicle cells that are known to express tsl (using Slbo-Gal4; Rorth 1998). Strikingly, these embryos showed full restoration of the ventral cuticle (Figure 1, E and G). These data strongly suggest that the ventral cuticular hole phenotype is due to a loss of maternal tsl from the same cells in which it is needed for terminal patterning.
Having established that loss of tsl was the cause of the cuticular holes, we next wanted to pinpoint the origins of the defect during embryogenesis. Given the ventral location of the holes, we reasoned that tsl might function in an aspect of early ventral development such as patterning or morphogenesis. Therefore, we first asked whether ventral cell fate is specified correctly in tslΔ embryos by examining snail (sna) expression, a well-known marker for ventral cell differentiation (Leptin et al. 1992). To ensure that any deviations from wild-type that we observed were not due to the terminal class mutant phenotype, we compared tslΔ embryos to control terminal class mutant embryos that did not display the cuticular holes. For this, we used either HA-Tsl; tslΔ/tslΔ embryos (as their genetic background is very close to that of tslΔ) or, when large numbers of embryos were required for precisely timed fixations, homozygotes of the tor null allele torXR1 (Sprenger et al. 1989).
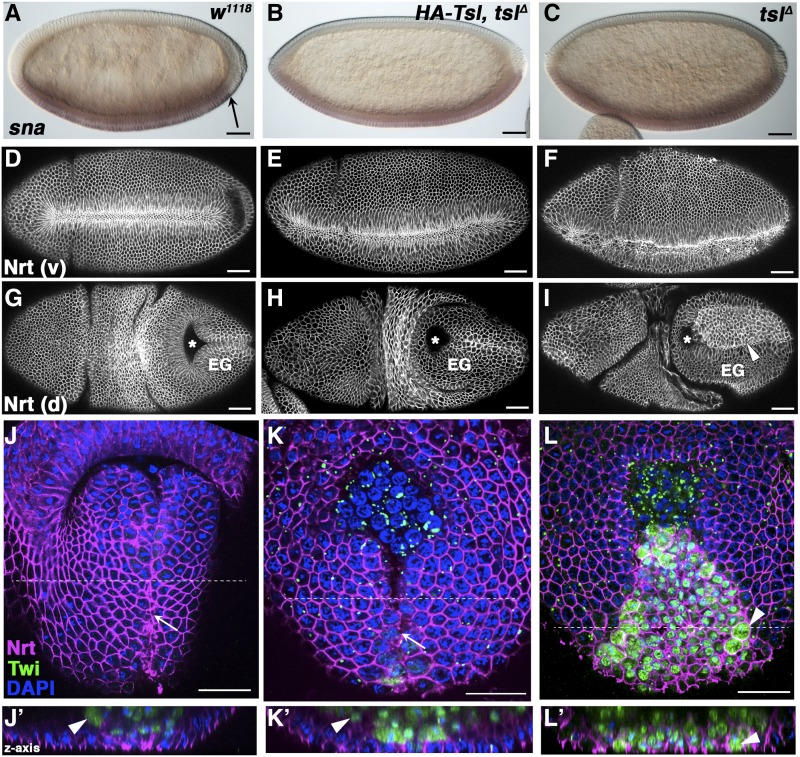
No obvious defects in the sna expression domain were seen in tslΔ embryos, with the exception of its extension posteriorly to the pole (a known consequence of lacking posterior Tor signaling), and also observed in the control terminal mutant embryos (Figure 2, A–C; Leptin and Grunewald 1990; Ray et al. 1991). These data suggest that the cuticular hole phenotype is unlikely to be due to a failure of ventral fate specification.
Figure 2.
The ventral cuticular holes in tslΔ embryos are caused by impaired mesoderm invagination during morphogenesis. Wild-type (w1118, left panels), terminal class mutant (HA-Tsl, tslΔ, center panels), and tslΔ (right panels) embryos stained with various markers of embryogenesis. (A–C) Transcript expression of the ventral cell fate marker Snail (sna) is repressed at the posterior of wild-type embryos by the terminal system (A, arrow). In tslΔ (B) and terminal class mutant (C) embryos, sna expression extends to the posterior pole. (D–F) Ventral (v) furrow formation in gastrulating embryos labeled with the membrane marker anti-Neurotactin (Nrt). Wild-type (D) and terminal class mutant (E) embryos form regular ventral furrows. The furrows from tslΔ embryos are irregular and incomplete (F). (G–I) Dorsal (d) views of gastrulated embryos stained with anti-Nrt. (G) Wild-type embryos correctly invaginate their posterior midgut (pole cell position indicated by asterisks) unlike terminal class mutant (H) and tslΔ (I) embryos due to terminal system failure. however, tslΔ embryos possess a large field of intensely labeled cells on the extended germband (EG) that are not seen in wild-type nor terminal class mutant embryos (arrowhead). Anteriors are to the left. (J–L) Posterior–dorsal surface views of gastrulae embryos stained with anti-Nrt (magenta), anti-Twist (Twi, green) to label mesodermal precursors, and DAPI (blue). The ventral furrow has closed at the midline (white arrow) in wild-type (J) terminal class mutant (K) embryos but remains open in tslΔ embryos (L), as indicated by the surface location of Twi-positive cells (white arrowheads). Anteriors are to the top. Lower panels (J’–L’) show the z-axis cross-section of the top panels at the position indicated by the dotted white line. Twi-positive nuclei are visible only below the dorsal surface in wild-type (J’) and terminal class mutant (K’) embryos indicating successful furrow invagination. Many of these cells remain at the dorsal surface of tslΔ embryos (L’). Maternal genotypes are shown. Bar, 50 μm.
To determine whether morphogenesis is instead affected by loss of tsl, tslΔ embryos were stained for the membrane marker Neurotactin (Nrt), which becomes concentrated at the apical region of constricting cells (Hortsch et al. 1990). In contrast to wild-type embryos, which formed normal furrows (Figure 2D), and to terminal class mutant embryos, that formed a largely normal but extended furrow (Figure 2E), tslΔ embryos formed irregular and often incomplete ventral furrows (Figure 2F). Imaging the dorsal side of ∼4.5-hr-old tslΔ embryos revealed defects in the extended germband. As expected for terminal class embryos, and also seen in the terminal class mutant control (Figure 2H), the posterior midgut invagination failed, leaving the pole cells at the dorsal surface rather than being internalized (Figure 2 compare G and H with I; Costa et al. 1994). By contrast, in tslΔ embryos an additional large field of cells posterior to the pole cells was observed (Figure 2I). We reasoned that these cells might be mesodermal tissue remaining at the embryo surface as a consequence of invagination failure. To confirm this, we stained tslΔ embryos with anti-Twist (Twi), a marker of the presumptive mesoderm (Leptin and Grunewald 1990). Twi-positive cells were not detected at the surface of wild-type nor terminal class mutant embryos following ventral furrow invagination, indicating their successful internalization (Figure 2, J and K). However, in tslΔ embryos, Twi-positive cells were readily visible at the surface indicating regional invagination failure (Figure 2L). Collectively, these data strongly suggest that tsl functions to promote invagination of the ventral mesoderm during morphogenesis.
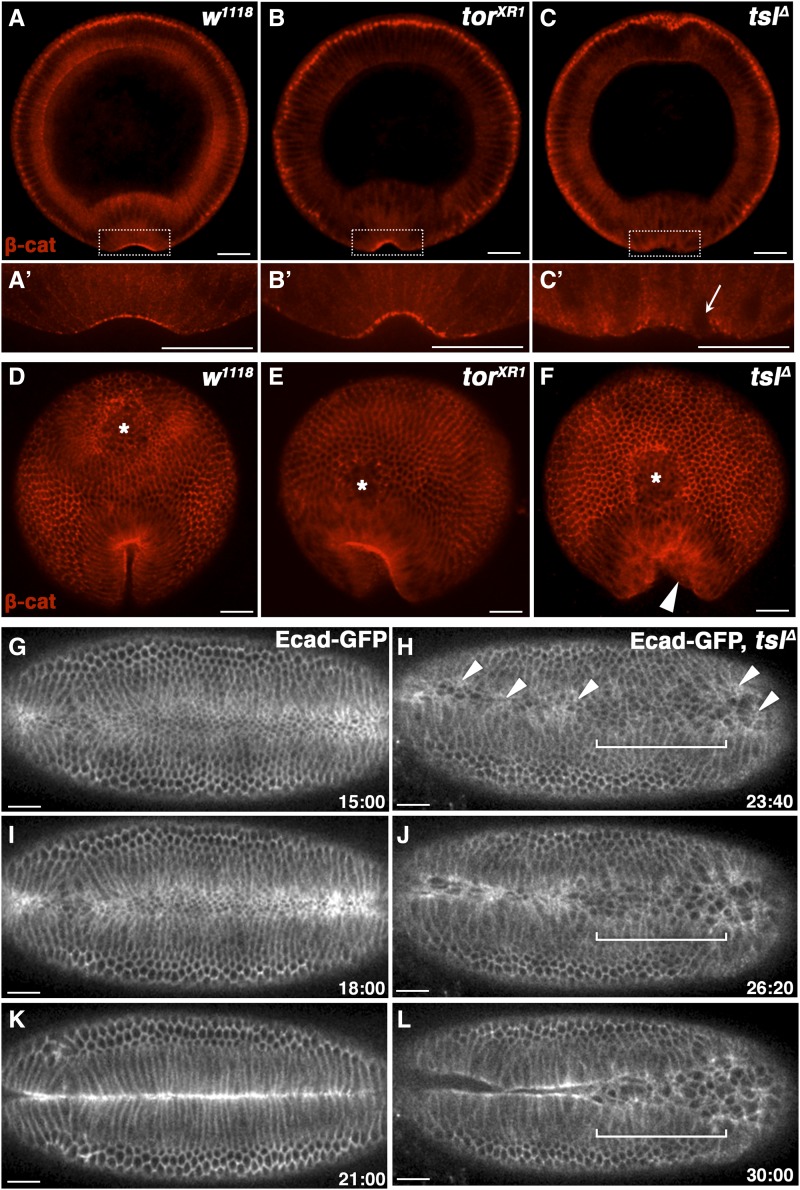
A failure of invagination is most commonly due to defects in apical constriction. Therefore, we assessed constriction at the onset of ventral furrow formation by staining embryos for β-catenin, an integral intracellular component of the AJ (Cox et al. 1996). In wild-type embryos, strong and uniform apical accumulation of β-catenin was observed in constricting ventral cells (Figure 3A). This was also observed in control terminal class mutant embryos that did not display the cuticular holes (Figure 3B). By contrast, in tslΔ embryos, ventral cells that were attempting furrow formation showed greatly reduced levels of apical β-catenin, often coinciding with incorrectly positioned nuclei close to the apical surface (Figure 3C). These cells were misaligned at their apical edges, suggesting apical constriction failure, and likely underlying the inconsistent and poorly formed early furrow. These defects were particularly evident at the posterior ends of tslΔ embryos (Figure 3, D–F), corresponding with the final position of the cuticular holes. Therefore, these data strongly suggest that the holes are caused by furrow defects.
Figure 3.
Tsl promotes and coordinates apical constriction during ventral furrow formation. (A–C) Cross-sections of fixed early gastrulae embryos sectioned at approximately two-thirds the embryo length (from the anterior to posterior) and labeled with anti-β-catenin to reveal adherens junctions (AJs). β-catenin localizes strongly to the apical surface in the early ventral furrow in wild-type (w1118, A) and terminal class mutant (torXR1, B) embryos, but poorly and irregularly in tslΔ embryos (C). Lower panels are (A’–C’) high-magnification images of the boxed area in the top panels. Areas of low apical β-catenin correspond to unconstricted cells indicated by apical nuclei (arrowed). Posterior view of embryos with closing ventral furrows. Uniform furrows and concentrated apical AJs are observed for wild-type (D) and terminal class mutant (E) embryos, despite the latter failing to internalize the posterior midgut (asterisks) and forming a posteriorly extended furrow as expected. tslΔ embryos fail similarly in these regards; however, they also display an irregular shaped posterior furrow (F, arrowhead). Ventral is to the bottom. (G–L) Live imaging stills taken at 3-min intervals of representative Ecad-GFP-expressing control (G, I, and K) and tslΔ (H, J, and L) embryos during ventral furrow formation and invagination. Furrow formation in tslΔ embryos is delayed by ∼8 min compared to controls. Cell constrictions in the ventral domain of tslΔ embryos are limited to sporadic patches of cells (arrowheads). Areas containing more constricted cells appear to initiate furrow formation first. Invagination is incomplete in this example due to a large population of cells toward the posterior (bracketed) remaining unconstricted. Time is in minutes postcellularization completion. Ventral side is shown with anteriors to the left. Maternal genotypes are indicated. Bar, 35 μm.
Our observation that not all tslΔ embryos displayed defects in furrow formation suggested that tsl is important but not essential for apical constriction, and thus may perform a regulatory role in morphogenesis. Therefore, we hypothesized that tsl might be required to coordinate the timing of apical constriction across the ventral domain. To investigate this, we imaged ventral morphogenesis live in embryos expressing Ecad-GFP (Oda and Tsukita 2001), a marker of the AJ. Control (Ecad-GFP) embryos showed the characteristic rapid and coordinated apical constriction and internalization of a band of ventral cells following cellularization (Figure 3, G, I, and K and File S2). In contrast, in tslΔ embryos there was a delay in the initiation of ventral apical constriction of ∼8 min. However, once constriction initiated in tslΔ embryos, the timing of tissue folding appeared relatively normal. We noted that the action of the furrow was wave-like rather than simultaneous, with the most constricted patches invaginating first, and appearing to pull less constricted cells into the furrow along with them. In agreement with the fixed embryo cross-sections, constriction was highly irregular and limited to seemingly random patches of cells across the ventral domain (Figure 3H and File S3). Furthermore, we commonly observed apical constriction failure in a large proportion of posterior–ventral cells, often corresponding with a failure of the entire posterior half of the embryo mesoderm to invaginate (Figure 3L, compare to K). Taken together, these support the idea that Tsl both promotes and coordinates apical cell constriction during early furrow formation.
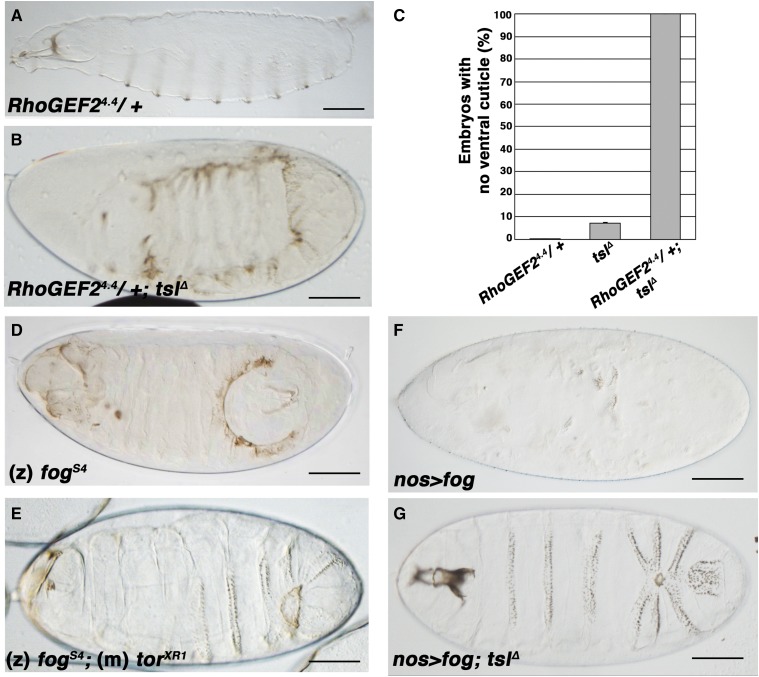
Cell shape changes that occur during Drosophila gastrulation require the activity of the Rho1 pathway (Barrett et al. 1997; Nikolaidou and Barrett 2004; Kolsch et al. 2007). Since loss of tsl causes defects in apical constriction, we next asked if Tsl might function in this pathway. To test this, we took advantage of the variation in the tslΔ cuticle phenotype and investigated whether reducing the gene dosage of RhoGEF2, which encodes the upstream activator of Rho1, could enhance its severity. Loss of either RhoGEF2 or Rho1 results in an identical phenotype whereby no ventral furrow forms (Barrett et al. 1997; Hacker and Perrimon 1998; Nikolaidou and Barrett 2004; Dawes-Hoang et al. 2005). We found that reducing RhoGEF2 gene dosage (RhoGEF24.4/+) strikingly enhanced the severity of the tslΔ cuticular phenotype, resulting in all embryos missing their entire ventral cuticle (Figure 4, B and C). This finding suggests that Tsl activity during ventral morphogenesis requires the function of RhoGEF2.
Figure 4.
tsl interacts with RhoGEF2 and fog in ventral morphogenesis. (A) Progeny from RhoGEF24.4 heterozygote females display a wild-type cuticle pattern. (B) Progeny from females heterozygous for RhoGEF24.4 and lacking tsl (tslΔ) display a severe loss of ventral cuticle. (C) A striking increase in the proportion of embryos lacking ventral cuticles is observed when RhoGEF2 dosage is reduced in a tslΔ background compared to tslΔ alone (tslΔ = 7.3% vs. RhoGEF24.4/+; tslΔ = 100%). Means are plotted and error bars represent one SE calculated from at least three cuticle preparations (> 100 cuticles scored for each). (D) Posterior–ventral holes are observed in cuticles of fogs4 embryos. (E) fogs4;torXR1 double-mutant embryos have a cuticle phenotype that closely resembles that of tslΔ. (F) Embryos from females expressing fog ubiquitously from the maternal germline (nos>) are severely compromised in their ability to produce ventral cuticle. (G) The cuticle phenotype of embryos that express fog in the absence of tsl closely resembles the tslΔ phenotype. Maternal genotypes are shown unless otherwise indicated (m, maternal; z, zygotic). Anteriors are to the left, ventral is down. Bar, 100 μm.
In terminal patterning Tsl acts extracellularly and upstream of the Tor receptor ligand Trk (Casanova and Struhl 1989; Stevens et al. 1990; Sprenger and Nusslein-Volhard 1992). Recently, we have reported that Tsl likely achieves this by controlling the extracellular accumulation of Trk (Johnson et al. 2015). Therefore, we hypothesized that in gastrulation Tsl may regulate a receptor/ligand pathway upstream of the effectors RhoGEF2 and Rho1. The only extracellular molecule known to act upstream of Rho1 in Drosophila ventral morphogenesis is the zygotically-expressed protein Fog (Costa et al. 1994; Dawes-Hoang et al. 2005). Loss of Fog has been reported to result in ventral defects similar to the phenotypes observed here for tslΔ, including uncoordinated ventral apical constriction (Costa et al. 1994; Oda and Tsukita 2001) and ventral cuticle holes (Wieschaus et al. 1984; Zusman and Wieschaus 1985). In addition, we noted that fog mutant (fogs4/Y) cuticle holes were often positioned at the posterior (Figure 4D), as observed in embryos laid by tslΔ females (Figure 1D), further indicating that the two genes may be acting in the same pathway.
If Tsl acts in the Fog pathway then we might expect that the combination of loss of fog and loss of terminal patterning would replicate the overall phenotype of tslΔ. Consistent with this idea, we found that embryos mutant for fog while also lacking the maternal contribution of tor produced cuticles that closely resemble the tslΔ cuticle (Figure 4E). To more directly determine whether Fog activity requires Tsl, we ectopically expressed fog from the female germline at high levels (nanos::VP16-Gal4; pUASP-fog), which has previously been shown to overactivate the Fog pathway (Dawes-Hoang et al. 2005). This resulted in a severely defective cuticle phenotype, with only small amounts of recognizable cuticle remaining (Figure 4F). Remarkably however, loss of tsl function strongly suppressed the effects of ectopic Fog, resulting in cuticles more closely resembling the tslΔ phenotype (Figure 4G). However, we note that despite being null for tsl, many of these embryos no longer had ventral cuticle holes, possibly due to a partial rescue of tslΔ by residual Fog activity. Together, these data strongly suggest that Fog activity and the Rho1 pathway depend upon the maternal action of Tsl for morphogenesis.
Discussion
The apical constriction of cells underlies the critical ability of animal tissues to fold and change shape during development. While the intracellular mechanisms that govern this process, including the involvement of the highly conserved Rho1 pathway, are quite well-characterized, relatively little is known about the extracellular signals that coordinate this process. Here, we make the unexpected finding that Tsl plays a second maternal role as a key extracellular component of the Fog/Rho1 pathway.
Extensive characterization of the role of Fog in the ventral furrow has demonstrated its importance in the coordination of cellular apical constriction in the cells of the ventral domain (Costa et al. 1994; Oda and Tsukita 2001; Dawes-Hoang et al. 2005; Fuse et al. 2013; Manning et al. 2013). Consistent with the phenotype of Fog mutants, we find that loss of maternal Tsl leads to irregular and uncoordinated ventral cell apical constrictions, incomplete furrow formation, and resultant ventral cuticle holes. Furthermore, we find that the activity of ectopic ubiquitous Fog delivered through the maternal germline is highly dependent on Tsl, suggesting that Tsl serves to regulate ventral Fog activity.
tsl has been studied for many years in its terminal patterning role, so it is somewhat surprising that the ventral cuticle defects described here have not been previously reported. This may be because most terminal patterning studies have utilized hypomorphic alleles of tsl. Here, we found that the ventral cuticle defects are only observed in embryos laid by homozygotes of the null allele, or when the null allele is placed in trans with those hypomorphic alleles of tsl that have been reported to be stronger with respect to terminal patterning (tsl4 and tsl5; Savant-Bhonsale and Montell 1993). We also made the surprising finding that the ventral morphogenesis defects we observed in tsl mutants can be rescued by expressing tsl in the same polar ovarian cells required for its function in terminal patterning. These data accordingly suggest that the same population of Tsl molecules is involved in both roles.
How might polar localized Tsl influence ventral Fog activity? Recent data on the role of Tsl in terminal patterning suggest that it mediates the extracellular accumulation of the ligand for the Tor receptor, Trk (Johnson et al. 2015). Thus, one possibility is that Tsl acts to directly mediate the secretion or activity of Fog. However, this idea seems unlikely as the overlap between the ventral cells that produce Fog and polar localized Tsl would be small or nonexistent. Furthermore, mosaic analyses by Costa et al. (1994) estimated that Fog could induce apical constriction only two to three cells away from its cell of origin. In addition, since immunostaining experiments by Mineo et al. (2015) have shown that Tsl remains localized to the embryo termini plasma membrane, we therefore reason that it is improbable that Tsl could directly influence Fog produced at the center of the embryo. Accordingly, an alternative idea is that Tsl is responsible for the extracellular accumulation of a hitherto unidentified molecule that can diffuse to the ventral region and control local Fog activity. A mechanism such as this might aid in coordinating the timing of apical constriction and subsequent furrow formation by controlling extracellular Fog activity uniformly across the cells of the ventral domain.
Is tsl also required for other fog-dependent morphogenetic events? Previous work has implicated fog in several other developmental roles, including morphogenesis of the larval wing disc (Nikolaidou and Barrett 2004; Manning et al. 2013), salivary gland formation during midembryogenesis (Lammel and Saumweber 2000), and invagination of the posterior midgut (the second major morphogenetic movement during gastrulation; Costa et al. 1994). While it remains possible that maternal tsl contributes to salivary gland morphogenesis and posterior midgut invagination, the dependence of these processes upon Tor signaling through the activities of target genes tailless and forkhead (Weigel et al. 1989; Costa et al. 1994; Wu and Lengyel 1998) precludes our ability to determine whether this is the case. In addition, since tslΔ adults have no discernable wing defects, we reason that Tsl is unlikely to play a role in wing morphogenesis. Determining if Tsl is required in other fog-mediated processes is thus challenging and will require sophisticated further studies.
The finding that maternal Tsl functions in two distinct processes during early Drosophila embryogenesis further raises the question as to whether Tsl was coopted from one role to the other during the course of evolution. Interestingly, bioinformatic studies of patterning pathway components in Drosophila and other insects have revealed that the function of Tsl in terminal patterning is likely a relatively recent adaptation (Duncan et al. 2013). For example, the honeybee uses an alternative terminal patterning system to Tor signaling, as its genome lacks tor- and trk-encoding sequences (Duncan et al. 2013). Further, in the honeybee, tsl is expressed ubiquitously in ovarian tissue, suggesting that its maternal function is not spatially restricted as it is in Drosophila. In addition, a recent study used RNA interference to knockdown tsl transcripts in the milkweed bug (Oncopeltus fasciatus), which like the honeybee also lacks the canonical terminal patterning pathway (Weisbrod et al. 2013). Intriguingly, rather than yielding terminal patterning defects, embryonic invagination defects were observed, indicating that Tsl may also function in morphogenesis in this insect. Together, these studies infer that the ancestral role of maternal Tsl in insects may have been in morphogenesis rather than terminal patterning. Furthermore, they raise the possibility that the localizing activity of Tsl, which has been its defining feature in terminal patterning, may instead represent a novel exploitation of its molecular function.
Tsl is a member of the pore forming perforin-like protein superfamily (Ponting 1999; Rosado et al. 2007). However, in contrast to most perforin-like proteins, which function as immune effectors or virulence factors, Tsl is instead critical for cell signaling during insect development. While we are yet to determine whether Tsl functions via pore formation or indeed via another mechanism of action, it is clear that its activity is crucial for several processes during fly development (Stevens et al. 1990; Grillo et al. 2012; Johnson et al. 2013; Forbes-Beadle et al. 2016). However, to date our ability to identify the commonalities and differences between these roles has been hampered by our lack of knowledge of the genetic pathways in which Tsl operates.
The data presented here suggest that, remarkably, Tsl serves to control two molecularly distinct signaling pathways in the context of the same early embryonic extracellular space. Tor is a receptor tyrosine kinase that signals through the Ras and mitogen-activated protein kinase cassette to influence cellular transcription (Sprenger et al. 1989), whereas Mist is a G-protein-coupled receptor that modulates the actomyosin cytoskeleton via G-protein signaling and the Rho1 GTPase (Manning et al. 2013). Therefore, we reason that the apparent lack of similarities between the Trk/Tor and Fog/Mist pathways, together with the influence of Tsl on extracellular Trk accumulation, is more in keeping with a function for Tsl in the regulation of either trafficking or secretion. Indeed, it will be interesting to learn how the active Mist ligand is generated and whether a Tsl-regulated event common to both ventral morphogenesis and terminal patterning is involved. Such information will undoubtedly provide valuable insights for our understanding of both the cellular coordination of tissue folding and of how perforin-like proteins function during development.
Supplementary Material
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.117.200576/-/DC1.
Acknowledgments
We thank Lauren Forbes Beadle and the Australian Drosophila Biomedical Research Facility (OzDros) for technical support, and also acknowledge support from Monash Micro Imaging and the Australian Research Council (ARC) Centre of Excellence in Advanced Molecular Imaging. We thank the Bloomington Drosophila Stock Centre for providing fly stocks, and the Developmental Studies Hybridoma Bank and Michael Murray for antibodies. We further thank Eric Wieschaus for discussions and Michelle Henstridge for critical reading of the manuscript. T.K.J. is an ARC Discovery Early Career Researcher Award Fellow. J.C.W. is a National Health and Medical Research Council of Australia Senior Principal Research Fellow.
Author contributions: T.K.J., J.C.W., and C.G.W. conceived the experiments and interpreted the data, co-led the work, and wrote the paper; T.K.J. and K.A.M. performed experiments.
Footnotes
Communicating editor: R. J. Duronio
Literature Cited
- Barrett K., Leptin M., Settleman J., 1997. The Rho GTPase and a putative RhoGEF mediate a signaling pathway for the cell shape changes in Drosophila gastrulation. Cell 91: 905–915. [DOI] [PubMed] [Google Scholar]
- Bischof J., Maeda R. K., Hediger M., Karch F., Basler K., 2007. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc. Natl. Acad. Sci. USA 104: 3312–3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova J., Struhl G., 1989. Localized surface activity of torso, a receptor tyrosine kinase, specifies terminal body pattern in Drosophila. Genes Dev. 3: 2025–2038. [DOI] [PubMed] [Google Scholar]
- Costa M., Wilson E. T., Wieschaus E., 1994. A putative cell signal encoded by the folded gastrulation gene coordinates cell shape changes during Drosophila gastrulation. Cell 76: 1075–1089. [DOI] [PubMed] [Google Scholar]
- Cox R. T., Kirkpatrick C., Peifer M., 1996. Armadillo is required for adherens junction assembly, cell polarity, and morphogenesis during Drosophila embryogenesis. J. Cell Biol. 134: 133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes-Hoang R. E., Parmar K. M., Christiansen A. E., Phelps C. B., Brand A. H., et al. , 2005. folded gastrulation, cell shape change and the control of myosin localization. Development 132: 4165–4178. [DOI] [PubMed] [Google Scholar]
- Duncan E. J., Benton M. A., Dearden P. K., 2013. Canonical terminal patterning is an evolutionary novelty. Dev. Biol. 377: 245–261. [DOI] [PubMed] [Google Scholar]
- Forbes-Beadle L., Crossman T., Johnson T. K., Burke R., Warr C. G., et al. , 2016. Development of the cellular immune system of Drosophila requires the membrane attack complex/perforin-like protein Torso-like. Genetics 204: 675–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuse N., Yu F., Hirose S., 2013. Gprk2 adjusts Fog signaling to organize cell movements in Drosophila gastrulation. Development 140: 4246–4255. [DOI] [PubMed] [Google Scholar]
- Grillo M., Furriols M., de Miguel C., Franch-Marro X., Casanova J., 2012. Conserved and divergent elements in Torso RTK activation in Drosophila development. Sci. Rep. 2: 762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker U., Perrimon N., 1998. DRhoGEF2 encodes a member of the Dbl family of oncogenes and controls cell shape changes during gastrulation in Drosophila. Genes Dev. 12: 274–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortsch M., Patel N. H., Bieber A. J., Traquina Z. R., Goodman C. S., 1990. Drosophila neurotactin, a surface glycoprotein with homology to serine esterases, is dynamically expressed during embryogenesis. Development 110: 1327–1340. [DOI] [PubMed] [Google Scholar]
- Ip Y. T., Gridley T., 2002. Cell movements during gastrulation: snail dependent and independent pathways. Curr. Opin. Genet. Dev. 12: 423–429. [DOI] [PubMed] [Google Scholar]
- Jimenez G., Gonzalez-Reyes A., Casanova J., 2002. Cell surface proteins Nasrat and Polehole stabilize the Torso-like extracellular determinant in Drosophila oogenesis. Genes Dev. 16: 913–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T. K., Crossman T., Foote K. A., Henstridge M. A., Saligari M. J., et al. , 2013. Torso-like functions independently of Torso to regulate Drosophila growth and developmental timing. Proc. Natl. Acad. Sci. USA 110: 14688–14692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T. K., Henstridge M. A., Herr A., Moore K. A., Whisstock J. C., et al. , 2015. Torso-like mediates extracellular accumulation of Furin-cleaved Trunk to pattern the Drosophila embryo termini. Nat. Commun. 6: 8759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam Z., Minden J. S., Agard D. A., Sedat J. W., Leptin M., 1991. Drosophila gastrulation: analysis of cell shape changes in living embryos by three-dimensional fluorescence microscopy. Development 112: 365–370. [DOI] [PubMed] [Google Scholar]
- Knust E., Muller H. J., 1998. Drosophila morphogenesis: orchestrating cell rearrangements. Curr. Biol. 8: R853–R855. [DOI] [PubMed] [Google Scholar]
- Kolsch V., Seher T., Fernandez-Ballester G. J., Serrano L., Leptin M., 2007. Control of Drosophila gastrulation by apical localization of adherens junctions and RhoGEF2. Science 315: 384–386. [DOI] [PubMed] [Google Scholar]
- Lammel U., Saumweber H., 2000. X-linked loci of Drosophila melanogaster causing defects in the morphology of the embryonic salivary glands. Dev. Genes Evol. 210: 525–535. [DOI] [PubMed] [Google Scholar]
- Lecuit T., Lenne P. F., 2007. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat. Rev. Mol. Cell Biol. 8: 633–644. [DOI] [PubMed] [Google Scholar]
- Leptin M., 1995. Drosophila gastrulation: from pattern formation to morphogenesis. Annu. Rev. Cell Dev. Biol. 11: 189–212. [DOI] [PubMed] [Google Scholar]
- Leptin M., Grunewald B., 1990. Cell shape changes during gastrulation in Drosophila. Development 110: 73–84. [DOI] [PubMed] [Google Scholar]
- Leptin M., Casal J., Grunewald B., Reuter R., 1992. Mechanisms of early Drosophila mesoderm formation. Dev. Suppl. 1992: 23–31. [PubMed] [Google Scholar]
- Manning A. J., Peters K. A., Peifer M., Rogers S. L., 2013. Regulation of epithelial morphogenesis by the G protein-coupled receptor mist and its ligand fog. Sci. Signal. 6: ra98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. C., Gelbart M., Fernandez-Gonzalez R., Kaschube M., Wieschaus E. F., 2010. Integration of contractile forces during tissue invagination. J. Cell Biol. 188: 735–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. R., Raibaud A., Ollo R., 1994. Terminal pattern elements in Drosophila embryo induced by the torso-like protein. Nature 367: 741–745. [DOI] [PubMed] [Google Scholar]
- Mineo A., Furriols M., Casanova J., 2015. Accumulation of the Drosophila Torso-like protein at the blastoderm plasma membrane suggests that it translocates from the eggshell. Development 142: 1299–1304. [DOI] [PubMed] [Google Scholar]
- Morize P., Christiansen A. E., Costa M., Parks S., Wieschaus E., 1998. Hyperactivation of the folded gastrulation pathway induces specific cell shape changes. Development 125: 589–597. [DOI] [PubMed] [Google Scholar]
- Nikolaidou K. K., Barrett K., 2004. A Rho GTPase signaling pathway is used reiteratively in epithelial folding and potentially selects the outcome of Rho activation. Curr. Biol. 14: 1822–1826. [DOI] [PubMed] [Google Scholar]
- Oda H., Tsukita S., 2001. Real-time imaging of cell-cell adherens junctions reveals that Drosophila mesoderm invagination begins with two phases of apical constriction of cells. J. Cell Sci. 114: 493–501. [DOI] [PubMed] [Google Scholar]
- Ponting C. P., 1999. Chlamydial homologues of the MACPF (MAC/perforin) domain. Curr. Biol. 9: R911–R913. [DOI] [PubMed] [Google Scholar]
- Ray R. P., Arora K., Nusslein-Volhard C., Gelbart W. M., 1991. The control of cell fate along the dorsal-ventral axis of the Drosophila embryo. Development 113: 35–54. [DOI] [PubMed] [Google Scholar]
- Rorth P., 1998. Gal4 in the Drosophila female germline. Mech. Dev. 78: 113–118. [DOI] [PubMed] [Google Scholar]
- Rorth P., Szabo K., Bailey A., Laverty T., Rehm J., et al. , 1998. Systematic gain-of-function genetics in Drosophila. Development 125: 1049–1057. [DOI] [PubMed] [Google Scholar]
- Rosado C. J., Buckle A. M., Law R. H., Butcher R. E., Kan W. T., et al. , 2007. A common fold mediates vertebrate defense and bacterial attack. Science 317: 1548–1551. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C., 1982. Genetic transformation of Drosophila with transposable element vectors. Science 218: 348–353. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Hong L., Brokstein P., Evans-Holm M., Frise E., et al. , 2000. A Drosophila complementary DNA resource. Science 287: 2222–2224. [DOI] [PubMed] [Google Scholar]
- Savant-Bhonsale S., Montell D. J., 1993. torso-like encodes the localized determinant of Drosophila terminal pattern formation. Genes Dev. 7: 2548–2555. [DOI] [PubMed] [Google Scholar]
- Sawyer J. M., Harrell J. R., Shemer G., Sullivan-Brown J., Roh-Johnson M., et al. , 2010. Apical constriction: a cell shape change that can drive morphogenesis. Dev. Biol. 341: 5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger F., Nusslein-Volhard C., 1992. Torso receptor activity is regulated by a diffusible ligand produced at the extracellular terminal regions of the Drosophila egg. Cell 71: 987–1001. [DOI] [PubMed] [Google Scholar]
- Sprenger F., Stevens L. M., Nusslein-Volhard C., 1989. The Drosophila gene torso encodes a putative receptor tyrosine kinase. Nature 338: 478–483. [DOI] [PubMed] [Google Scholar]
- Stevens L. M., Frohnhofer H. G., Klingler M., Nusslein-Volhard C., 1990. Localized requirement for torso-like expression in follicle cells for development of terminal anlagen of the Drosophila embryo. Nature 346: 660–663. [DOI] [PubMed] [Google Scholar]
- Sweeton D., Parks S., Costa M., Wieschaus E., 1991. Gastrulation in Drosophila: the formation of the ventral furrow and posterior midgut invaginations. Development 112: 775–789. [DOI] [PubMed] [Google Scholar]
- Tomancak P., Beaton A., Weiszmann R., Kwan E., Shu S., et al. , 2002. Systematic determination of patterns of gene expression during Drosophila embryogenesis. Genome Biol. 3: RESEARCH0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel D., Jurgens G., Kuttner F., Seifert E., Jackle H., 1989. The homeotic gene fork head encodes a nuclear protein and is expressed in the terminal regions of the Drosophila embryo. Cell 57: 645–658. [DOI] [PubMed] [Google Scholar]
- Weisbrod A., Cohen M., Chipman A. D., 2013. Evolution of the insect terminal patterning system–insights from the milkweed bug, Oncopeltus fasciatus. Dev. Biol. 380: 125–131. [DOI] [PubMed] [Google Scholar]
- Wieschaus E., Nüsslein-Volhard C., 1986. Looking at embryos, pp. 199–227 in Drosophila: A Practical Approach, edited by Roberts D. B. IRL Press, Oxford. [Google Scholar]
- Wieschaus E., Nüsslein-Volhard C., Jürgens G., 1984. Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster. Rouxs Arch. Dev. Biol. 193: 296–307. [DOI] [PubMed] [Google Scholar]
- Wu L. H., Lengyel J. A., 1998. Role of caudal in hindgut specification and gastrulation suggests homology between Drosophila amnioproctodeal invagination and vertebrate blastopore. Development 125: 2433–2442. [DOI] [PubMed] [Google Scholar]
- Zusman S. B., Wieschaus E. F., 1985. Requirements for zygotic gene activity during gastrulation in Drosophila melanogaster. Dev. Biol. 111: 359–371. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and reagents are available upon request. Supplemental Material, File S1 contains one figure and the legends for File S2 and File S3.