Abstract
Prion diseases are associated with the misfolding of the prion protein (PrP) from its normal cellular form (PrPC) to its infectious scrapie form (PrPSc). Posttranslational modifications of PrP in vivo can play an important role in modulating the process of misfolding. To gain more insight into the effects of posttranslational modifications on PrP structure and dynamics and to test the hypothesis that such modifications can interact with the protein, we have performed molecular dynamics simulations of diglycosylated human PrPC bound to a lipid bilayer via a glycophosphatidylinositol anchor. Multiple simulations were performed at three different pH ranges to explore pH effects on structure and dynamics. In contrast to simulations of protein-only PrPC, no large effects were observed upon lowering the pH of the system. The protein tilted toward the membrane surface in all of the simulations and the putative PrPSc oligomerization sites became inaccessible, thereby offering a possible protective mechanism against PrPSc-induced misfolding of PrPC.
Keywords: Prion protein misfolding, Membrane simulation, Molecular dynamics
Graphical Abstract
Diglycosylated human prion protein PrPC bound to a lipid bilayer did not misfold upon lowering the pH in molecular dynamics simulations, in contrast to the non-glycosylated protein in water, but the protein tilted toward the membrane surface and putative sites for misfolding and oligomerization became inaccessible, thereby offering a possible protective mechanism against misfolding.

Introduction
Transmissible spongiform encephalopathies (TSEs) are fatal neurodegenerative diseases associated with misfolding of the prion protein (PrP). TSEs include a range of diseases in humans and other mammals, such as Creutzfeldt-Jakob disease (CJD) and bovine spongiform encephalopathy (BSE or mad cow disease). These diseases are caused by the misfolding of the prion protein from its native conformation (PrPC) to a disease-associated form (PrPSc) (Van der Kamp and Daggett, 2009; Prusiner et al., 1998; Gambetti et al., 2003). PrPC is a glycosylphosphatidylinositol (GPI) anchor membrane-bound protein and it is usually diglycosylated at Asn181 and Asn197 (DeArmond et al., 1997, 1999). The native structure of PrPC consists of a flexible N-terminal region (residues 23–127) and a structured C-terminal globular domain (residues 128–228). There are three helices – HA (residues 144–154), HB (residues 173–194), and HC (residues 200–228) – and two β-strands – S1 (residues 128–131) and S2 (residues 161–164) – in the structured globular domain (Figure 1). PrPC is presented at the cell surface of neurons, and it has been implicated in signal transduction (Mouillet-Richard et al., 2000; Linden et al., 2008; Resenberger et al., 2011) and metal metabolism (Burns et al., 2003; Millhauser, 2007; Klewpatinond et al., 2008; Singh et al., 2009).
Figure 1. PrPmem system used for MD simulations.
(A) Cyan and purple colored surfaces are the glycans attached to Asn181 and Asn197, respectively. The GPI anchor is represented as the light-blue surface. HA (residues 144–154), N-terminus (residues 90–127), S1 and S2 (residues 128–131 and 161–164) are colored in cyan, green, and yellow, respectively. The HB (residues 173–194) and HC (residues 200–228) helices are in blue. Remaining loop regions are colored gray. The carbon and oxygen atoms of the phosphatidylcholine (POPC) molecules are indicated as thin cyan and red lines, respectively. Nitrogen and phosphorus atoms are indicated by blue and brown spheres, respectively. The water is not displayed. (B) Schematic depiction of individual residues in the glycan attached to Asn 181 and Asn 197.
The central hypothesis of prion diseases is that they are protein-only diseases, whereby the prion protein is the only agent required for propagation and transmission of the disease (Prusiner et al., 1998). Recombinant PrP, which lacks glycans and the GPI anchor, is the primary construct used in experimental studies. However, non-protein moieties affect the disease process in vivo. The N-linked glycans influence PrP expression, distribution (within regions of the brain and among different types of neuronal cells) and deposition of PrPSc in vivo (DeArmond et al., 1997, 1999). The glycans have also been observed to modify the conformation of PrPC and/or affect the affinity of PrPC for a particular strain of PrPSc (Rudd et al., 1999; DeMarco and Daggett, 2005; Collinge et al., 1996; Safar et al., 1998). Nonetheless, based on the protein structure alone, recombinant PrPC appears to be a good model for biologically relevant forms of PrPC, because the structures of diglycosylated human PrPC and recombinant PrPC are similar, as indicated by circular dichroism (CD) and Nuclear Magnetic Resonance Spectrometry (NMR) (Hornemann et al., 2004).
Most of the molecular dynamics (MD) simulation studies of PrP only focus on the protein-only portion (PrPprot), i.e. the simulations include neither the posttranslational modifications nor the membrane environment. Various computational studies have been performed on PrPprot at acidic pH ranges (Alonso et al., 2001; Cheng and Daggett, 2014; Van der Kamp and Daggett, 2010). Experiments indicate that low pH cellular environments, such as endocytic organelles and lipid rafts, play a role in misfolding (Borchelt et al., 1992; Arnold et al., 1995). In order to provide a more realistic simulation environment of PrPC to determine the pH effects on misfolding, it is necessary to include the non-protein moieties in simulations.
Thus far there has been only one membrane MD simulation study of GPI-anchored diglycosylated PrPC in which both PrPprot and the full scrapie-competent construct (i.e. with the N-terminus built on to the NMR fragment) including the membrane, glycans and GPI anchor (PrPmem) were simulated (DeMarco and Daggett, 2009). In this case the simulations were performed at neutral and low pH at 25°C and they began with the protein perpendicular to the membrane to allow for interactions between the two to evolve naturally. However, the PrPmem simulations were only 15 ns. Here we build upon that work by performing multiple, longer simulations that include the glycans, GPI anchor and lipid membrane at 37°C to better explore the conversion pathway of PrPC and its structural dynamics under more physiological conditions. We performed simulations of the system in triplicate at low, mid, and neutral pH. Each PrPmem simulation was 80 ns long, increasing sampling time from 30 ns to 720 ns, and we have 750 ns of control free PrPC MD at this same temperature and pH regimes, for a total of ~1.5 μs. With these new simulations we have both confirmed and extended our earlier findings and observed interactions between the protein and non-protein moieties that potentially modulate conversion of PrPC. We found that the structured C-terminal domain of PrP is more stable with glycans and membrane than without such non-protein moieties. The glycans did not form contacts with the membrane, but the Asn181 glycan formed contacts with the flexible N-terminus of PrP. In addition, PrP tilted towards the membrane such that the putative oligomerization sites on the protein were shielded by the membrane surface. Our findings shed light on why conversion of PrPC to PrPSc is more efficient with the bare recombinant protein than with glycosylated, membrane-bound PrPC.
Materials and Methods
Starting structures of PrPprot and PrPmem
For PrPprot, the starting coordinates for the globular region (residues 125–228) were obtained from the human NMR structure (Zahn et al., 2000) (PDBID: 1QLX). The NMR structure was then extended to include residues 90–230; this longer construct is necessary because the N-terminal residues not present in the NMR structures are critical to infectivity, and the shorter NMR construct is not scrapie competent. In order to model residues 90–230 of PrP, the missing N-terminal residues were added to the structure such that the N-terminus extended away from the protein to avoid bias, as described previously (Van der Kamp and Daggett, 2010; DeMarco and Daggett, 2009). To construct PrPmem (Figure 1A), non-protein moieties of the system were added to the PrPprot system, as described previously (DeMarco and Daggett, 2009). Briefly, two 13-residue glycans were attached to Asn181 and Asn197. A triantennary glycoform scaffold common to mouse PrPC and PrPSc was chosen (Stimson et al., 1999). This glycan is representative of human N-glycans (Varki et al., 2003) (Figure 1B). This glycan contains one sialic group and the acid was deprotonated in all simulations as its pKa is ~2.5, which is below the pH regimes investigated here. The GPI anchor was attached to the C-terminus of PrP. Further details regarding building of the glycan and GPI components are provided by DeMarco and Daggett (2009). The GPI anchor was embedded in a 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) membrane bilayer, which consists of 335 lipid-molecules. The lipid bilayer was modelled based on the structure built by the Schulten group (Heller et al., 1993). POPC was chosen as is has been identified as the most abundant lipid around PrP (Brugger et al, 2004).
MD simulations
For both PrPprot and PrPmem simulations, the starting structures were solvated in a pre-equilibrated rectangular water box (Levitt et al., 1997) with a density of 0.993 g/ml, which is the experimental density for water at 37 °C (Kell, 1967). Detailed methods of the PrPprot simulations have been described previously (Van der Kamp and Daggett, 2010). For the PrPmem system simulations, the periodic box, which extended >20 Å from any solute atom above and below, had the following dimensions: 94 × 98 × 147 Å. Neutral, mid, and low pH ranges were modelled by altering protonation states of histidine, aspartate, and glutamate residues, as described previously (Van der Kamp and Daggett, 2010). Unfortunately, pKa values are not available for these groups, so the pH ranges fall in the following approximate ranges: neutral, pH ≥ ~6; mid, 4 ≤ pH ≤ 6; and low, 3 ≤ pH ≤ 4. These ranges are approximate and individual pKas can certainly differ from the ‘standard’ values, but this approach has correctly modeled pH-dependent conformational behavior in a variety of systems, including the prion protein. The Levitt et al. (1995) force field was employed for all MD simulations, and simulations were performed at 37°C using in lucem molecular mechanics (ilmm) (Beck et al., 2000–2017) and standard procedures (Beck and Daggett, 2004). The lipid (Smolin et al, 2008) parameters have been presented previously and they were derived to be consistent with our force field. For the PrPmem system, triplicate simulations were performed for each pH using a different random number seed, and each simulation was performed for 80 ns. Performing multiple independent simulations improves sampling over a single simulation (e.g. 3 x 80 ns should provide better sampling and improved probability of observing conformational transitions compared with a single simulation of 240 ns). For the PrPprot system, five simulations were performed for each pH regime, and each simulation was 50 ns long. In total we have ~1.5 μs of sampling: 720 ns for PrPmem and 750 ns for PrPprot.
MD simulation analysis
Analyses such as, solvent accessible surface area (SASA), dictionary definition of secondary structure of proteins (DSSP), Cα root-mean square deviation (RMSD), and Cα root-mean square fluctuation (RMSF) were performed with ilmm as previously described (Van der Kamp and Daggett, 2010). NOE restraint data (BMRB 4641) were obtained from human PrP E200K (Zhang et al., 2000). NOE restraint satisfaction of PrPmem simulations was measured for the combined simulations in each pH regime with ilmm. An NOE restraint was considered satisfied if the <r−6> weighted distance between the nearest protons associated with the NOE was less than the upper bound or 5 Å, whichever is greater. The HC tilt angle was measured between a vector fit through Cα atoms of residues 200–214 on HC and the x-y plane. The x-y plane was an approximation of the membrane surface. Heavy atom contacts were considered intact when two carbon atoms were ≤ 5.4 Å apart or when other interacting heavy atoms were ≤ 4.6 Å apart. The population of a contact (percentage time present over the simulation) was averaged across simulation replicates for each pH regime. The 40–80 ns of PrPmem simulations and the 10–50 ns time periods of PrPprot simulations were used to calculate the average contact occupancies.
Statistics
Data are presented as the mean and associated standard deviation. Overall statistics over time amounted to analysis of 1,500,000 time points (or structures). Statistical properties and frequencies over the specific time periods provided in the previous section included 40,000 samples for each simulation.
Results and Discussion
The starting structure of the PrPprot system (bare protein in water) was created using the human NMR PrPC structure (PDB:1QLX). The missing N-terminal residues 90–127 were added to the structure, as they are important to PrP conversion and pathology (Swietnicki et al., 1997; Luhrs et al., 2006). These N-terminal residues were built to extend away from the structured globular domain to avoid bias in sampling of the N-terminus, as described previously (Van der Kamp and Daggett, 2010; DeMarco and Daggett, 2009). The PrPmem system (including glycans, the GPI anchor and the membrane), in addition to the NMR structure, was created by placing PrP on a phosphatidylcholine (POPC) lipid bilayer (Figure 1A). The GPI anchor was embedded in the lipid bilayer and attached to the C-terminus of the PrP. Glycans were attached to the glycosylation sites at Asn181 and Asn197. Simulations of both the PrPprot and PrPmem systems were performed at neutral, mid and low pH. The pH environment was modeled by altering the protonation states of charged residues such that low pH corresponds to pH below the pKa of Asp and Glu (approximately pH 4), mid pH corresponds to pH above 4 and below the pKa of His, and neutral pH is above the pKa of His (see Materials and Methods). All simulations were performed at 37 °C.
Misfolding in Protein-only PrPC simulations
PrPprot simulations (without GPI anchor, glycans and membrane) at neutral, mid and low pH yielded structures that deviate significantly from the starting structure. The Cα root-mean square deviation (RMSD) was used to detect gross conformational changes in simulations. The average Cα RMSD for the globular domain (residues 128–228) for neutral, mid and low pH simulations were 2.12 ± 0.5 Å, 2.59 ± 0.38 Å and 2.96 ± 1.27Å, respectively, showing increasing structural changes upon lowering the pH. One of the major contributors to the increase in Cα RMSD at low pH was the HA helix region: the HA helix along with its preceding loop (S1-HA loop) was displaced from its native position, resulting in significant disruption in hydrophobic contacts (Figure 2A,B). Such a conformational change was also observed in previous simulations of human PrP pathogenic mutants (Van der Kamp and Daggett, 2010; Cheng and Daggett, 2014; Chen et al., 2010) and low pH simulations of human PrP (Van der Kamp and Daggett, 2010). Hydrophobic packing is crucial to protein stability, and disruption of the S1-HA loop packing increases the aggregation tendency of PrP. In addition, detachment of the S1-HA loop from the HC helix is necessary for PrPSc fibrillation, as indicated in both in vitro (Eghiaian et al., 2007; Hafner-Bratkovic et al., 2011) and in vivo (Hafner-Bratkovic et al., 2011) disulfide engineering experiments. These experiments support our earlier hypothesis that PrPC misfolding involves the exposure of the hydrophobic S1-HA loop, with the formation of a new hydrophobic strand that is a site for PrPSc-oligomerization. We denoted this oligomerization site as the E4 strand (Figure 2B), since it consistently forms an extended structure in simulations of various PrP species at low pH (Alonso et al., 2001; DeMarco and Daggett, 2004; DeMarco et al., 2006; Scouras and Daggett, 2008).
Figure 2. Misfolding in PrPprot simulations.
(A) Left: starting structure. Right: helix HA structure for the last 25 ns of all PrPprot simulations at 1 ns granularity. Structures were aligned to the starting structure at the stable core residues 174–186 and 200–219, as shown in blue. Cyan, orange, and red cartoons represents structures from neutral, mid and low pH simulations, respectively. (B) Left: starting structure. Right: mid pH simulation 3 at 49.9 ns. Hydrophobic residues 134, 137, 139, 141, 205, 209 and 213 are shown with translucent surface and stick representations of the side chains. E4 region is colored in orange. (C) Formation of the E1 strand (in red). Left: crystal structure (PDBID: 4KML (Abskharon et al., 2014)). Right: a representative MD-derived misfolded structure from PrPprot simulations. (D) An MD-derived converted PrPSc structure was used to construct a threefold screw-axis spiral model. The oligomerization site occurs between the E1 and E4 strands of the adjacent monomers. The structures are not to scale with panels A–C.
In addition to the E4 strand, another nonnative strand (denoted E1) forms at the N-terminus in the PrPprot simulations (Figure 2C). The E1 strand was recruited by the native strand S1, and our previous simulations also have formed this E1 strand, which ranges from residues 113–122 (DeMarco and Daggett, 2004; Scouras and Daggett, 2008; Alonso et al., 2001). We hypothesized that the formation of the E1 strand is another hallmark of PrP misfolding, and that the E1 strand is also a site for PrPSc-oligomerization. Our results are in agreement with experiments indicating that the PrP peptides within residues 106–127 of the N-terminus aggregate and form β-sheet-rich oligomers and fibrils (Lee et al., 2008; Silva et al., 2003; Walsh et al., 2010; Cheng et al., 2011). Interestingly, a recent crystal structure of PrP in complex with a PrPSc-inhibitory nanobody also has an E1 strand consisting of N-terminal residues 118–122 (Figure 2C) (Abskharon et al., 2014), lending further support to the MD-derived misfolded structures and our prediction and characterization of this strand before the structure was determined (Alonso et al., 2001).
In order to interpret our MD-derived misfolded PrPSc structures in the context of the oligomerization process, we have previously proposed the spiral model. In the spiral model, MD-derived misfolded PrPSc structures were assembled by docking the E1 strand of one monomer to an adjacent neighbor at the E4 strand, and in this way the monomers are arranged along a three-fold screw-axis (Figure 2D). The spiral model has been previously validated by various experimental data (DeMarco and Daggett, 2004; DeMarco et al., 2006), and recent experimental data on PrPSc soluble oligomers lend further support to our model (Cheng and Daggett, 2017, in preparation).
Although misfolding was observed in the PrPprot simulations, the experiments indicate that membrane-bound PrPC is more resistant to misfolding, since neither acidic treatment (Baron et al., 2002) nor denaturants (Lin et al., 2013) have been shown to induce misfolding and oligomerization of membrane-bound PrPC. Furthermore, unlike bare recombinant PrP, membrane-bound PrPC appears to be resistant to PrPSc-induced misfolding unless it is released from membranes by phospholipase C digestion (Baron et al, 2002; Baron and Caughey, 2003). We hypothesize two possible mechanisms by which the posttranslational modifications and the membrane environment contribute to the misfolding-resistance: (1) the glycans and membrane environment provide contacts that stabilize the native structure of PrPC; and (2) the glycans and membrane environment shield the putative oligomerization sites, strands E1 and E4, thereby providing a steric barrier to misfolding and oligomerization. In the following results from our PrPmem simulations, we evaluate the stability of PrP using Cα RMSD, Nuclear Overhauser Effect Crosspeak (NOE) satisfaction and contact analyses. Details regarding the contacts and relative positions between PrP and the glycans, and between PrP and the membrane, are also provided. We conclude by discussing how the glycans and the membrane may contribute to protective mechanisms against PrP misfolding.
Conformational changes in PrPmem simulations
Gross conformational changes of PrP in the PrPmem simulations are reflected in the Cα RMSD relative to the starting structure. In all pH regimes, the average Cα RMSDs of the globular domain of PrP (residues 128–228) were within the range of 1.5–2.0 Å (Figure S1), reflecting the retention of the native-like globular domain structure. The Cα root-mean square fluctuations (RMSF) about the average structure after equilibration reflect the mobility of the protein. The Cα RMSF values of the residues in the globular domain were less than 2 Å in all pH regimes, whereas those of the residues in the flexible N-terminus were in the range of 2 – 5 Å (Figure S2).
NOE satisfaction by PrPmem simulations
The PrP structures in the PrPmem simulations were compared to the Nuclear Overhauser Effect crosspeaks (NOE) reflecting the proximity of protons throughout the structure by comparing with the NOE restraints of the human PrP E200K mutant (Zhang et al., 2000), which is structurally similar to wild type human PrP, using a previously described method (Van der Kamp and Daggett, 2010). The NOE restraints are available for residues 125–230, which cover the structured, helical domain. Structures from the neutral, mid, and low pH PrPmem simulations satisfied 88% of the 2641 NOEs, in all pH regimes. Therefore, PrP structures from our PrPmem simulations are similar to the NMR structures of human recombinant PrP regardless of the pH environment. This is in agreement with CD experiments that confirm that PrPC retains a helix-rich native structure when bound to POPC vesicles (Sanghera and Pinheiro, 2002).
Acidic pH effects on PrPmem simulations
There were contact differences in acidic and neutral pH simulations (Table S1). The mid pH simulations lost the Glu152-His155 contact when the His140-Asp147 as well as other interactions formed. As for the low pH simulations, only two residue contacts were significantly disrupted: Leu125-Ile182 and Tyr157-Phe198. The Leu125-Ile182 contact was affected primarily by the loss of a hydrogen bond between side chains of Tyr128 and Asp178 upon protonation of the Asp. As for the Tyr157-Phe198 contact, Phe198 became more exposed and lost hydrophobic packing with Tyr157. As a consequence of these changes, residues 154–157 at the C-terminus of HA lost helicity in both the mid and low pH simulations compared to neutral pH (Table S1). These minor structural and intramolecular contact changes were all directly affected by the protonated residues at mid and low pH. These contact changes were localized and did not have a significant impact on the structure, as indicated by the globular Cα RMSD (Figure S1). These results are consistent with the experiments, which indicates that low pH misfolding is inefficient for membrane-bound PrP (Baron et al., 2002).
PrPmem vs PrPprot: Major differences in intramolecular interactions
There were three PrP regions with significant contact differences between PrPmem and PrPprot simulations: the Phe198 hydrophobic pocket, the native sheet packing with the HB and HC helices, and the packing between HC and the S1-HA loop. A list of relevant contact occupancies are provided in Table S2. Overall, the contacts in the PrPmem simulations were much more stable than those in PrPprot. These three areas that differ are discussed in detail below.
In the PrPmem simulations, Phe198 was buried and its average side chain solvent accessible surface area (SASAs) for neutral, mid, and low pH simulations was only 4, 13, and 19 Å2, respectively. On the other hand, the SASA of Phe198 in the PrPprot simulations was at least three to four times greater (Figure S3). As Phe198 became exposed, the contacts between Phe198 and Met206 were lost in the mid and low pH PrPprot simulations. The exposure of Phe198 was accompanied by the formation of the nonnative His187-Asp202 salt bridge. The side chains of His187 and Asp202 occupied the native side chain position of Phe198 (Figure 3A). This nonnative salt bridge kept the side chain of Phe198 in a solvent exposed conformation, which is consistent with our previous PrPprot simulations (Van der Kamp and Daggett, 2010). On the other hand, in all PrPmem simulations, Phe198 retained stable core contacts with neighboring residues, including His187 and Met206. The side chain motion of Phe198 was deterred by the bulky glycan attached to Asn197. As a result, the side chain of Phe198 remained buried in between HB and HC in all PrPmem simulations.
Figure 3. Differences between PrPmem and PrPprot simulations.
Structures are taken from mid pH simulations. Relevant residue contact occupancies are displayed. Standard deviations are computed for both PrPmem and PrPprot (For each pH, n = 3 for PrPmem, and n = 5 for PrPprot). (A) Phe198 hydrophobic pocket. The black oval indicates the nonnative salt bridge that formed in PrPprot simulation. (B) Native sheet packing with HB and HC. The black ovals indicate the loss of hydrogen bonds in PrPprot simulations. (C) S1-HA loop packing with HC. The S1-HA loop detaches from HC and loses hydrophobic packing in PrPprot.
In all PrPmem simulations, the native S2 strand was tightly packed to HB and HC, such that Thr183 formed a stable contact with Gln160 (Figure 3B). The residue contact between Gln160 and Met213 on HC was populated on average >80% of the time in the neutral and mid pH PrPmem simulations (Table S1), but this interaction was less stable in the low pH simulations (36% occupancy). On the other hand, all of the PrPprot simulations, regardless of pH, showed a significant loss in both Gln160-Thr183 and Gln160-Met213 contacts. Other than the packing between S2 and the helices HB and HC, there were two hydrogen bonds that contributed to the stability of this region: Tyr128-Asp178 and Tyr162-Thr183. The side chain of Tyr128 on S1 stabilized the native sheet by hydrogen bonding to the side chain of Asp178 on HB (Figure 3B). As for the side chain of Thr183, it hydrogen bonded with the backbone amide of Tyr162 (Figure 3B). These hydrogen bonds were more prevalent in PrPmem simulations than PrPprot simulations. Overall, the native sheet in PrPmem formed stable packing interactions and hydrogen bonds while such interactions were lost in the PrPprot simulations.
Contacts in the S1-HA loop were more variable due to the conformational flexibility of this loop. One of the relatively robust contacts formed in PrPmem simulations was Arg136-Val209, in which the hydrophobic portion of the side chain of Arg136 packed against the side chain of Val209 on HC (Figure 3C). In PrPprot, however, this contact was diminished and highly variable across simulations. Such loss of contacts between S1-HA loop and HC was expected, since the HA helix of the low pH PrPprot simulations was highly mobile and occasionally detached from HC (Figure S4).
Interactions between the membrane and the flexible N-terminus
The flexible N-terminus (residues 90–127) formed contacts with the membrane surface in the PrPmem simulations (Figure S5). As can be seen in Figure S5, The N-terminus made extensive contacts with the membrane with respect to both the number of residues involved and the extent of time that contacts were formed in all simulations at mid and low pH. This was also the case in two of the three simulations at neutral pH. As an illustration of the particularly prevalent interactions, His96 and Lys101 each formed long-lasting salt bridges with the phosphate groups on the membrane surface at low and mid pH (Figure 4A,B). These two positively charged residues are within residues 90–110, which represent one of the charged clusters of PrP (Wang et al., 2010). These charge clusters are important for electrostatic interactions involved in membrane association (Boland et al., 2010). At acidic pH, PrP binds to anionic lipids with higher affinity (Morillas et al., 1999). The pH effect was observed in our simulations, as reflected in the heightened interactions with the membrane at mid and low pH (Figure S5), and illustrated more specifically for a stable salt bridge between the doubly-protonated His96 with a phosphate group on the membrane (Figure 4A).
Figure 4. Contacts between N-terminus and the membrane surface at mid and low pH.

Contacts between residues 90–117 and the membrane are shown on the right. (A) Structure of membrane-bound PrP in mid pH simulation 3 at 80 ns. His96 formed a stable salt bridge with phosphate head group on the membrane. (B) Structure of membrane-bound PrP in low pH simulation 2 at 80 ns. Lys106 formed stable salt bridge with phosphate head group on the membrane.
Interactions between the glycans and the flexible N-terminus
The N-terminus of PrP interacted with the Asn181 glycan via Lys residues, but not with the Asn197 glycan. For example, at neutral pH, Lys101 formed stable contacts with the Asn181 glycan (Figure 5A), preventing the N-terminus from forming contacts with the membrane (Figure S5). At mid pH, Lys104 also formed stable contacts with the Asn181 glycan in a similar conformation (Figure 5B). At low pH, Lys104 bound to the Asn181 glycan in a conformation different from that of the neutral and mid pH simulations but with the same effect (Figure 5C).
Figure 5. Contacts between the Asn183 glycan and lysine residues at the flexible N-terminus.
Contacts between residues 90–117 and the Asn181 glycan are shown in the right column. Relevant lysine residues, N-acetylneuraminate (NeuNAc), galactose (Gal), N-acetylglucosamine (GlcNAc), and fucose (Fuc) are shown as sticks. (A) Neutral pH simulation 3 at 80 ns. (B) Mid pH simulation 2 at 80 ns. (C) Low pH simulation 3 at 80 ns.
Such consistent interactions between lysine residues and the Asn181 glycan were surprising and heretofore unrecognized. However, it is known that the charge clusters at the flexible N-terminus of PrP are important for binding glycosaminoglycans (GAGs), such as heparin, chondroitin sulfate, and hyaluronic acid (Pan et al., 2002). Given the similar constituents of GAGs and glycans, it is reasonable to expect that the charge cluster of the flexible N-terminus can bind to the glycans, as indicated in our simulations. Glycosylation of PrP impedes the conversion efficiency in in vitro conversion assays (Priola and Lawson, 2001). While these large glycans can sterically inhibit PrPSc-induced misfolding, our results suggest that glycans can also bind to N-terminal lysine residues, thereby deterring the N-terminus from forming the E1 strand, which is a hallmark for misfolding in our MD-simulated conversion. Other than retardation of the misfolding process, glycans also play a complicated role in prion propagation (Zahn et al., 2000). Given that the glycans can form stable interactions with the flexible N-terminus of PrP, different glycans may induce different N-terminal conformations, thus influencing strain-specific prion propagation, which is sensitive to conformational changes in PrPSc. Another complication is that while the glycans appear to stabilize and deter conversion of PrPC, the glycans are also involved in recognition and targeting of PrPSc to PrPC glycoforms in different regions of the brain (DeArmond et al., 1999). Unfortunately, the PrPC simulations presented here cannot directly address potential PrPC-PrPSc interactions, but our results suggest that the glycans have a number of effects on PrPC as well as its interactions with PrPSc. While speculative, PrPSc binding may compete with favorable glycan-PrPC interactions; MD simulations investigating these possible mechanisms are in progress.
Tilting of the structured C-terminal domain on the membrane
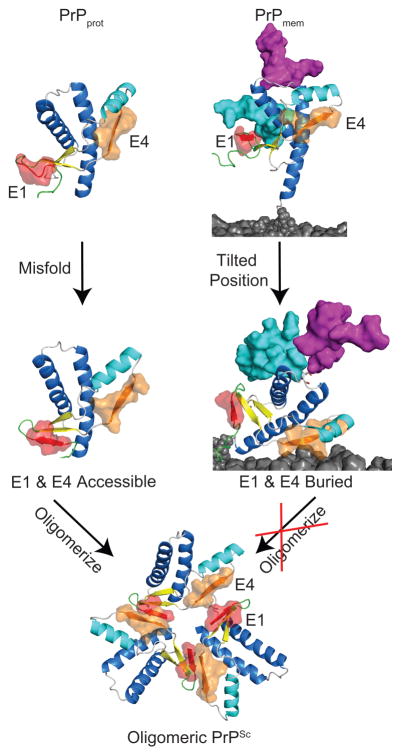
In all of the PrPmem simulations, PrP tilted toward the membrane. For example, the angle between HC and the membrane surface dropped from 90 to ~30o by the end of the simulations (Figure 6A,B). This tilting motion placed the putative E1 and E4 oligomerization sites on or very near the membrane surface. Exposure of residues 135–140 (the E4 region) was probed, and in low pH simulation 1, as an example, its exposure dropped from ~500 Å2 to < 200 Å2 by 45 ns. This significant reduction was due to the tilting motion of PrP, which buried the E4 epitope (Figure 6B). This tilted conformation was stable and buried the E4 epitope around 45 ns and remained as such until the end of the simulation. The other putative oligomerization site, the E1 strand, was formed only in low pH simulation 3 (Figure 7). However, with a 30o tilt, the E1 strand was buried on the membrane surface. We hypothesize that the tilting motion of membrane-bound PrPC retards PrPSc-induced misfolding by masking putative oligomerization sites on PrPC (Figure 8). Experiments broadly support this hypothesis, given that membrane-bound PrP is more resistant to misfolding than free PrP (Baron et al., 2002; Baron and Caughey, 2003). In addition, an antibody that targets residues 138–141 shows significantly decreased binding of membrane-bound PrPC compared with soluble PrPC (Leclerc et al., 2003), consistent with steric occlusion of E4 in the MD simulations.
Figure 6. PrP tilting on the membrane protecting the E4 putative oligomerization site. (A).
HC tilt angle for PrPmem simulation 1 (blue), 2 (green) and 3 (orange) at neutral, mid and low pH. (B) Before and after the tilting motion in low pH simulation 1.
Figure 7. Buried E1 epitope putative oligomerization site.

(A) Structure of the membrane-bound PrP in low pH simulation 3 at 80ns. The orange epitope is E1, which is buried on the membrane surface in grey. (B) Secondary structure of the simulation over time.
Figure 8. Schematic of PrP conversion.
Without glycans and the membrane environment, PrPprot misfolds and both E1 and E4 strands are accessible, allowing for oligomerization. As for PrPmem, the oligomerization sites E1 and E4 are buried or near the membrane surface, which would interfere of PrPSc to PrPC and propagation of PrPSc.
Conclusions
Overall, the PrPmem simulations were extremely stable and preserved a native-like PrPC structure in all pH regimes, unlike the PrPprot simulation of free unglycosylated PrPC. In most of the PrPmem simulations, the flexible N-terminus formed sporadic contacts with the membrane except for a few lysine and histidine residues in the N-terminus that formed stable salt bridges with the phosphate groups on the membrane. The glycans may modulate the misfolding process by interfering with E1 formation at the N-terminus through direct contacts with lysine residues at the N-terminus. The tilting motion of membrane-bound PrP buried the E1 and E4 regions, both of which are putative oligomerization sites. This led us to hypothesize that the tilted conformation of membrane-bound PrPC protects critical regions of PrPC and deters PrPSc-induced misfolding, explaining why recombinant PrPC converts more readily.
Supplementary Material
Acknowledgments
We are grateful for financial support from the National Institutes of Health (GM 95808 to VD). HK was supported through a PhD-stipend from the Lundbeck Foundation, the Danish Council for Independent Research | Natural Science and through grants from the Carlsberg Foundation and the Danish Center for Scientific Computing (DCSC). The authors have no conflict of interest to declare.
Abbreviations used
- MD
Molecular Dynamics
- TSE
Transmissible spongiform encephalopathy
- PrP
prion protein
- PrPC
cellular prion protein, biologically active form
- PrPSc
scrapie prion protein, infectious conformer
- CJD
Creutzfeldt-Jakob disease
- BSE
bovine spongiform encephalopathy
- GPI
Glycosylphosphatidylinositol
- NMR
Nuclear Magnetic Resonance Spectrometry
- DSSP
Dictionary of secondary structure of proteins
- SASA
Solvent accessible surface area
References
- Abskharon RNN, Giachin G, Wohlkonig A, Soror SH, Pardon E, Legname G, Steyaert J. Probing the N-Terminal β-Sheet Conversion in the Crystal Structure of the Human Prion Protein Bound to a Nanobody. J Am Chem Soc. 2014;136:937–944. doi: 10.1021/ja407527p. [DOI] [PubMed] [Google Scholar]
- Alonso DOV, DeArmond SJ, Cohen FE, Daggett V. Mapping the early steps in the pH-induced conformational conversion of the prion protein. Proc Natl Acad Sci USA. 2001;98:2985–2989. doi: 10.1073/pnas.061555898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold JE, Tipler C, Laszlo L, Hope J, Landon M, Mayer RJ. The abnormal isoform of the prion protein accumulates in late-endosome-like organelles in scrapie-infected mouse brain. J Pathol. 1995;176:403–411. doi: 10.1002/path.1711760412. [DOI] [PubMed] [Google Scholar]
- Baron GS, Caughey B. Effect of Glycosylphosphatidylinositol Anchor-dependent and -independent Prion Protein Association with Model Raft Membranes on Conversion to the Protease-resistant Isoform. J Biol Chem. 2003;278:14883–14892. doi: 10.1074/jbc.M210840200. [DOI] [PubMed] [Google Scholar]
- Baron GS, Wehrly K, Dorward DW, Chesebro B, Caughey B. Conversion of raft associated prion protein to the protease-resistant state requires insertion of PrP-res (PrPSc) into contiguous membranes. EMBO J. 2002;21:1031–1040. doi: 10.1093/emboj/21.5.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck DAC, Daggett V. Methods for molecular dynamics simulations of protein folding/unfolding in solution. Methods. 2004;34:112–20. doi: 10.1016/j.ymeth.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Beck DAC, McCully ME, Alonso DOV, Daggett V. in lucem Molecular Mechanics (ilmm) University of Washington; Seattle: 2000–2017. [Google Scholar]
- Boland MP, Hatty CR, Separovic F, Hill AF, Tew DJ, Barnham KJ, Haigh CL, James M, Masters CL, Collins SJ. Anionic Phospholipid Interactions of the Prion Protein N Terminus Are Minimally Perturbing and Not Driven Solely by the Octapeptide Repeat Domain. J Biol Chem. 2010;285:32282–32292. doi: 10.1074/jbc.M110.123398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchelt DR, Taraboulos A, Prusiner SB. Evidence for synthesis of scrapie prion proteins in the endocytic pathway. J Biol Chem. 1992;267:16188–16199. [PubMed] [Google Scholar]
- Brugger B, Graham C, Leibrecht I, Mombelli E, Jen A, Wieland F, Morris R. The membrane domains occupied by glycosylphosphatidylinositol-anchored prion protein and Thy-1 differ in lipid composition. J Biol Chem. 2004;279:7530–7536. doi: 10.1074/jbc.M310207200. [DOI] [PubMed] [Google Scholar]
- Burns CS, Aronoff-Spencer E, Legname G, Prusiner SB, Antholine WE, Gerfen GJ, Peisach J, Millhauser GL. Copper Coordination in the Full-Length, Recombinant Prion Protein. Biochemistry. 2003;42:6794–6803. doi: 10.1021/bi027138+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Van der Kamp MW, Daggett V. Diverse Effects on the Native β-Sheet of the Human Prion Protein Due to Disease-Associated Mutations. Biochemistry. 2010;49:9874–9881. doi: 10.1021/bi101449f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CJ, Daggett V. Different misfolding mechanisms converge on common conformational changes: Human prion protein pathogenic mutants Y218N and E196K. Prion. 2014;8:1–11. doi: 10.4161/pri.27807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CJ, Daggett V. Molecular Dynamics Simulations Capture the Misfolding of the Bovine Prion Protein at Acidic pH. Biomolecules. 2014;4:181–201. doi: 10.3390/biom4010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CJ, Daggett V. Comparison of PrPSc models from a Soluble Oligomer Perspective, in preparation 2017 [Google Scholar]
- Cheng HM, Tsai TWT, Huang WYC, Lee HK, Lian HY, Chou FC, Mou Y, Chan JCC. Steric Zipper Formed by Hydrophobic Peptide Fragment of Syrian Hamster Prion Protein. Biochemistry. 2011;50:6815–6823. doi: 10.1021/bi200712z. [DOI] [PubMed] [Google Scholar]
- Collinge J, Sidle KCL, Meads J, Ironside J, Hill AF. Molecular analysis of prion strain variation and the aetiology of “new variant” CJD. Nature. 1996;383:685–690. doi: 10.1038/383685a0. [DOI] [PubMed] [Google Scholar]
- DeArmond SJ, Sánchez H, Yehiely F, Qiu Y, Ninchak-Casey A, Daggett V, Camerino AP, Cayetano J, Rogers M, Groth D, Torchia M, Tremblay P, Scott MR, Cohen FE, Prusiner SB. Selective Neuronal Targeting in Prion Disease. Neuron. 1997;19:1337–1348. doi: 10.1016/s0896-6273(00)80424-9. [DOI] [PubMed] [Google Scholar]
- DeArmond SJ, Qiu Y, Sànchez H, Spilman PR, Ninchak-Casey A, Alonso D, Daggett V. PrPc glycoform heterogeneity as a function of brain region: implications for selective targeting of neurons by prion strains. J Neuropathol Exp Neurol. 1999;58:1000–1009. doi: 10.1097/00005072-199909000-00010. [DOI] [PubMed] [Google Scholar]
- DeMarco ML, Daggett V. From conversion to aggregation: Protofibril formation of the prion protein. Proc Natl Acad Sci USA. 2004;101:2293–2298. doi: 10.1073/pnas.0307178101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarco ML, Daggett V. Local environmental effects on the structure of the prion protein. Comp Rend Biol. 2005;328:847–62. doi: 10.1016/j.crvi.2005.05.001. [DOI] [PubMed] [Google Scholar]
- DeMarco ML, Daggett V. Characterization of cell-surface prion protein relative to its recombinant analogue: insights from molecular dynamics simulations of diglycosylated, membrane-bound human prion protein. J Neurochem. 2009;109:60–73. doi: 10.1111/j.1471-4159.2009.05892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarco ML, Silveira J, Caughey B, Daggett V. Structural properties of prion protein protofibrils and fibrils: An experimental assessment of atomic models. Biochemistry. 2006;45:15573–15582. doi: 10.1021/bi0612723. [DOI] [PubMed] [Google Scholar]
- Eghiaian F, Daubenfeld T, Quenet Y, van Audenhaege M, Bouin AP, Van der Rest G, Grosclaude J, Rezaei H. Diversity in prion protein oligomerization pathways results from domain expansion as revealed by hydrogen/deuterium exchange and disulfide linkage. Proc Natl Acad Sci USA. 2007;104:7414–7419. doi: 10.1073/pnas.0607745104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambetti P, Kong Q, Zou W, Parchi P, Chen SG. Sporadic and familial CJD: classification and characterisation. Br Med Bull. 2003;66:213–239. doi: 10.1093/bmb/66.1.213. [DOI] [PubMed] [Google Scholar]
- Hafner-Bratkovič I, Bester R, Pristovšek P, Gaedtke L, Veranič P, Gašperšič J, Manček-Keber M, Avbelj M, Polymenidou M, Julius C, Aguzzi A, Vorberg I, Jerala R. Globular domain of the prion protein needs to be unlocked by domain swapping to support prion protein conversion. J Biol Chem. 2011;286:12149–12156. doi: 10.1074/jbc.M110.213926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller H, Schaefer M, Schulten K. Molecular dynamics simulation of a bilayer of 200 lipids in the gel and in the liquid crystal phase. J Phys Chem. 1993;97:8343–8360. [Google Scholar]
- Hornemann S, Schorn C, Wüthrich K. NMR structure of the bovine prion protein isolated from healthy calf brains. EMBO Rep. 2004;5:1159–1164. doi: 10.1038/sj.embor.7400297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kell GS. Precise representation of volume properties of water at one atmosphere. J Chem Eng Data. 1967;12:66–69. [Google Scholar]
- Klewpatinond M, Davies P, Bowen S, Brown DR, Viles JH. Deconvoluting the Cu2+ Binding Modes of Full-length Prion Protein. J Biol Chem. 2008;283:1870–1881. doi: 10.1074/jbc.M708472200. [DOI] [PubMed] [Google Scholar]
- Leclerc E, Peretz D, Ball H, Solforosi L, Legname G, Safar J, Serban A, Prusiner SB, Burton DR, Williamson RA. Conformation of PrPC on the cell surface as probed by antibodies. J Mol Biol. 2003;326:475–483. doi: 10.1016/s0022-2836(02)01365-7. [DOI] [PubMed] [Google Scholar]
- Lee SW, Mou Y, Lin SY, Chou FC, Tseng WH, Chen C, Lu CYD, Yu SSF, Chan JCC. Steric Zipper of the Amyloid Fibrils Formed by Residues 109–122 of the Syrian Hamster Prion Protein. J Mol Biol. 2008;378:1142–1154. doi: 10.1016/j.jmb.2008.03.035. [DOI] [PubMed] [Google Scholar]
- Levitt M, Hirshberg M, Sharon R, Daggett V. Potential energy function and parameters for simulations of the molecular dynamics of proteins and nucleic acids in solution. Comput Phys Commun. 1995;91:215–231. [Google Scholar]
- Levitt M, Hirshberg M, Sharon R, Laidig KE, Daggett V. Calibration and testing of a water model for simulation of the molecular dynamics of proteins and nucleic acids in solution. J Phys Chem B. 1997;101:5051–5061. [Google Scholar]
- Lin SJ, Yu KH, Wu JR, Lee CF, Jheng CP, Chen HR, Lee CI. Liberation of GPI-Anchored Prion from Phospholipids Accelerates Amyloidogenic Conversion. Int J Mol Sci. 2013;14:17943–17957. doi: 10.3390/ijms140917943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden R, V, Martins R, Prado MAM, Cammarota M, Izquierdo I, Brentani RR. Physiology of the Prion Protein. Physiol Rev. 2008;88:673–728. doi: 10.1152/physrev.00007.2007. [DOI] [PubMed] [Google Scholar]
- Millhauser GL. Copper and the Prion Protein: Methods, Structures, Function, and Disease. Ann Rev of Phys Chem. 2007;58:299–320. doi: 10.1146/annurev.physchem.58.032806.104657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morillas M, Swietnicki W, Gambetti P, Surewicz WK. Membrane Environment Alters the Conformational Structure of the Recombinant Human Prion Protein. J Biol Chem. 1999;274:36859–36865. doi: 10.1074/jbc.274.52.36859. [DOI] [PubMed] [Google Scholar]
- Mouillet-Richard S, Ermonval M, Chebassier C, Laplanche JL, Lehmann S, Launay JM, Kellermann O. Signal Transduction Through Prion. Protein Science. 2000;289:1925–1928. doi: 10.1126/science.289.5486.1925. [DOI] [PubMed] [Google Scholar]
- Pan T, Wong BS, Liu T, Li R, Petersen RB, Sy MS. Cell-surface prion protein interacts with glycosaminoglycans. Biochem J. 2002;368:81–90. doi: 10.1042/BJ20020773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priola SA, V, Lawson A. Glycosylation influences cross-species formation of protease-resistant prion protein. EMBO J. 2001;20:6692–6699. doi: 10.1093/emboj/20.23.6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner SB, Scott MR, DeArmond SJ, Cohen FE. Prion Protein Biology Cell. 1998;93:337–348. doi: 10.1016/s0092-8674(00)81163-0. [DOI] [PubMed] [Google Scholar]
- Resenberger UK, Harmeier A, Woerner AC, Goodman JL, Müller V, Krishnan R, Vabulas RM, Kretzschmar HA, Lindquist S, Hartl FU, Multhaup G, Winklhofer KF, Tatzelt J. The cellular prion protein mediates neurotoxic signalling of β-sheet-rich conformers independent of prion replication. EMBO J. 2011;30:2057–2070. doi: 10.1038/emboj.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd PM, Endo T, Colominas C, Groth D, Wheeler SF, Harvey DJ, Wormald MR, Serban H, Prusiner SB, Kobata A, Dwek RA. Glycosylation differences between the normal and pathogenic prion protein isoforms. Proc Natl Acad Sci USA. 1999;96:13044–13049. doi: 10.1073/pnas.96.23.13044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safar J, Wille H, Itri V, Groth D, Serban H, Torchia M, Cohen FE, Prusiner SB. Eight prion strains have PrPSc molecules with different conformations. Nat Med. 1998;4:1157–1165. doi: 10.1038/2654. [DOI] [PubMed] [Google Scholar]
- Sanghera N, Pinheiro TJT. Binding of prion protein to lipid membranes and implications for prion conversion. J Mol Biol. 2002;315:1241–1256. doi: 10.1006/jmbi.2001.5322. [DOI] [PubMed] [Google Scholar]
- Scouras AD, Daggett V. Species variation in PrPSc protofibril models. J Mater Sci. 2008;43:3625–3637. [Google Scholar]
- Silva RAGD, Barber-Armstrong W, Decatur SM. The Organization and Assembly of a β-Sheet Formed by a Prion Peptide in Solution: An Isotope-Edited FTIR Study. J Am Chem Soc. 2003;125:13674–13675. doi: 10.1021/ja036725v. [DOI] [PubMed] [Google Scholar]
- Singh A, Isaac AO, Luo X, Mohan ML, Cohen ML, Chen F, Kong Q, Bartz J, Singh N. Abnormal Brain Iron Homeostasis in Human and Animal Prion Disorders. PLoS Pathog. 2009;5:e1000336. doi: 10.1371/journal.ppat.1000336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolin N, Li B, Beck DAC, Daggett V. Side-chain dynamics are critical for water permeation through aquaporin-1. Biophysical Journal. 2008;95:1089–1098. doi: 10.1529/biophysj.107.125187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimson E, Hope J, Chong A, Burlingame AL. Site-specific characterization of the N-linked glycans of murine prion protein by high-performance liquid chromatography electrospray mass spectrometry and exoglycosidase digestions. Biochemistry. 1999;38:4885–4895. doi: 10.1021/bi982330q. [DOI] [PubMed] [Google Scholar]
- Swietnick W, Peterson R, Gambetti P, Surewicz WK. pH-dependent Stability and Conformation of the Recombinant Human Prion Protein PrP(90–231) J Biol Chem. 1997;272:27517–27520. doi: 10.1074/jbc.272.44.27517. [DOI] [PubMed] [Google Scholar]
- Van der Kamp MW, Daggett V. The consequences of pathogenic mutations to the human prion protein. Protein Eng Des Sel. 2009;22:461–468. doi: 10.1093/protein/gzp039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kamp MW, Daggett V. Influence of pH on the human prion protein: insights into the early steps of misfolding. Biophys J. 2010;99:2289–98. doi: 10.1016/j.bpj.2010.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kamp MW, Daggett V. Pathogenic mutations in the hydrophobic core of the human prion protein can promote structural instability and misfolding. J Mol Biol. 2010;404:732–748. doi: 10.1016/j.jmb.2010.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A, Cummings R, Esko J, Freeze H, Hart G, Marth J. The Essentials of Glycobiology. Cold Spring Harbor Laboratory Press; New York: 2003. [PubMed] [Google Scholar]
- Walsh P, Neudecker P, Sharpe S. Structural Properties and Dynamic Behavior of Nonfibrillar Oligomers Formed by PrP(106–126) J Am Chem Soc. 2010;132:7684–7695. doi: 10.1021/ja100431q. [DOI] [PubMed] [Google Scholar]
- Wang F, Yin S, Wang X, Zha L, Sy MS, Ma J. Role of the highly conserved middle region of prion protein (PrP) in PrP-lipid interaction. Biochemistry. 2010;49:8169–8176. doi: 10.1021/bi101146v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R, Liu A, Lührs T, Riek R, von Schroetter C, López García F, Billeter M, Calzolai L, Wider G, Wüthrich K. NMR solution structure of the human prion protein. Proc Natl Acad Sci USA. 2000;97:145–50. doi: 10.1073/pnas.97.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Swietnicki W, Zagorski MG, Surewicz WK, Sönnichsen FD. Solution Structure of the E200K Variant of Human Prion Protein Implications for the Mechanism of Pathogenesis in Familial Prion Diseases. J Biol Chem. 2000;275:33650–33654. doi: 10.1074/jbc.C000483200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.